CD4+CD25+FOXP3+ regulatory T cells (CD4+ Treg cells) are thought to differentiate in the thymus and immigrate from the thymus to the periphery. Treg cells can regulate both acquired and innate immunity through multiple modes of suppression. The cross-talk between Treg cells and targeted cells, such as antigen-presenting cells (APCs) and T cells, is crucial for ensuring suppression by Treg cells in the appropriate microenvironment. Emerging evidence suggests that Treg compartmentalization and trafficking may be tissue or/and organ specific and that distinct chemokine receptor and integrin expression may contribute to selective retention and trafficking of Treg cells at sites where regulation is required. In this review, the cellular and molecular signals that control specialized migration and retention of Treg cells are discussed.
Introduction
Appropriate trafficking and retention are indispensable for immune cells to mediate efficient immune responses in vivo.1 Emerging evidence demonstrates that regulatory T cells, particularly CD4+CD25+ regulatory T cells (CD4+ Treg cells), play a central role in the immunopathogenesis of autoimmune diseases, tumors, and organ transplantation.2-8 It is speculated that Treg cells modulate immune responses through selective migration and accumulative retention at sites where regulation is required. Current data suggest that the migratory capacity of Treg cells is controlled by distinct signals from chemokines/chemokine receptors and integrins/integrin ligands. Several recent reviews have extensively analyzed Treg cells in the context of basic biology,4,9-12 autoimmune diseases,3 organ transplantation,10 allergies,13 infectious diseases,14,15 and tumor immunity.8 In this review, we solely discuss the cellular and molecular signals responsible for specialized Treg migration and retention. We suggest that selective targeting of Treg-cell trafficking and compartmentalization would be therapeutically beneficial.
Treg-cell homeostatic compartmentalization and lymphoid homing
Naturally occurring murine Treg cells differentiate in the thymus.2-5 In homeostatic conditions, Treg cells are found primarily in thymus, peripheral blood, lymph nodes, and spleen.4,10 Interestingly, 6% to 10% of CD4+ T cells are Treg cells in both human and mouse thymus, blood, lymph nodes, and spleen.4,10,16-18 Thus, it appears that there is equal distribution of Treg cells among the different lymphoid compartments. Strikingly, more than 25% of all CD4+ T cells are phenotypically and functionally Treg cells in both normal human and murine bone marrow.16 This suggests that during homeostasis Treg cells are actively, rather than passively, retained in the bone marrow and bone marrow may function as an immune regulatory organ via Treg-cell recruitment and retention. We will discuss the potential migratory mechanisms for Treg-cell trafficking to various organs including bone marrow.
Although it is generally accepted that Treg cells are derived in the thymus then exit from thymus and migrate into the periphery including lymphoid organs,4,10 it is poorly understood how thymic-derived CD4+ Treg cells emigrate into peripheral organs and whether this mechanism is distinct from that of conventional T cells.
The integrin CD62L is a crucial lymphoid homing molecule for immune cells including conventional T cells. Recent reports suggest that both murine CD4+CD25+CD62L+ T cells and CD4+CD25+CD62L– T cells are capable of suppressing T-cell activation in vitro with similar suppressive capacity.19,20 However, it is the CD4+CD25+CD62L+ subset, rather than the CD4+CD25+CD62L– T cells, that efficiently protect from lethal acute graft-versus-host disease (GVHD)21,22 and delay diabetes in prediabetic nonobese diabetic (NOD) mice.20 It is possible that the in vivo suppressive activity of CD4+CD25+CD62L+ Treg cells may be superior to that of CD4+CD25+CD62L– Treg cells; however, it is more likely that the different in vivo suppressive activity may be due to distinct lymphoid homing capacity. CD4+CD25+CD62L+ Treg cells may migrate into draining lymph nodes more efficiently than CD4+CD25+CD62L– Treg cells. In support of this, administration of neutralizing CD62L-specific antibody blocks expansion of Treg cells in draining lymph nodes and results in allogeneic graft rejection.23 This indirect evidence suggests that CD62L may be essential for murine CD4+ Treg cells to efficiently traffic into the draining lymph nodes.
The chemokine receptor CCR7 is another crucial lymphoid homing molecule for immune cells. Although human peripheral blood Treg cells express CCR7 to some degree in an in vitro migration assay, they do not efficiently migrate in response to CCL19, the ligand for CCR7.17,24 Recently, it was reported that human CD4+CD25+CD69– tonsillar T cells migrated to CCL19, a chemokine expressed in the T-cell zone.25 These data suggest that a CCL19/CCR7 signal could facilitate CD4+CD25+CD69– Treg-cell migration toward the T-cell zones within lymphoid organs. Furthermore, upon activation, the chemokine receptor expression on Treg cells is switched from CCR7 to CXCR5. This change in chemokine receptor expression is consistent with reduced migration toward CCL19 and increased migration toward CXCL13, a chemokine expressed in the B-cell zone. This suggests that upon activation, Treg-cell migration toward the T-cell zone changes to trafficking toward B-cell follicles.
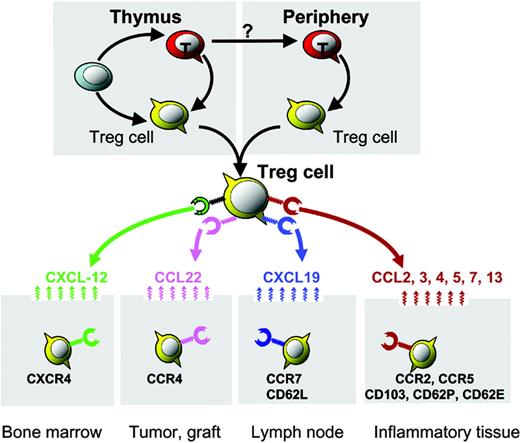
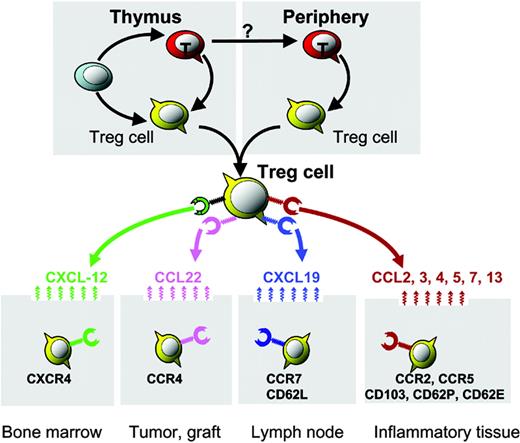
Organ/tissue trafficking and distribution of Treg cells. Distinct chemokine receptors and integrin molecules implicated in Treg-cell organ/tissue trafficking and compartmentalization. Bone marrow–derived CXCL12 mediates Treg-cell bone marrow trafficking. Environmental CCL22 mediates Treg-cell trafficking into human ovarian cancer and mouse cardiac grafts. The lymphoid homing molecules CCR7 and CD62L may facilitate lymphoid homing of Treg cells. Certain CC chemokines and integrins may mediate Treg-cell trafficking into inflammatory tissues/organs.
Organ/tissue trafficking and distribution of Treg cells. Distinct chemokine receptors and integrin molecules implicated in Treg-cell organ/tissue trafficking and compartmentalization. Bone marrow–derived CXCL12 mediates Treg-cell bone marrow trafficking. Environmental CCL22 mediates Treg-cell trafficking into human ovarian cancer and mouse cardiac grafts. The lymphoid homing molecules CCR7 and CD62L may facilitate lymphoid homing of Treg cells. Certain CC chemokines and integrins may mediate Treg-cell trafficking into inflammatory tissues/organs.
Collectively, although a direct link between CD62L, CCR7, and Treg-cell lymphoid homing is missing, current studies suggest that CD62L and CCR7 could be important lymphoid homing molecules for CD4+ Treg cells (Figure 1; Table 1). Further definitive experiments are warranted, such as adoptively transferring CD62L–/– and CCR7–/– Treg cells to study their lymphoid homing capacity in vivo. Interestingly, recent reports demonstrated that CCR7 was essential for conventional T cells in exiting from the periphery and entering the afferent lymphatics.35,36 As Treg cells are found in peripheral tissues, it is unknown whether peripheral Treg cells would contra-flow into lymphoid organs, including thymus, lymph nodes, and spleen, and, if so, whether CCR7 is also implicated in this process.
Treg-cell bone marrow trafficking
Bone marrow is vascularized by blood, not by lymphatic vessels, and is a part of the lymphocyte recirculation network,37 with billions of lymphocytes recirculating through it each day. Bone marrow has long been known to be a primarily hematopoietic organ. However, there has been a growing realization that bone marrow may also play a critical role in immunity. For example, long-lived, antibody-secreting plasma cells reside in bone marrow.38 Furthermore, a number of reports have demonstrated that functional memory T cells exist in bone marrow.39,40 Bone marrow can serve as a site for naive tumor-associated antigen (TAA)–specific T-cell priming.39-42 Interestingly, TAA-specific T cells isolated from the bone marrow of tumor-bearing mice and cancer patients are functional in vitro and are able to prevent tumor growth when transferred to another host. These data suggest that these TAA-specific T cells are functionally suppressed in the bone marrow.40-44 Therefore, it is evident that the bone marrow plays an active role in humoral and cellular lymphocyte immunity.
This notion was further supported by our recent observation that large numbers of functional Treg cells accumulate in the bone marrow of healthy volunteers and mice.16 This observation was confirmed in a FOXP3 bicistronic reporter knock-in mouse model.45 In this model, a bicistronic reporter expressing a red fluorescent protein has been knocked into the endogenous FOXP3 locus. High levels of FOXP3-expressing T cells (with red fluorescence) are found in the bone marrow.45 Bone marrow Treg cells express functional CXCR4, the receptor for CXCL12, and Treg-cell release from bone marrow is achieved through granulocyte colony-stimulating factor (G-CSF), reducing marrow expression of CXCL12.16 Interestingly, activation of Treg cells upregulates CXCR4 expression and enables them to migrate to the bone marrow through CXCL12.16 Thus, CXCR4/CXCL12 signals are crucial for activated Treg-cell bone marrow trafficking. This suggests that bone marrow is a preferential site for migration and/or selective retainment of CD4+ Treg cells and bone marrow may function as an immunoregulatory organ16 (Figure 1; Table 1).
Treg cells differentiate in the thymus. The murine thymus produces Treg cells as a functionally separate lineage that express FOXP3.46,47 However, thymic function is largely reduced after adolescence whereas Treg cells persist throughout the human lifespan. Although the half-life for Treg cells remains undefined, it is critical to understand how the Treg-cell pool is maintained. Indeed, Treg cells may have to be induced or/and expanded in the periphery, such as in the bone marrow. Although little is known about homeostatic expansion Treg cells, based on the literature, memory CD8+ T cells are maintained in the absence of antigen through a process of homeostatic proliferation.48,49 Recent work using IL-15–/– and IL-7–/– mice has demonstrated that these 2 cytokines are critical for memory CD8+ T-cell maintenance. IL-15 is primarily responsible for driving memory cells into cell cycle.50-52 More strikingly, it was shown recently that bone marrow is the preferential site for homeostatic proliferation and recruitment of CD8+ memory T cells.39,40 Thus, it will be interesting to determine whether Treg cells preferentially expand in the bone marrow.
On the other hand, bone marrow is a common site for human tumor metastasis, suggesting that bone marrow would provide an immunosuppressive environment for tumor retention and growth. Accumulation of Treg cells in the bone marrow may provide an immune shield to facilitate tumor bone marrow metastasis.
Treg-cell tumor trafficking
Tumors express TAAs that should render them objects of immune attack. Cancer-bearing hosts often exhibit detectable tumor-specific immunity, although spontaneous immunologic clearance of cancer is rare. Thus, boosting antitumor immunity with various vaccine strategies is a logical approach to improving current cancer treatments. However, recent evidence demonstrates that tumors actively defeat TAA-specific immunity. Mechanisms of active immune evasion are the subject of much recent research. Most tumor-associated antigens are self-antigens and it has been postulated that patients develop tolerance to their TAAs (and hence their tumor) through Treg cells. In support of this hypothesis, we recently demonstrated that in all ovarian tumor stages there are consistently fewer Treg cells in tumor-draining lymph nodes than in lymphoid organs unrelated to the tumor. This suggests that Treg cells may traffic to tumors from lymph nodes. In support of this concept, ovarian tumor Treg cells express functional CCR4, the receptor for CCL22, and migrate toward tumor microenvironmental CCL22 in vitro and in vivo. The source of tumor CCL22 appears to be cancer cells and tumor-associated macrophages.17 Therefore, tumor environmental CCL22 mediates Treg-cell tumor trafficking (Figure 1; Table 1). These ovarian tumor Treg cells are functionally suppressive and able to block tumor-specific immunity, foster tumor growth, and predict poor patient survival.17
Notably, in addition to Treg cells, other T cells may express CCR4 and would migrate into the tumor through CCL22. Hence, the suppressive effects would depend on the balance between Treg cells and effector T cells in the tumor microenvironment.7 In addition to human ovarian cancer,17,53 a higher frequency of CD4+CD25+ T cells in both peripheral blood and tumors was reported in patients with a variety of cancers including breast cancer,54 colorectal cancer,55,56 esophageal cancer,57 gastric cancer,57,58 hepatocellular carcinoma,59 leukemia,60 lung cancer,53,56 lymphoma,60,61 melanoma,62,63 and pancreatic cancer.54 This list continues to grow following the current interest of studying Treg cells in human tumors. Altogether, these data suggest that specific recruitment of Treg cells into tumors may defeat TAA-specific T-cell immunity.
Treg-cell trafficking into transplanted organs
The induction and maintenance of immune tolerance to transplanted tissues is an active process involving multiple cooperative mechanisms to prevent graft rejection. It has been repeatedly demonstrated that Treg cells play an important role in allotolerance.2-5 Early studies showed that specific tolerance can be induced through adoptively transferring Treg cells from spleen and lymph nodes of tolerant mice to naive mice. Treg cells are found largely in the draining lymph nodes. Tolerance was initially thought to occur mainly in lymphoid organs. Subsequently, it was found that transplanted organs including kidney,64 skin,65 and cardiac tissues27 harbor functional Treg cells. For example, in a murine model of allogeneic skin transplantation, when tolerated skin grafts are retransplanted onto T-cell–depleted hosts, graft-infiltrating T cells including Treg cells exit the graft and recolonize the new host. These colonizing T cells can be shown to contain members with regulatory function, as they can prevent nontolerant lymphocytes from rejecting fresh skin allografts, without hindrance of rejection of third-party skin.65 Thus, Treg cell–mediated suppression of graft rejection is an active process that operates beyond secondary lymphoid tissue and involves the persistent presence of Treg cells at the site of the tolerated transplant.
How do Treg cells migrate into transplanted organs? In line with our study of ovarian cancer patients, a recent study demonstrated that Treg cells are present within cardiac allografts and that their homing to allografts is CCR4 dependent. Interestingly, there was also increased expression of one of the 2 main CCR4 ligands, CCL22, but not thymus-and-activation–regulated chemokine (TARC; CCL17) in the grafts.27 We also observed selective expression of CCL22 in the ovarian tumor environment, which attracted Treg cells into tumors.17 In addition to CCR4, another study using mouse CCR5–/– Treg cells demonstrated that CCR5 is essential for the recruitment of Treg cells to both lymphoid tissues and GVHD target organs including liver, lung, and spleen.28 These studies highlight that Treg cells can be recruited into various transplanted organs and tissues with distinct mechanisms.
Treg-cell trafficking into inflammatory tissues
Treg cells were initially found to efficiently control inflammation mediated by autoimmune diseases.2-5 Subsequently, in addition to autoimmune diseases, Treg cells were implicated in controlling inflammation mediated by infectious pathogens and allergens (Table 2). As Treg cells reside in lymphoid organs, early studies suggested that Treg cells predominantly inhibit naive T-cell priming in the draining lymph nodes. Further studies show that Treg cells infiltrate and traffic into inflammatory sites in various inflammatory diseases and suppress effector T-cell function31,73-77 (Table 2). It was suggested that distinct expression of chemokine receptors and integrin molecules on Treg cells was responsible for lymphoid homing versus variable peripheral tissue trafficking. For example, the chemokine receptor CCR2 mediates Treg-cell trafficking into inflammatory joints in mice with collagen-induced arthritis,26 and we previously discussed the potential role of the chemokine receptor CCR7 in Treg-cell lymphoid homing. In addition to chemokine receptors, integrin molecules are crucial for guiding Treg-cell migration in vivo. For example, αE (CD103)– Treg cells highly express CD62L and CCR7, efficiently migrate into lymph nodes, and predominantly suppress naive T-cell activation within the lymph nodes, whereas CD103+ Treg cells express low levels of CD62L, efficiently migrate into inflammatory sites, and predominantly suppress peripheral inflammation.32-34 In support of this notion, CD103 was reported to be necessary for the homing of Treg cells at sites of Leishmania major infection and in turn contributes to the outcome of chronic infection.31 Strikingly, CD103 expression by dendritic cells is reported to be essential for CD103+ Treg-cell homing into intestinal tissues, and CD103 expression on dendritic cells influences the balance between effector and regulatory T-cell activity in the intestine in the mouse colitis model.78 However, it remains to be defined how and why CD103 on dendritic cells influences Treg-cell migration and function, whereas mouse Treg cells deficient in CD62P and CD62E are incapable of migrating into inflammatory skin and fail to inhibit tissue inflammation.29,30 Together, these data suggest that Treg cells can be recruited into variable inflammatory tissues and distinct migratory molecular patterns are responsible for Treg-cell migration (Table 1).
IL-2 therapy and Treg-cell tumor trafficking
IL-2 has been used to treat HIV-infected patients and patients with various tumors. Studies of the mechanisms of IL-2 action revealed that IL-2 restores the ability of the immune system to produce CD4+ T cells from T-cell precursors, stimulates CD4+ T-cell expansion, prolongs the half-life of CD4+ T cells, and activates natural killer (NK) cells.79-81 Interestingly, recent data indicates that in patients with HIV, the long-term effect of IL-2 treatment is a decrease of T-cell proliferation and activation in vivo accompanied by an increase of CD4+CD25+ T cells.82 CD4+CD25+ Treg cells are capable of inhibiting HIV-specific T-cell activation.83 Therefore, it remains to be determined whether functional suppressive Treg cells are enriched in these IL-2–induced CD4+CD25+ T cells.
Several groups recently observed that administration of IL-2 significantly increased the number of CD4+CD25+ T cells in patients with ovarian cancer (S.W., I.K., and W.Z., unpublished data, January-August 2005), melanoma,84 Ewing sarcoma, and alveolar rhabdomyosarcoma.85 These CD4+CD25+ T cells highly expressed FOXP3 and efficiently suppressed polyclonal and TAA-specific T-cell responses. This indicates that IL-2–induced CD4+CD25+ T cells may be predominantly suppressive Treg cells. In line with these findings, recent studies in mice have indicated that IL-2 contributes to Treg-cell differentiation, expansion, maintenance, and suppressor function in mice.86-89 Therefore, the data reveal a critical role of IL-2 in human Treg-cell differentiation, expansion, and function.
On the other hand, despite recent advances in our knowledge, relatively little is known regarding the regulation of Treg-cell trafficking.16,17 We demonstrated that G-CSF can mobilize Treg cells from bone marrow to periphery.16 Human blood Treg cells express CCR4 and CCR8 and migrate in response to their ligands in vitro.24 High levels of CCL22 and CXCL12 are found in human ovarian cancer17,90,91 but peripheral blood Treg cells efficiently migrate in response to CCL22 not CXCL12. Tumor environmental CCL22 mediates Treg-cell trafficking into ovarian tumors in vivo through CCR4.17 We now have data demonstrating that IL-2 promotes Treg-cell tumor migration through CXCR4. The notion is supported by 2 lines of evidence. First, IL-2 stimulates CXCR4 expression on Treg cells in vitro and in vivo. Second, IL-2–conditioned Treg cells efficiently migrate toward recombinant and tumor-derived CXCL12 in vitro. Thus, administration of IL-2 would accelerate CD4+ Treg tumor trafficking (S.W., I.K., and W.Z., unpublished data, January-August 2005).
IL-7, IL-15, and IL-21 share γc receptor and certain properties with IL-2. They stimulate innate immunity and enhance CD8+ T-cell–mediated antitumor activity.92-94 Furthermore, IL-7 and IL-15 together are able to abrogate the suppressive activity of Treg cells in vitro.95 Therefore, IL-2 may be therapeutically replaced by other γc cytokines. The γc cytokines such as IL-7, IL-15, and IL-21, alone or in concert, may be useful as a novel immunotherapeutic strategy. Notably, administration of IL-2 results in clinical therapeutic efficacy in a small population of patients with melanoma and renal cell carcinoma. It suggests that in addition to Treg cells, the effects of IL-2 on other immune cells need to be considered. Nonetheless, our data provide an urgent rationale for reevaluating the clinical benefit of IL-2 treatment in patients with cancer and AIDS.
Concluding remarks
Treg cells regulate both acquired and innate immunity through multiple modes of suppression. Appropriate Treg-cell migration and retention ensure Treg-cell suppression occurs in the microenvironment where regulation is required. Emerging evidence suggests that Treg-cell compartmentalization and trafficking may exhibit a tissue or/and organ specificity and could be modulated. Distinct expression patterns of chemokine receptors and integrins and their responsiveness contribute to selective retention and trafficking of Treg cells. If this concept is correct, we can hypothesize that Treg-cell mobilization or blockade of Treg-cell trafficking would be therapeutically beneficial.
Prepublished online as Blood First Edition Paper, DOI 10.1182/blood-2006-01-0177.
Supported by grants from the National Institutes of Health (CA092562, CA100227, CA099985) and the Department of Defense (DC020173).
We would like to thank our collaborators for their intellectual input and hard work.