Abstract
There has been interest in generating T cells expressing chimeric artificial receptors (CARs) targeting CD19/CD20 antigens to treat B-cell lymphomas. If successful, however, this approach would likely impair humoral immunity because T cells may persist long-term. Most low-grade lymphoma and chronic lymphocytic leukemia (B-CLL) cells express monoclonal immunoglobulins carrying either κ or λ light chains. We, therefore, explored whether T lymphocytes could be genetically modified to target the tumor-associated light chain, sparing B lymphocytes expressing the reciprocal light chain, and consequently reduce impairment of humoral immunity. We found that T lymphocytes expressing the anti-κ light chain CAR showed cytotoxic activity against Igκ+ tumor cell lines and B-CLL cells both in vitro and in vivo. We also found that the incorporation of the CD28 endodomain within the CAR enhanced the in vitro and in vivo expansion of transgenic T cells after tumor-associated antigen stimulation. Free Igκ+ did not compromise the ability of redirected T lymphocytes to eliminate Igκ+ tumors because these free immunoglobulins served to sustain proliferation of CAR-CD28 transgenic T cells. Thus, adoptive transfer of T lymphocytes targeting the appropriate light chain could be a useful immunotherapy approach to treat B-lymphocyte malignancies that clonally express immunoglobulin without entirely compromising humoral immunity.
Introduction
Low-grade non-Hodgkin lymphomas (B-NHLs) and B-cell chronic lymphocytic leukemia (B-CLL) are generally characterized by a smoldering clinical course.1,2 Nonetheless, these diseases slowly progress and require intervention. Although remission can be obtained with chemotherapy and antibody directed to B-cell antigens such as CD20, most patients ultimately have relapses.3-5 More aggressive treatments including allogeneic stem cell transplantation may eradicate disease, apparently in part by a T cell–mediated graft-versus-leukemia (GVL) effect.6-8 Unfortunately, their high rate of morbidity and mortality limits their application to younger patients.9,10 Because these malignancies are sensitive to both T cell–mediated and antibody-mediated cytotoxic effector functions, there has been increasing interest in combining these approaches and recruiting the host immune system to help eradicate the disease that remains after conventional treatments. Anti-idiotype vaccine or whole tumor cell–based vaccines have been used in several clinical trials, but although antitumor activity was observed, the effects were often limited and transient.11-14 An alternative means of recruiting both the cellular and humoral arms of the immune response is to adoptively transfer T cells genetically modified to express a B cell–specific antibody incorporated in an artificial chimeric T-cell receptor (CAR).15,16 These molecules combine the antigen-binding property of monoclonal antibodies with the lytic capacity and potential longevity of T lymphocytes to provide an enhanced antitumor effect.16
Because B-NHL and B-CLL stably express CD19 or CD20 antigens, adoptive transfer of CD19- or CD20-specific CARs to T lymphocytes has been proposed.17-20 However, adoptively transferred T cells, unlike monoclonal antibodies, may have almost indefinite persistence21 so that success of this approach would likely be associated with long-term impairment of humoral immunity. We now propose an alternative target for chimeric T cells. B lymphocytes express surface monoclonal immunoglobulins with either κ or λ light chains. Because expression of κ/λ is clonally restricted, and because low-grade B-NHL and B-CLL are themselves clonal, the malignant cells in a given individual will express either κ or λ light chain.22 Chimeric T lymphocytes targeting the light chain expressed by the tumor should spare normal B cells expressing the reciprocal light chain. Because no functional differences have been found between antibodies containing the κ or λ chains23 and because κ light chain deficiency has been described in animals24 and humans24,25 without increased susceptibility to infection, sparing the normal population of B lymphocytes expressing the nontargeted light chain should have minimal adverse effects on patient immunity. We now demonstrate the feasibility of this approach using a κ light chain–specific chimeric T-cell receptor.
Materials and methods
Cell lines and tumor cells
Daudi, BJAB, K562, Raji, and CCL-120 were obtained from the American Type Culture Collection (ATCC; Rockville, MD). JAKO-1 was obtained from the German Collection of Cell Cultures (DMSZ, Braunschweig, Germany). The SP53 was kindly provided by Dr Amin Hesham (M. D. Anderson Cancer Center, Houston, TX). All cells were maintained in culture with RPMI 1640 medium (Gibco-BRL, Gaithersburg, MD) containing 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 25 IU/mL penicillin, and 25 mg/mL streptomycin (all from BioWhittaker, Walkersville, MD). Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C. Primary leukemic cells were obtained from peripheral blood of patients with B-CLL. T lymphocytes were isolated from these samples using CD3 microbead antibody (Miltenyi Biotec, Bergisch Gladbach, Germany). The protocol for collection of peripheral blood from healthy donors and patients with B-CLL was approved by the institutional review board (IRB) and ethics review committees at the Baylor College of Medicine (Houston, TX).
Cloning of the single-chain antibody
We cloned the antibody targeting the κ light chain of human immunoglobulins produced by the CRL-1758 hybridoma (ATCC) as a single chain (scFv). The genes coding the variable regions of the heavy chain (VH) and light chain (VL) of the monoclonal antibody were cloned by reverse transcription–polymerase chain reaction (RT-PCR) using a set of murine variable domain-specific primers modified to generate SfiI restriction sites at the 5′ end of the amplified VL and 3′ end of the amplified VL.26 Combinatorial scFv genes were generated by splicing-by-overlap PCR and then ligated into SfiI sites of the replicative form of fUSE5 vector phage DNA (kindly provided by Dr George P. Smith, University of Missouri, Columbia, MO). Ninety-six clones were screened for specific binding to human κ light chain-expressing Daudi target cells by whole-cell enzymelinked immunosorbent assay (ELISA). The specificity of selected clones was confirmed by flow cytometry. Positive clones were sequenced using insert-flanking primers, and the sequences blasted (National Center for Biotechnology Information [NCBI]) to confirm that the PCR fragments corresponded to mouse immunoglobulin sequences.
Generation of retroviral constructs
The scFv sequence was cloned in frame with the human IgG1-CH2CH3 domain and with the ζ chain of the TCR/CD3 complex in the SFG retroviral backbone, previously established in our laboratory.27 The CD28 domain within the construct was included as previously described.28 Three additional retroviral vectors were constructed to label tumor cells and transgenic T lymphocytes for in vivo study. The first 2 vectors encode for the Renilla luciferase (RLuc) gene and the firefly luciferase gene (FFLuc), respectively. These vectors were used for stable transduction of tumor cell lines. After transduction, cells were selected in puromycin (Sigma, St Louis, MO). The third vector encoded the fusion protein eGFP-firefly luciferase (eGFP-FFLuc) and was used to transduce the T lymphocytes.
Retrovirus production and transduction of T lymphocytes
To produce the retroviral supernatant, 293T cells were cotransfected with retroviral vectors, Peg-Pam-e plasmid containing the sequence for MoMLV gag-pol, and the DRF plasmid containing the sequence for the RD114 envelope,29 using the Fugene6 transfection reagent (Roche, Indianapolis, IN), according to the manufacturer's instruction. Supernatant containing the retrovirus was collected 48 and 72 hours later. For transduction, 0.5 × 106/ mL peripheral-blood mononuclear cells (PBMCs) activated with OKT3 (Ortho Biotech, Bridgewater, NJ) and CD28 (Becton Dickinson, Mountain View, CA) antibodies and recombinant human interleukin-2 (rhIL-2; 100 U/mL; Proleukin; Chiron, Emeryville, CA) were plated in complete media (RPMI 1640 [Gibco-BRL] 45%, Click medium [Irvine Scientific, Santa Ana, CA] 45%, supplemented with 10% FCS and 2 mM l-glutamine) in 24-well plates precoated with a recombinant fibronectin fragment (FN CH-296; Retronectin; Takara Shuzo, Otsu, Japan). After the addition of viral supernatant, the cells were spun and incubated at 37°C in 5% CO2. CAR expression on T lymphocytes was measured 72 hours later and the cells maintained in culture in complete media with the addition of rIL-2 (50 U/mL) every 3 days.
Immunophenotyping
Phycoerythrin (PE)–conjugated, fluorescein isothiocyanate (FITC)–conjugated, and peridinin chlorophyll protein (PerCP)–conjugated CD3, CD4, CD8, and CD56 monoclonal antibodies were used to stain T lymphocytes, whereas CD5, CD19, CD20, anti-Igκ, and anti-Igλ antibodies were used to stain tumor cells. All antibodies were from Becton Dickinson. Control samples labeled with an appropriate isotype-matched antibody were included in each experiment. To detect the expression of CARs, T lymphocytes were stained with a monoclonal antibody Fc-specific cyanine-Cy5-conjugated (Fc-γCy5) provided by Jackson ImmunoResearch (West Grove, PA), which recognized the IgG1-CH2CH3 component of the artificial receptor. Cells were analyzed by fluorescence-activated cell sorting FACScan (Becton Dickinson) equipped with the filter set for 4 fluorescence signals. A flow cytometry read-out was also used to measure the cytotoxic activity of transgenic T lymphocytes against primary B-CLL cells.30 Briefly, 2 × 107/mL B-CLL cells were incubated for 10 minutes at room temperature with 1.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes Europe, Leiden, The Netherlands). These target cells were cocultured with T lymphocytes at a 20:1 effector-target (E/T) ratio for 6 to 8 hours at 37°C in 5% CO2. Before and after incubation, the CFSE-labeled cells were counted by FACScan gating on the viable population using a fixed number (5000) of unconjugated beads (BD Biosciences, San Diego, CA) as a standard to allow a precise and consistent quantification of cell death between experiments. The percentage of killing was calculated as follows: [100 – (a/b × 100)] – [100 – c/b × 100], where a is the viable B-CLL absolute cell count 6 to 8 hours after culture with T lymphocytes, b is the viable absolute count at time 0, and c is the viable absolute B-CLL cell count after 6 to 8 hours of culture alone, without T lymphocytes.
Chromium-release assay
The cytotoxic specificity of control and CAR+ T lymphocytes was evaluated in a standard 4-hour 51Cr-release assay, as previously described.31 The targets tested included Daudi, JEKO-1, BJAB, CCL-120, SP53, Raji, and K562. Target cells incubated in media alone or in 1% Triton X-100 (Sigma) were used to determine spontaneous and maximum 51Cr release, respectively. The mean percentage of specific lysis of triplicate wells was calculated as follows: [(test counts – spontaneous counts)/(maximum counts – spontaneous counts)] × 100%.
Coculture experiments
Control and CAR+ T lymphocytes were cultured at 1 × 106 cells/well with serial dilution of tumor cells with or without a low dose of rhIL-2 (25 U/mL). Serial dilutions of human plasma obtained from healthy donors were added to the cocultures to evaluate the inhibitory effect of the soluble immunoglobulins on the antitumor activity of transgenic T cells. After 5 to 7 days of coculture, cells were collected, stained with monoclonal antibodies to detect both T lymphocytes (CD3) and tumor cells (CD19/CD20), and then analyzed by flow cytometry (FACScan; Becton Dickinson). To evaluate the expansion of transgenic T lymphocytes in response to specific antigen, control T lymphocytes and CAR+ T lymphocytes obtained from patients with B-CLL were stimulated weekly with autologous tumor cells (ratio, 1:1) without addition of exogenous cytokines. T lymphocytes were counted and stimulated every week.
Proliferation assay with free immunoglobulins
To evaluate whether soluble immunoglobulins affected the proliferation and expansion of CAR+ T lymphocytes, we cultured cells at 1 × 105 cells/well either with serial dilution of human plasma obtained from healthy donors or serial dilution of purified human immunoglobulins (Jackson ImmunoResearch) without any addition of exogenous cytokines. After 72 hours, the cells were pulsed with 1 μCi (0.037 MBq) methyl-3[H]thymidine (Amersham Pharmacia Biotech, Piscataway, NJ) and cultured for additional 15 hours. The cells were then harvested onto filters and dried, and counts per minute were measured in a β-scintillation counter (TriCarb 2500 TR; Packard BioScience, Meridien, CT). The experiments were performed in triplicate. In other experiments, control and CAR+ T lymphocytes were cultured either with media alone or with media in which serial dilution of plasma or purified immunoglobulins were added every second day. Cells were then counted every third day using trypan blue exclusion.
T lymphocytes redirected to express κ light chain-specific CAR kill Igκ+ tumor cell lines. (A) Immunophenotype of tumor cell lines stained with CD19, anti-κ and anti-λ light-chain–specific antibodies. (B) Cytotoxic activity of T lymphocytes obtained from healthy donors and transduced either with control-GFP vector (□) or CAR46ζ (▦) or CAR46/28ζ (▪). Cytotoxic activity was evaluated in a standard 51Cr-release assay, and results are shown at an E/T ratio of 20:1. Data represent the mean ± SD of 6 different donors.
T lymphocytes redirected to express κ light chain-specific CAR kill Igκ+ tumor cell lines. (A) Immunophenotype of tumor cell lines stained with CD19, anti-κ and anti-λ light-chain–specific antibodies. (B) Cytotoxic activity of T lymphocytes obtained from healthy donors and transduced either with control-GFP vector (□) or CAR46ζ (▦) or CAR46/28ζ (▪). Cytotoxic activity was evaluated in a standard 51Cr-release assay, and results are shown at an E/T ratio of 20:1. Data represent the mean ± SD of 6 different donors.
Cytometric bead array
To measure the release of cytokines (IL-2, IFN-γ, and TNF-α) by T lymphocytes cocultured with tumor cells or purified immunoglobulins, we used the BD Human Th1/Th2 Cytokine cytometric bead array (CBA) kit (Becton Dickinson). The assay was performed according to the manufacturer's instructions on supernatants collected 24 hours after coculture of T lymphocytes with target cells or soluble immunoglobulins. Supernatants were stained with the mixture of Human Cytokine Capture Bead suspension and the PE Detection Reagent (both from Becton Dickinson). After 3 hours of incubation, samples were washed and then analyzed by using Becton Dickinson CBA software.32 Human Th1/Th2 cytokine standards provided with the kit were appropriately diluted and used in parallel to samples for preparation of the standard curves.
In vivo study in a xenogeneic SCID mouse model
To assess the expansion, persistence, and antitumor effect of CAR+ T lymphocytes in vivo, we used a SCID mouse model and an in vivo imaging system. In the first set of experiments, tumor cells were transduced with a retrovirus coding for the RLuc gene and selected under puromycin, whereas control and CAR+ T lymphocytes were transduced a second time with the vector coding for the eGFP-FFLuc gene. SCID mice (8-10 weeks old) were sublethally irradiated (250 rad) and injected intraperitoneally with 5 × 106 tumor cells suspended in Matrigel (Becton Dickinson). Seven to 10 days later when the tumor was detectable, 5 × 106 T lymphocytes were injected intraperitoneally. No exogenous cytokines were administered. For in vivo imaging of T lymphocytes expressing eGFP-FFLuc and tumor cells expressing RLuc, mice were injected intraperitoneally with d-luciferin (150 mg/kg) and coelenterazine (1 mg/kg), respectively. Mice were analyzed using the Xenogen-IVIS Imaging System (kindly provided by Dr Spencer, Baylor College of Medicine, Houston, TX). Briefly, a constant region-of-interest (ROI) was drawn either over the tumor region or T-lymphocyte region and the intensity of the signal measured as total photon/s/cm2/sr (p/s/cm2/sr) was obtained as previously validated.33 In a second set of experiments we assessed the antitumor effect of CAR+ T lymphocytes. Daudi cells labeled with FFLuc gene were injected intraperitoneally (0.5 × 106 cells) and 10 to 11 days later when the tumor was detectable, mice received either control T lymphocytes or CAR+ T lymphocytes (5 × 106). For these experiments T lymphocytes were not labeled. Tumor growth was evaluated using the same bioluminescence approach as described. In a third group of experiments, mice bearing Daudi tumor cells and treated with CAR+ T cells also received human immunoglobulins (Jackson ImmunoResearch). Based on pharmacokinetic studies,34 we injected the human immunoglobulin 3 times weekly intraperitoneally (100 μL at 11 mg/mL). Mouse experiments were performed in accordance with Baylor College of Medicine Animal Husbandry guidelines.
Statistical analysis
All in vitro data are presented as mean ± 1 SD. The Student t test was used to determine the statistical significance of differences between samples, and P values less than .05 were accepted as indicating a significant difference. For the bioluminescent experiments, intensity signals were log-transformed and summarized using mean ± SD at baseline and multiple subsequent time points for each group of mice. Changes in intensity of signal from baseline at each time point were calculated and compared using paired t tests or the Wilcoxon signed rank test.
Results
Cloning of the scFv for anti-κ light chain from the CRL-1758 hybridoma
Six of 96 clones screened by phage display specifically bound to Daudi tumor cells by ELISA. Binding specificity was confirmed by FACSscan analysis. After sequencing and identification of the open reading frame, the scFv (CAR46) was cloned in 2 different retroviral constructs. In the first (CAR46ζ), the scFv was cloned in-frame with the hinge-CH2CH3 region of the IgG1 and with the ζ chain of the TCR/CD3 complex. In the second (CAR46/28ζ), we included the CD28 endodomain between the hinge-CH2CH3 region and the ζ chain.28 For both constructs we used the SFG backbone retroviral vector.
CAR46ζ+ and CAR46/28ζ+ T lymphocytes kill human κ+ tumor cell lines
T lymphocytes obtained from 6 healthy donors were transduced with CAR46ζ or CAR46/28ζ or a control vector coding for GFP. Seventy percent of T lymphocytes (range, 68%-76%) expressed CAR46/28ζ and both CD4+ and CD8+ T cells were transduced. The CAR46ζ construct also transduced both CD4+ and CD8+ cells although efficiency was less, ranging from 30% to 45%. Control T lymphocytes transduced with a GFP vector were more than 50% GFP+. The ability of redirected and control transduced T lymphocytes to kill B-tumor cell lines that are κ+ or λ+ is shown in Figure 1. CAR46/28ζ+ T cells specifically killed Daudi, JEKO-1, BJAB, and CCL-120 cell lines (76% ± 13%, 76% ± 4%, 51% ± 12%, and 48% ± 5%, respectively at an E/T ratio of 20:1), whereas less than 15% killing was observed for SP53 (which is λ+ but κ–), or RAJI (which is λ– and κ–) or the erythroleukemia-derived K562 cell line. T lymphocytes expressing CAR46/ζ lacking the CD28 domain showed the same pattern of specific killing (Figure 1). Nontransduced or GFP-transduced control T cells did not induce specific Cr51 release (< 15%) from any target. Killing was mediated by both CD8+ and CD4+ CAR+ T lymphocytes (data not shown). The amount of specific Cr51 release by both CAR46ζ+ and CAR46/28ζ+ T lymphocytes was proportional to the level of κ+ immunoglobulin expression on the target-cell surface; BJAB and CCL-120 cell lines, which are κ+dim were less efficiently killed (51% ± 10% and 41% ± 11%, respectively, at an E/T ratio of 20:1) than Daudi and JEKO-1, which are κ+bright (69% ± 12% and 75% ± 8%, respectively, at an E/T ratio of 20:1; P = .001 and P < .001, respectively). Nonetheless, even low-expressing target cells are effectively killed because coculture of CAR46ζ+ or CAR46/28ζ+ T lymphocytes with the κ+dim CCL-120 cell line (ratio, 5:1) eliminates viable tumor cells by days 5 to 7, whereas tumor growth continues unabated in cocultures containing control T cells (Figure 2).
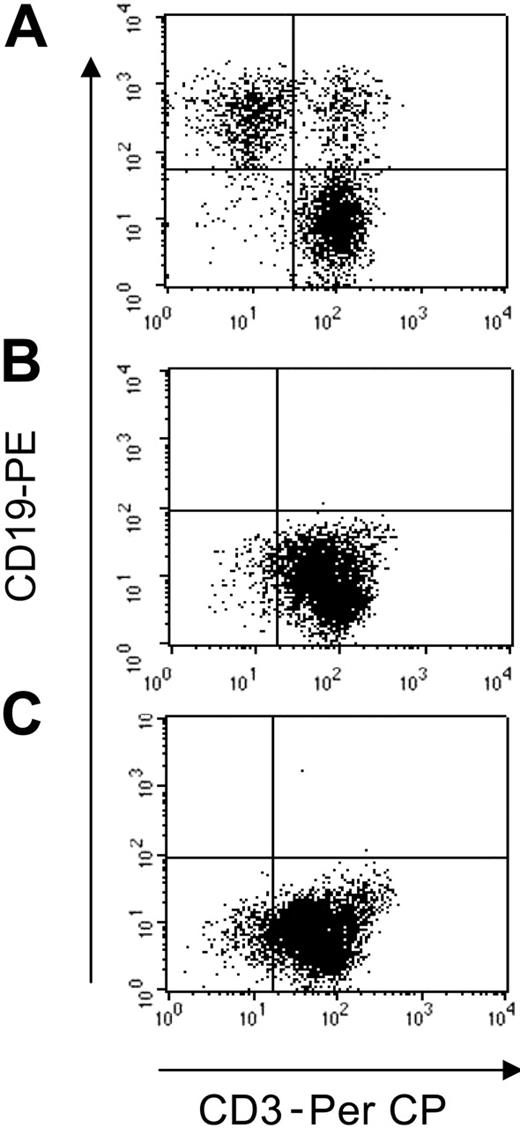
T lymphocytes redirected to express the κ-specific CAR eliminate Igκ+dimtumor cells. T lymphocytes obtained from healthy donors and transduced either with control-GFP vector (A), CAR46ζ (B), or CAR46/28ζ (C) were cocultured with the CCL-120 tumor cell line (ratio, 5:1), which is Igκ+dim. After 5 to 7 days of culture, cells were collected and stained with CD3-PerCP and CD19-PE to evaluate the growth of CD19+ tumor cells. No CD19+ cells were detectable after coculture with CAR46ζ+ or CAR46/28ζ+ T lymphocytes, whereas CD19+ cells were detectable when tumor cells were cocultured with control T cells.
T lymphocytes redirected to express the κ-specific CAR eliminate Igκ+dimtumor cells. T lymphocytes obtained from healthy donors and transduced either with control-GFP vector (A), CAR46ζ (B), or CAR46/28ζ (C) were cocultured with the CCL-120 tumor cell line (ratio, 5:1), which is Igκ+dim. After 5 to 7 days of culture, cells were collected and stained with CD3-PerCP and CD19-PE to evaluate the growth of CD19+ tumor cells. No CD19+ cells were detectable after coculture with CAR46ζ+ or CAR46/28ζ+ T lymphocytes, whereas CD19+ cells were detectable when tumor cells were cocultured with control T cells.
Redirected T lymphocytes isolated from patients with B-CLL kill primary κ+ B-CLL cells and expand in the presence of autologous tumor cells
CD3+ T lymphocytes were isolated from the peripheral blood of 4 patients with κ+ B-CLL and transduced with CAR46ζ or CAR46/28ζ or control GFP vector as described. Because B-CLL cells do not express costimulatory molecules,35 the presence of the CD28 endodomain within the CAR construct was essential to sustain the expansion of redirected T lymphocytes when stimulated with autologous tumor cells. As illustrated in Figure 3, CAR46/28ζ+ T lymphocytes stimulated once a week with autologous B-CLL cells (ratio, 1:1) without any addition of exogenous cytokines expanded 14-fold (range, 6-25) within 3 weeks, whereas neither T lymphocytes carrying CAR46ζ nor the control T lymphocytes expanded significantly. The growth of CAR46/28ζ+ T lymphocytes was sustained by the autocrine production of IL-2 (3276 ± 1494 pg/mL) after stimulation with autologous tumor cells (Figure 3).
In addition to IL-2 release, we observed significant production of IFN-γ and TNF-α. Because B-CLL cells are poorly suited as targets in a 51Cr-release assay, we evaluated the capacity of redirected and expanded T cells to specifically kill autologous κ+ B-CLL cells, using a cytotoxicity assay based on CFSE staining and FACS analysis. As shown in Figure 3, CAR46/28ζ+ T cells efficiently killed both autologous (55%; range, 28%-71%) and allogeneic κ+ B-CLL cells (54%; range, 38%-64%), because CAR-mediated recognition is not MHC restricted, but they did not kill allogeneic λ+ B-CLL cells (11%; range, 1%-17%) after 6 hours of incubation. Control T lymphocytes did not significantly kill tumor cells.
Soluble immunoglobulins do not affect the capacity of redirected T cells to eliminate κ+ tumor cell lines
Because immunoglobulins are abundantly present as soluble molecules in vivo, we evaluated whether free Igκ+ could compete with cell-surface Igκ+ and inhibit binding and killing by CAR-expressing T cells. In a 4-hour 51Cr-release assay, CAR46ζ+ or CAR46/28ζ+ T lymphocytes were incubated with Daudi cells (E/T ratio, 20:1) in the presence of increasing concentrations of normal plasma. Although specific killing persisted even in the presence of plasma, it was substantially reduced (Figure 4). For CAR46/28ζ+ cells, inhibition of specific killing reached 75% ± 6% and 78% ± 9% when cells were tested in 50% and 100% plasma, respectively. Plasma did not modify the specificity of the killing because control GFP+ T lymphocytes did not kill Daudi cells and CAR+ T cells remained unable to kill Igκ– tumor cells or K562 even in the presence of plasma (data not shown).
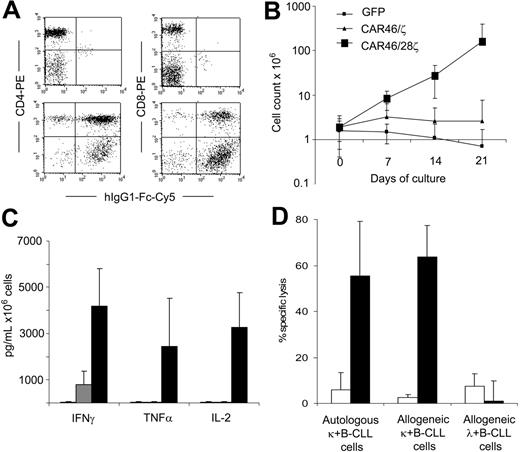
T lymphocytes from B-CLL patients expressing the κ-specific CAR kill primary κ+ B-CLL cells and expand when CD28 endodomain is present in the CAR. CD3+ T lymphocytes were isolated from patients with κ+ B-CLL. After activation with CD3/CD28 antibodies, T lymphocytes were transduced either with CAR46ζ or CAR46/28ζ or control GFP vector. After transduction, T lymphocytes were stained either with CD4-PE or CD8-PE antibodies and with Fc-γCy5 antibody to detect the expression of the CAR. Panel A illustrates the profile for Fc-γCy5 antibody in control T lymphocytes (top panels) and CAR46/28ζ+ T lymphocytes (bottom panels). Both CD4 and CD8 cells were transduced. Control, CAR46ζ+ and CAR46/28ζ+ T lymphocytes were stimulated weekly with autologous B-CLL cells (ratio, 1:1) without exogenous cytokines. Panel B illustrates that only CAR46/28ζ+ T lymphocytes expanded (for at least 3 weeks) after antigenic stimulation. Panel C shows that CAR46/28ζ+ T lymphocytes (▪) released significantly more IFN-γ (P = .01) and IL-2 (P = .02) than CAR46ζ T cells (▦) or control T cells (□) after stimulation with autologous B-CLL cells. Data represent mean ± SD of 4 different donors. The specificity of expanded T lymphocytes was then evaluated using a CFSE-based cytotoxicity assay (E/T ratio, 20:1). Panel D illustrates that CAR46/28ζ T lymphocytes (▪) killed autologous and allogeneic κ+ B-CLL cells, but not allogeneic κ+ B-CLL cells. In contrast, no significant killing was observed for control transduced T lymphocytes expanded in the presence of exogenous IL-2 (□). Data represent mean ± SD of 3 different donors.
T lymphocytes from B-CLL patients expressing the κ-specific CAR kill primary κ+ B-CLL cells and expand when CD28 endodomain is present in the CAR. CD3+ T lymphocytes were isolated from patients with κ+ B-CLL. After activation with CD3/CD28 antibodies, T lymphocytes were transduced either with CAR46ζ or CAR46/28ζ or control GFP vector. After transduction, T lymphocytes were stained either with CD4-PE or CD8-PE antibodies and with Fc-γCy5 antibody to detect the expression of the CAR. Panel A illustrates the profile for Fc-γCy5 antibody in control T lymphocytes (top panels) and CAR46/28ζ+ T lymphocytes (bottom panels). Both CD4 and CD8 cells were transduced. Control, CAR46ζ+ and CAR46/28ζ+ T lymphocytes were stimulated weekly with autologous B-CLL cells (ratio, 1:1) without exogenous cytokines. Panel B illustrates that only CAR46/28ζ+ T lymphocytes expanded (for at least 3 weeks) after antigenic stimulation. Panel C shows that CAR46/28ζ+ T lymphocytes (▪) released significantly more IFN-γ (P = .01) and IL-2 (P = .02) than CAR46ζ T cells (▦) or control T cells (□) after stimulation with autologous B-CLL cells. Data represent mean ± SD of 4 different donors. The specificity of expanded T lymphocytes was then evaluated using a CFSE-based cytotoxicity assay (E/T ratio, 20:1). Panel D illustrates that CAR46/28ζ T lymphocytes (▪) killed autologous and allogeneic κ+ B-CLL cells, but not allogeneic κ+ B-CLL cells. In contrast, no significant killing was observed for control transduced T lymphocytes expanded in the presence of exogenous IL-2 (□). Data represent mean ± SD of 3 different donors.
Soluble immunoglobulins do not impair the capacity of transgenic T cells to eliminate Igκ+tumor cells. The inhibitory effect of free immunoglobulins was tested using a standard 4-hour 51Cr-release assay, in which either T lymphocytes CAR46ζ+ (▦) or CAR46/28ζ+ (▪) were incubated with Daudi cells (E/T ratio, 20:1) in the presence of serial dilutions of plasma obtained from healthy donors. The top panel illustrates the mean ± SD of residual killing by transgenic T lymphocytes in presence of the plasma for 4 different donors. The bottom panels illustrate the effects of coculturing either control T lymphocytes or CAR46ζ+ or CAR46/28ζ+ T lymphocytes with Daudi cells at a 10:1 E/T ratio, in complete media (top panels) or in 100% human plasma (bottom panels). After 5 to 7 days of culture, cells were stained with CD3-PerCP and CD19-PE to enumerate CD19+ tumor cells. CAR46/28ζ+ T lymphocytes were able to prevent the growth of tumor cells even in the presence of human plasma. This is representative of 5 independent experiments.
Soluble immunoglobulins do not impair the capacity of transgenic T cells to eliminate Igκ+tumor cells. The inhibitory effect of free immunoglobulins was tested using a standard 4-hour 51Cr-release assay, in which either T lymphocytes CAR46ζ+ (▦) or CAR46/28ζ+ (▪) were incubated with Daudi cells (E/T ratio, 20:1) in the presence of serial dilutions of plasma obtained from healthy donors. The top panel illustrates the mean ± SD of residual killing by transgenic T lymphocytes in presence of the plasma for 4 different donors. The bottom panels illustrate the effects of coculturing either control T lymphocytes or CAR46ζ+ or CAR46/28ζ+ T lymphocytes with Daudi cells at a 10:1 E/T ratio, in complete media (top panels) or in 100% human plasma (bottom panels). After 5 to 7 days of culture, cells were stained with CD3-PerCP and CD19-PE to enumerate CD19+ tumor cells. CAR46/28ζ+ T lymphocytes were able to prevent the growth of tumor cells even in the presence of human plasma. This is representative of 5 independent experiments.
We subsequently performed long-term experiments to determine whether the partial inhibition of the function of the redirected T cells observed in these short-term assays would compromise the ability of T cells to eliminate tumor cells in longer-term culture. Control and redirected T lymphocytes were cocultured with tumor cells (ratio, 5:1) for 5 to 7 days with or without the addition of human plasma at increasing concentrations. We found that T lymphocytes carrying either CAR46/ζ or CAR46/28ζ were able to eliminate Igκ+ Daudi cells from the culture by days 5 to 7 when the cells were cultured in media alone (CD19+ cells < 1%; Figure 4). Even when the cells were cultured in 100% human plasma, the capacity of CAR46/28ζ+ T lymphocytes to eliminate the tumor cells was unimpaired (CD19+ cells < 1%; Figure 4). In contrast, CAR46/ζ+ T lymphocytes lacking the CD28 endodomain (CD19+ cells > 4%) were unable to control tumor outgrowth once plasma was present. Control T lymphocytes were similarly ineffective (CD19+ cells > 13%; Figure 4).
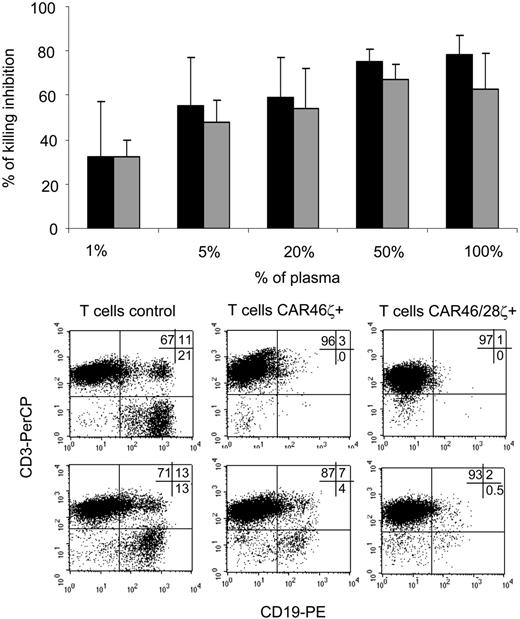
Soluble immunoglobulins sustain the expansion of CAR46/28ζ+T cells. Control T cells (□), CAR46ζ+ T lymphocytes (▦), and CAR46/28ζ+ T lymphocytes (▪) were incubated either with serial dilutions of plasma (A) or purified immunoglobulins (B). After 72 hours, the cells were pulsed with 1 μCi (0.037 MBq) methyl-3[H]thymidine and cultured for an additional 15 hours. Only T cells carrying the CAR46/28ζ proliferate in response to soluble immunoglobulins. Data represent mean ± SD for 3 donors. CAR46ζ+ (C) and CAR46/28ζ+ (D) T lymphocytes were also cultured for 2 weeks with the addition of plasma or soluble immunoglobulins every 3 days. Only CAR46/28ζ+ T cells expanded in presence of plasma or purified immunoglobulins. Data represent mean ± SD for 3 donors.
Soluble immunoglobulins sustain the expansion of CAR46/28ζ+T cells. Control T cells (□), CAR46ζ+ T lymphocytes (▦), and CAR46/28ζ+ T lymphocytes (▪) were incubated either with serial dilutions of plasma (A) or purified immunoglobulins (B). After 72 hours, the cells were pulsed with 1 μCi (0.037 MBq) methyl-3[H]thymidine and cultured for an additional 15 hours. Only T cells carrying the CAR46/28ζ proliferate in response to soluble immunoglobulins. Data represent mean ± SD for 3 donors. CAR46ζ+ (C) and CAR46/28ζ+ (D) T lymphocytes were also cultured for 2 weeks with the addition of plasma or soluble immunoglobulins every 3 days. Only CAR46/28ζ+ T cells expanded in presence of plasma or purified immunoglobulins. Data represent mean ± SD for 3 donors.
Soluble immunoglobulins partially sustain the proliferation of CAR46/28ζ+ T lymphocytes
One explanation for the apparently unimpeded elimination of cocultured tumor cells by CAR46/28ζ+ T cells, even in the presence of high levels of soluble Igκ+, is that the soluble ligand augments the proliferation/activation of these lymphocytes by triggering the costimulatory CD28 endodomain. This effect may compensate for their competition with CAR+ T-cell and tumor-cell–ligand interactions. We therefore cultured control or CAR46ζ+ or CAR46/28ζ+ T lymphocytes in the presence of serial dilutions of normal human plasma or of purified human immunoglobulins and measured proliferation (Figure 5). T cells containing the CD28 endodomain proliferated significantly in the presence of plasma or purified immunoglobulins. In contrast, plasma or immunoglobulins induced no significant proliferation in control or CAR46/ζ+ T lymphocytes. The activation induced by free immunoglobulins significantly expanded CAR46/28ζ+ T lymphocytes, an effect that was sustained for at least 12 days (Figure 5). Stimulation by free immunoglobulins also induced IL-2 and IFN-γ production by CAR46/28ζ+ T lymphocytes (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article).
Coexpression of CD28 endodomain sustains the in vivo expansion and persistence of transgenic T lymphocytes
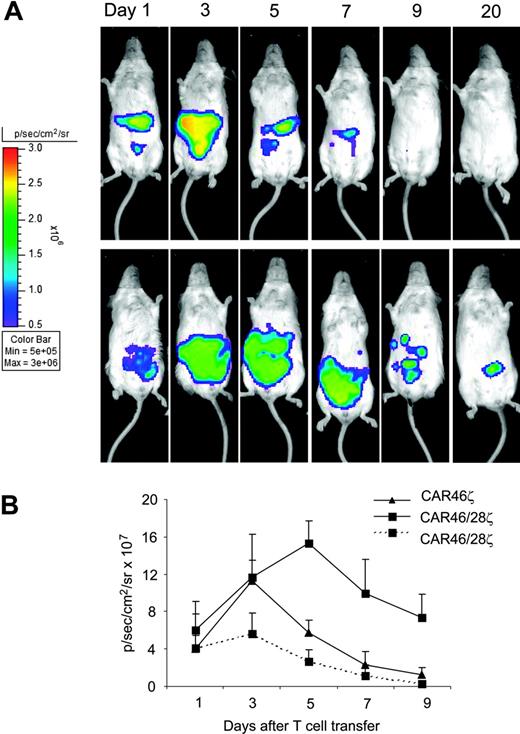
To evaluate the effect of the CD28 endodomain on the in vivo expansion, survival, and function of CAR+ T cells, we established a xenograft model in SCID mice and used a bioluminescence system to track CAR46ζ+ and CAR46/28ζ+ T lymphocytes as well as tumor cells in vivo. In the first set of experiments, we used T lymphocytes that were doubly transduced to express both the CAR and the eGFP-FFLuc genes, to evaluate whether the incorporation of the CD28 endodomain within the CAR enhanced expansion and survival of transgenic T lymphocytes in mice bearing κ+ Daudi tumor cells transduced with the vector carrying the RLuc gene. T cells expressing either CAR46ζ or CAR46/28ζ were adjusted to be comparable for CAR expression before transduction with the luciferase gene. After the second transduction with eGFP-FFLuc both CAR46ζ+ and CAR46/28ζ+ T lymphocytes showed the same expression of GFP (30% ± 10%). As illustrated in Figure 6, CAR46/28ζ+ T lymphocytes showed in mice bearing the κ+ Daudi tumor cells an increased and prolonged light emission within the first 10 days after infusion, compared to CAR46ζ+ T lymphocytes, suggesting enhanced expansion/survival (P < .001 for average intensity using a longitudinal model). The signal for CAR46/28ζ+ T lymphocytes persisted for more than 30 days after 1 single-cell infusion without exogenous cytokines. Our experiments also showed that the improved survival/expansion of CAR46/28ζ+ T lymphocytes was antigen dependent, because signal from CAR46/28ζ+ T lymphocytes did not increase/persist when the cells were injected in mice bearing the Igλ+/κ– SP53 tumor cell line. As an additional control we also injected T lymphocytes expressing eGFP-FL alone in mice bearing the Daudi cell line. Again, signal was only transient (data not shown).
T lymphocytes carrying CAR46/28ζ expand and persist in vivo after antigenic stimulation. Activated CD3+ lymphocytes expressing either CAR46ζ or CAR46/28ζ and double transduced to express eGFP-FLuc gene (5 × 106 cells) were injected intraperitoneally into SCID mice bearing either κ+ Daudi cells or λ+ SP53 cells (5 × 106 cells) labeled with RLuc gene. No exogenous cytokines were injected into the mice. Survival and expansion of transgenic T cells were monitored using an in vivo imaging system (Xenogen-IVIS Imaging System). Panel A illustrates that the persistence and intensity of the signal measured as maximum photon/sec/cm2/sr (p/s/cm2/sr) was less in mice receiving CAR46ζ+ T cells (top panels) than in mice given CAR46/28ζ+ T cells (bottom panels). Panel B illustrates the mean ± SD of 4 mice per group (P < .001). This panel also shows that CAR46/28ζ+ T cells did not expand/survive in mice bearing the κ–/λ+ SP53 tumor cell line (broken line).
T lymphocytes carrying CAR46/28ζ expand and persist in vivo after antigenic stimulation. Activated CD3+ lymphocytes expressing either CAR46ζ or CAR46/28ζ and double transduced to express eGFP-FLuc gene (5 × 106 cells) were injected intraperitoneally into SCID mice bearing either κ+ Daudi cells or λ+ SP53 cells (5 × 106 cells) labeled with RLuc gene. No exogenous cytokines were injected into the mice. Survival and expansion of transgenic T cells were monitored using an in vivo imaging system (Xenogen-IVIS Imaging System). Panel A illustrates that the persistence and intensity of the signal measured as maximum photon/sec/cm2/sr (p/s/cm2/sr) was less in mice receiving CAR46ζ+ T cells (top panels) than in mice given CAR46/28ζ+ T cells (bottom panels). Panel B illustrates the mean ± SD of 4 mice per group (P < .001). This panel also shows that CAR46/28ζ+ T cells did not expand/survive in mice bearing the κ–/λ+ SP53 tumor cell line (broken line).
Redirected T cells provide antitumor effect in vivo
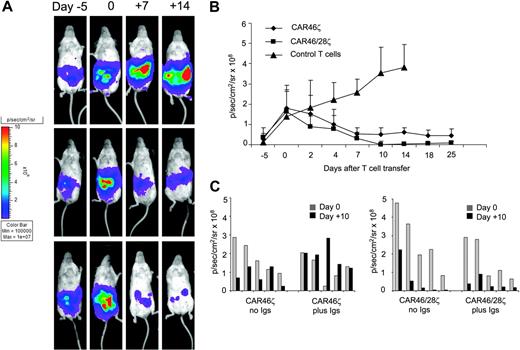
In the second set of experiments, we evaluated the antitumor activity of either CAR46ζ+ or CAR46/28ζ+ T lymphocytes in vivo compared to control T lymphocytes. For these experiments mice were given intraperitoneal injections of Daudi cells (0.5 × 106 cells) transduced with the vector coding for FFLuc gene. When the tumor signal was consistently detectable and increasing in at least 2 consecutive luminescence analyses (usually 10-11 days after tumor injection), T lymphocytes (5 × 106 cells) without exogenous cytokines were injected. T lymphocytes were not marked in these experiments. Tumor growth was tracked by the bioluminescence system. As shown in Figure 7, the tumor signal was consistently increasing in all mice (6 of 6) receiving control T cells. All control mice were humanely killed by day 15 after T-cell transfer and in all of them we confirmed that the signal corresponded to the presence of a tumor mass. Similar tumor growth was observed in mice given tumor cells alone (data not shown). In contrast, mice receiving either CAR46ζ+ T lymphocytes (6 of 6) or CAR46/28ζ+ T lymphocytes (6 of 6) showed a significant decrease of the tumor signal (P < .001). No significant differences were observed between mice receiving CAR46ζ+ or CAR46/28ζ+ T lymphocytes. We also evaluated the effects of soluble immunoglobulins on tumor-cell killing in vivo. Mice bearing κ+ Daudi cells labeled with FFLuc gene were treated either with CAR46ζ+ or CAR46/28ζ+ T cells as described. In addition, they were receiving human immunoglobulins 3 times a week. The presence of human immunoglobulin was able to completely inhibit the activity of CAR46ζ+ T cells lacking the CD28 endodmain, but had no effect on CAR+ T cells when the CD28 endodomain was incorporated within the receptor (Figure 7).
Discussion
Adoptive transfer of T lymphocytes genetically modified to express CARs targeting lineage-restricted antigens is an attractive approach for the treatment of B cell-derived malignancies,17-20 but risks producing persistent humoral immune compromise. We have taken advantage of the clonal restriction of κ and λ light-chain expression on normal and malignant B cells to target all malignant cells, while sparing normal B cells expressing the reciprocal light chain. To assess the feasibility of this approach, we cloned a novel CAR targeting the κ light chain of human immunoglobulins and demonstrated in vitro, and in a xenograft model, that T lymphocytes carrying this receptor efficiently kill κ+ tumor cell lines and primary tumor cells, while sparing B lymphocytes expressing the λ light chain. The incorporation of CD28 endodomain within our novel CAR construct proved crucial not only for the expansion of transgenic T cells in the presence of autologous tumor cells, but also for optimal function in the presence of soluble κ+ immunoglobulins, which otherwise substantially inhibits these retargeted CARs.
Tumor-associated antigens that are secreted or cleaved from the cell membrane are generally considered suboptimal candidates for passive immunotherapy, particularly using monoclonal antibodies, because the soluble form of the antigen reduces the bioavailability of the antibody and impairs its antitumor effect.36 T lymphocytes expressing a CAR derived from such antibodies may also suffer from the competitive effects of soluble versus cell-bound antigenic targets, although the effects are often less striking, perhaps because of the higher avidity of binding when the target antigen is presented as a surface array.27,37,38 For our κ light chain-specific CAR an additional mechanism appears to ensure adequate functionality even in the presence of free immunoglobulins. Whereas free immunoglobulins discernibly compete with the membrane-bound form of the antigen in short-term analyses of anti-κ CAR binding and function, longer term the presence of free immunoglobulins/plasma has little effect on target-cell destruction ex vivo and in vivo, provided the chimeric receptor contains the CD28 endodomain. The incorporation of costimulatory molecule components in cis in chimeric T-cell receptors has been shown to improve the persistence and expansion of transgenic T cells after engagement with the antigen expressed by the tumor cells.20,39 Our data confirm that not only does incorporation of the CD28 endodomain support expansion of transgenic T cells after stimulation with autologous tumor cells, it also transforms the overall effect mediated by free immunoglobulins from one of inhibition of target-cell binding/killing to that of chimeric T-cell activation and expansion, with attendant restoration of effector-cell antitumor activity.
CAR+ T lymphocytes control tumor growth in vivo. Activated CD3+ lymphocytes expressing CAR46ζ or CAR46/28ζ, or control T lymphocytes (5 × 106 cells) were injected intraperitoneally into SCID mice bearing κ+ Daudi cells (0.5 × 106 cells) labeled with FFLuc gene. No exogenous cytokines were injected into the mice. Day 0 is the day of adoptive transfer of T cells, 10 to 11 days after tumor implant. Tumor growth was monitored using an in vivo imaging system (Xenogen-IVIS Imaging System). Panels A illustrates that tumor growth measured as intensity of the signal (p/s/cm2/sr) was significantly greater in mice receiving control T cells (top panels) by day 25 after T-cell infusion compared to mice receiving CAR46ζ+ T lymphocytes (middle panels) or CAR46/28ζ+ T cells (bottom panels). Panel B illustrates the mean ± SD of 6 mice/group (P < .001). Panel C compares outcomes in mice bearing κ+ Daudi cells labeled with FFLuc gene and treated with either CAR46ζ+ or CAR46/28ζ+ T cells and receiving intraperitoneal injections of human immunoglobulins 3 times a week or no treatment. Tumor growth was monitored using an in vivo imaging system (Xenogen-IVIS Imaging System). Data are results of 5 mice/group.
CAR+ T lymphocytes control tumor growth in vivo. Activated CD3+ lymphocytes expressing CAR46ζ or CAR46/28ζ, or control T lymphocytes (5 × 106 cells) were injected intraperitoneally into SCID mice bearing κ+ Daudi cells (0.5 × 106 cells) labeled with FFLuc gene. No exogenous cytokines were injected into the mice. Day 0 is the day of adoptive transfer of T cells, 10 to 11 days after tumor implant. Tumor growth was monitored using an in vivo imaging system (Xenogen-IVIS Imaging System). Panels A illustrates that tumor growth measured as intensity of the signal (p/s/cm2/sr) was significantly greater in mice receiving control T cells (top panels) by day 25 after T-cell infusion compared to mice receiving CAR46ζ+ T lymphocytes (middle panels) or CAR46/28ζ+ T cells (bottom panels). Panel B illustrates the mean ± SD of 6 mice/group (P < .001). Panel C compares outcomes in mice bearing κ+ Daudi cells labeled with FFLuc gene and treated with either CAR46ζ+ or CAR46/28ζ+ T cells and receiving intraperitoneal injections of human immunoglobulins 3 times a week or no treatment. Tumor growth was monitored using an in vivo imaging system (Xenogen-IVIS Imaging System). Data are results of 5 mice/group.
Although the significant activation of transgenic T cells by free immunoglobulins is of potential benefit for antitumor activity, it raises 2 potential concerns. The first is that persistence of this phenomenon will lead to uncontrolled proliferation of the transgenic T lymphocytes and ultimately to autonomous growth. Fortunately, our experiments all indicate that soluble immunoglobulins sustain the proliferation of anti-κ–CAR+ T cells with the CD28 endodomain only for 2 to 3 weeks. After this initial expansion, T cells survive and retain their capacity to kill Igκ+ tumor cells, but undergo no measurable further expansion in the absence of exogenous cytokines. These data suggest that the benefits of CD28 signaling—though substantial—are of finite duration. Nevertheless, a suicide gene could in principle be incorporated within the CAR to control the expansion of the transgenic T cells.40 A second concern relates to rapid functional exhaustion of the transgenic T cells leading to impaired antitumor activity, secondary to chronic antigen stimulation.41 However, we found no evidence for this phenomenon in this system.
Our in vivo experiments showed that transgenic T-cell expansion was superior in the presence of the CD28 endodomain potentially in the presence of human immunoglobulins. Our experiments also showed that the improved survival/expansion of CAR46/28ζ+ T lymphocytes was antigen dependent and not due to spontaneous dimerization of the CD28 endodomain. Signal from CAR46/28ζ+ T lymphocytes did not increase/persist when the cells were injected in mice with tumor that lacked target antigen expression, even when these tumor cells expressed the costimulatory molecules CD80 and CD86. Transgenic T cells were able to significantly control growth of κ+ tumor cells for more than 3 weeks after one single dose of T cells, without any support with exogenous cytokines.
Our data suggest that a strategy based on the generation of transgenic T cells expressing CAR targeting the light chains of the immunoglobulins could represent a means to treat low-grade NHL and B-CLL. Somatic depletion of B cells that express one type of light chain might impair the response against some specific epitopes for which the immune response is dominated by the targeted light chain. However, the humoral immune response against an antigen is typically polyclonal and contains both κ and λ light chains, so that the persistence of immunoglobulins expressing the nontargeted light chain and recognizing different antigenic epitopes should largely compensate for the deficit. Nonetheless, clinical studies will be required to demonstrate that targeting to light-chain molecules has this hoped-for advantage for humoral immunity rather than targeting a pan–B-cell antigen.
Authorship
J.V., B.S., S.V., and E.B. performed the in vitro experiments; J.V. and B.S. performed the experiments in animals; G.D. cloned the scFv; M.P. and C.R. provided expertise for the generation of the CAR; G.D., B.S., J.V., and M.K.B. designed the research and analyzed the data; H.E.H. and C.M.R. provided expertise in T-cell generation and reviewed the manuscript; G.D., B.S., J.V., and M.K.B. wrote the paper; and J.W. performed the statistical analysis.
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 22, 2006; DOI 10.1182/blood-2006-04-017061.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported in part from Leukemia and Lymphoma Society Specialized Center of Research (SCOR; grant no. 7018) and 5R21AI65549. G.D. is supported by the Methodist Hospital Foundation award and by a Chao Scholar grant. J.V. and H.E.H. are supported by a Doris Duke Distinguished Clinical Scientist Award (H.E.H.).


![Figure 5. Soluble immunoglobulins sustain the expansion of CAR46/28ζ+ T cells. Control T cells (□), CAR46ζ+ T lymphocytes (▦), and CAR46/28ζ+ T lymphocytes (▪) were incubated either with serial dilutions of plasma (A) or purified immunoglobulins (B). After 72 hours, the cells were pulsed with 1 μCi (0.037 MBq) methyl-3[H]thymidine and cultured for an additional 15 hours. Only T cells carrying the CAR46/28ζ proliferate in response to soluble immunoglobulins. Data represent mean ± SD for 3 donors. CAR46ζ+ (C) and CAR46/28ζ+ (D) T lymphocytes were also cultured for 2 weeks with the addition of plasma or soluble immunoglobulins every 3 days. Only CAR46/28ζ+ T cells expanded in presence of plasma or purified immunoglobulins. Data represent mean ± SD for 3 donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/12/10.1182_blood-2006-04-017061/4/m_zh80230604270005.jpeg?Expires=1769770689&Signature=uNKpQrUt8MNnskFYj3h2dlsQtkNbvwEMeZ0hVYsvBIhrJ8GHnF5B3xzl3BOBqfv04I0aMrtR6Quya7SSe2xwH0igOYWFIJK-BgFBV6Adq9v2w9Pg-36P8sxOThSSlp9Kvn~YL0GyuIeIpeDpEE2sjAsW56Uo-PG7Gq2otRlJwaAlUXAZqe4Ka8wJ68PTHoFuiGiNTzqxOm3JsspMFYn4iIGAk72BI8TUxwuTP8SnTdSsnz4FJX1TadmezOhTOOjlXpY5RmnWBFdqmXSipWjPlspqllRcaBOtcvE522lkolBhBJKQas2iE6Z5FI9z62MfQlzpa-cefJTeH6je9vldNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)