Abstract
Activating mutations of the FLT3 gene occur because of an internal tandem duplication of the juxta-membrane domain (FLT3/ITD) or point mutation of the activation loop domain (FLT3/ALM). The presence of FLT3 mutations as well as the allelic ratio of FLT3/ITD (ITD-AR, mutant–wild type ratio) may have prognostic significance. FLT3 mutation status of 630 children with de novo acute myeloid leukemia (AML) treated on CCG-2941 and -2961 was determined, and ITD-AR was calculated for patients with FLT3/ITD. Clinical characteristics and outcomes for patients with FLT3/ALM and FLT3/ITD at varying ITD-ARs was determined and compared with those without FLT3 mutations (FLT3/WT). FLT3/ITD and FLT3/ALM were detected in 77 (12%) and 42 (6.7%) of the patients. Progression-free survival (PFS) was similar in patients with FLT3/ALM and FLT3/WT (51% versus 55%, P = .862). In contrast, PFS at 4 years from study entry for patients with FLT3/ITD was inferior to that of patients with FLT3/WT (31% versus 55%, P < .001). PFS decreased with increasing FLT3/ITD-AR (P < .001), and those with ITD-AR greater than 0.4 had a significantly worse PFS than those with lower ITD-AR (16% versus 72%, P = .001) or with FLT3/WT (55%, P < .001). ITD-AR defines the prognostic significance in FLT3/ITD-positive AML, and ITD-AR greater than 0.4 is a significant and independent prognostic factor for relapse in pediatric AML.
Introduction
Activating mutations of the FLT3 receptor gene lead to constitutive activation of the FLT3 receptor tyrosine kinase and cause autonomous, cytokine-independent proliferation in vitro.1 FLT3 mutations are the most common somatic mutations observed in acute myeloid leukemia (AML) and their presence may be a prognostic factor for poor outcome. Adult studies have shown a prevalence of 20% to greater than 35% for the FLT3/internal tandem duplication (ITD) and an additional 7% for FLT3/point mutation of the activation loop domain (ALM).2-9 The prevalence of FLT3/ITD in pediatric AML is approximately 15%, lower than in adults,10-12 and prevalence of FLT3/ALM is 7%, similar to that in adults.13 Early studies showed a poor outcome in patients carrying FLT3/ITD6 ; however, larger studies using more contemporary, intensive chemotherapies have shown a more modest impact on outcome.7,14 Furthermore, varying allelic ratios of FLT3/ITD have been shown to carry prognostic significance, although a clinically useful threshold for the allelic ratio of FLT3 to ITD (ITD-AR) has not been established. Furthermore, the clinical significance of FLT3/ALM has not been well established, although early adult studies suggest that such mutations may carry clinical significance.8,15
There have been no large-scale pediatric studies designed to evaluate the prognostic significance of FLT3 mutations in childhood AML, and given the lower prevalence of FLT3 mutations in children, subclassification to define clinically meaningful risk groups within the patients with FLT3 mutations has not been possible. Two important questions that remain to be answered are whether presence of FLT3/ALM is a prognostic factor, as well as whether there is a clinically useful threshold for the ITD-AR. In this study we define the prevalence and clinical significance of FLT3/ITD and FLT3/ALM in a group of 630 children with de novo AML and evaluate the prognostic significance of FLT3/ALM and determine an ITD allelic ratio threshold, which defines differing risk groups in the FLT3/ITD-positive patient population.
Patients, materials, and methods
Patients and treatments
Pediatric patients with newly diagnosed de novo AML between September 1995 and December 2001 and registered on Children's Cancer Group (CCG) pediatric AML clinical protocols CCG-2941 and CCG-2961 were included in this study. Treatment protocols CCG-2961 and its preceding pilot protocol CCG-2941 have been described in detail elsewhere.16-18 Patients with myelodysplastic syndrome, secondary AML, acute promyelocytic leukemia, or Down syndrome were excluded. Six hundred thirty available cryopreserved marrow specimens were obtained from the Children's Oncology Group (COG–AML) reference laboratory. The study was approved by the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board and the COG Myeloid Disease Biology Committee. Informed consent was provided according to the Declaration of Helsinki. The evaluation of the European cohort was approved by the Berlin-Frankfurt-Münster (BFM)/Dutch AML committee. The diagnosis of AML was made according to the French-American-British (FAB) classification and was confirmed by the CCG-Central Review Committee.
Mutational analysis and ITD-AR determination
Mutational analysis of the FLT3/ITD is detailed elsewhere.6,10 All patient samples showing aberrant FLT3 amplification products were confirmed by sequencing. FLT3/ITD-positive samples were further examined using Genescan analysis (Applied Biosystems, Foster City, CA) to determine the ITD allelic ratio as defined previously.19 ITD-AR was calculated by dividing the peak height of the ITD product to that of the normal WT product. In cases in which more than one ITD product was present, ITD peak heights were added and divided by the WT peak height. Mutational analysis of FLT3/ALM is detailed elsewhere.8,20
Statistical methods
Data were analyzed from CCG-2941 and -2961 through April 2005 and June 2005, respectively. The distributions of categorical and continuous variables were compared between FLT3/ITD-positive and FLT3/ITD-negative groups. Pearson chi-square statistic was used to test for differences in the distribution of categorical variables. However, when data were sparse, Fisher exact test was used. The Mann-Whitney test was used to test for differences in the medians of continuous variables. The Kaplan-Meier method was used to estimate overall survival (OS).21 OS was defined as time from study entry until death, censoring for patients identified as having a matched family donor (MFD) at the end of 2 courses of therapy. Estimates of progression-free survival (PFS) were obtained using methods that account for competing events.22 PFS was defined as time from study entry until remission failure or relapse whereby patients who died as a result of causes other than AML were competing events. Relapse risk (RR) was defined as time from remission to relapse whereby deaths from nonprogressive disease were considered competing events. Six patients without a previous documented relapse who had disease at the time of death were considered to have disease progression and thus were considered events in the PFS and RR analyses. Patients having an MFD were censored at the end of 2 courses of therapy. Patients lost to follow-up were censored at their date of last known contact or at a cutoff 6 months before the date the data were frozen to compensate for the tendency of deaths and relapses being reported sooner than ongoing follow-up. The significance of predictor variables was tested with the log-rank statistic. Confidence intervals for survival estimates were calculated using Greenwood estimate of the standard error. A Cox proportional hazards model23 was used for multivariate analyses using significant predictors from univariate tests.
Results
Study population
All patients enrolled on CCG-2941 and -2961 with de novo AML with available diagnostic marrow specimens were evaluated for FLT3 mutations. Of the 988 children with de novo AML treated on CCG-2941 and -2961 between September 1995 and December 2001, 630 (64%) had diagnostic marrow specimens available for analysis. Those patients without available diagnostic specimens (n = 358) had similar clinical outcomes with 4-year overall survival of 57% ± 6% compared with that of 51% ± 5% for those who were analyzed (P = .263). Complete remission (CR) rate, PFS, and EFS from study entry were also similar. The study population differed from those not tested mainly in regard to age and diagnostic white count, whereby those not tested were younger (5.7 versus 10.5 years; P < .001) and had lower median diagnostic white blood cell (WBC) counts (13.6 × 109/L versus 21.6 × 109/L; P = .001). In addition, the population that was not tested had a higher proportion of patients with megakaryocytic leukemia (10% versus 3%; P < .001).
FLT3 mutation analysis
Six hundred thirty patients with de novo AML with available diagnostic marrow specimens were evaluated for the presence of FLT3 mutations. Seventy-seven (12%) of the 630 patients tested had FLT3/ITD, and an additional 42 (6.7%) had FLT3/ALM. The presence of the mutations was mutually exclusive, and in total, 119 (19%) of the 630 patients had an FLT3 mutation. In the patients with FLT3/ITD, the size of the duplicated region varied from 15 to 174 base pairs. Exon 14 of the FLT3 gene was involved in every patient, and in 2 patients it extended across intron 14 into exon 15. In patients with FLT3/ALM, 4 patients had silent mutations and were subsequently excluded from analysis. Of the remaining 38 patients (6%), 25 had D835Y, 7 with I386H, 2 with D835E, 2 with D835H, and 2 with D835N.
Characteristics of the study population
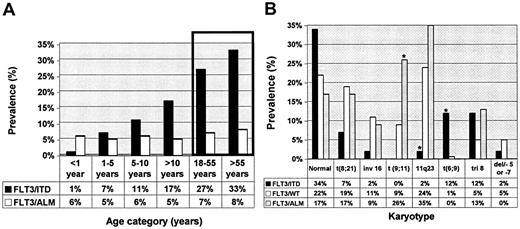
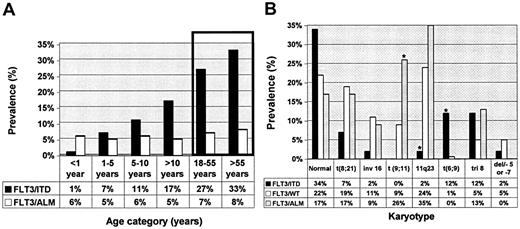
Laboratory and clinical characteristics of patients with and without FLT3 mutations were compared (Table 1). Compared with patients with wild-type FLT3 (FLT3/WT), patients with FLT3/ITD were older (12.6 years versus 10 years; P < .001), and its presence in infants was rare (1.3% versus 9.5%; P = .01). There was a stepwise increase in the prevalence of FLT3/ITD by age as the prevalence of FLT3/ITD increased from 1.5% in infant AML to 7% for patients aged 1 to 5 years to nearly 17% in patients aged 10 to 20 years (Figure 1A). This increase in prevalence by age was not observed in the patients with FLT3/ALM, whereby its prevalence was 5% to 8% in all age groups studied. Patients with FLT3/ITD had a significantly elevated diagnostic WBC count with a median diagnostic WBC count of 71.9 × 109/L, compared with 19.1 × 109/L for the patients with FLT3/WT (P < .001). Median diagnostic WBC count in patients with FLT3/ALM was 19.85 × 109/L, similar to that of patients with FLT3/WT (P = .74). Patients with FLT3/ITD or FLT3/ALM had a significantly higher marrow blast percentage of 85% and 84% compared with that of 67% for patients with FLT3/WT (P < .001 and P = .004, respectively). Patients with FLT3/ITD were more likely to have FAB M1 phenotype than those with FLT3/WT (32.5% versus 14.6%; P < .001). FAB M6 or M7 was rare in patients with FLT3 mutations; only 2 patients with FLT3 mutation had FAB M6 or FAB M7 (Table 1).
Informative cytogenetic information was available from 382 (60%) of 630 patients of whom 41 (11%) were positive for FLT3/ITD, 23 (5%) for FLT3/ALM, and 318 (83%) for FLT3/WT (Figure 1B). FLT3/ITD was more common in those with normal karyotype compared with that of FLT3/WT or FLT3/ALM. FLT3/ITD was less frequent in patients with t(8;21) or inv(16), and, as a collective group, patients with core binding factor (CBF) leukemias were underrepresented in the FLT3/ITD-positive group (10%), compared with the FLT3/WT population (30%, P = .058; Figure 1B). Prevalence of CBF leukemias in the patients with FLT3/ALM was similar to that in the FLT3/WT cohort (26% versus 30%; P = .9).
MLL gene rearrangements were rare in the FLT3/ITD population in which only one patient (2%) had 11q23 abnormality, compared with 75 (24%) of 318 in the FLT3/WT group (P = .001; Figure 1B). In contrast, patients with FLT3/ALM had a higher prevalence of 11q23 abnormalities than did the patients with FLT3/ITD, similar to that of patients with FLT3/WT (35% versus 24%; P = .3). Within the cohort with 11q23 abnormalities, patients with FLT3/ALM had a significantly higher prevalence of t(9;11) translocations than did patients with FLT3/WT (26% versus 8.5%, P = .016). Conversely, nearly 20% of all patients with t(9;11) had FLT3/ALM. High-risk cytogenetics were rare in the patients with FLT3 mutations, because no one with FLT3 mutation had monosomy 7, and 1 patient with FLT3/ITD had del 5q. The most striking correlation of a cytogenetic abnormality with FLT3 mutation was that of t(6;9). Of the 7 patients with t(6;9) in the entire study, 5 had FLT3/ITD (12% versus 0.6%; P < .001). Thus, with the exception of significant association with t(6;9), FTL3/ITD was not associated with any particular cytogenetic class, whereas there was a significant association between FLT3/ALM and MLL translocations, especially in the younger patients.
Clinical outcome
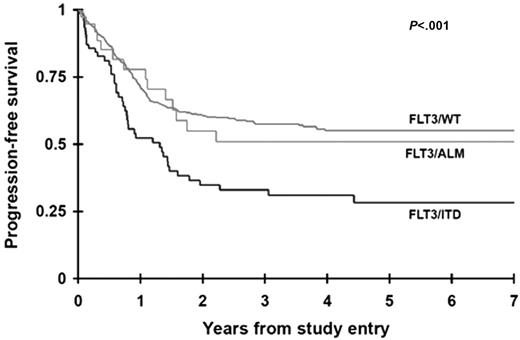
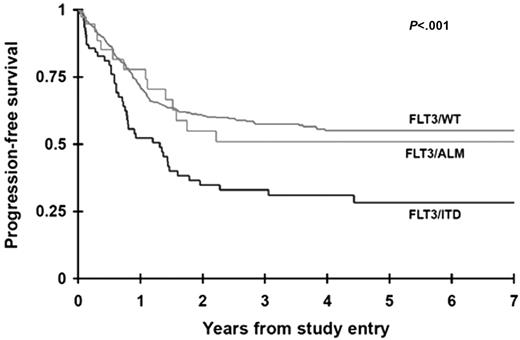
The CR rate was determined for patients with and without FLT3 mutations at the end of one course of therapy. Sixty-one (81%) of the 75 patients with FLT3/ITD and 32 (89%) of the 36 patients with FLT3/ALM achieved a CR compared with 440 (88%) of the 502 patients with FLT3/WT (P = .185 and P = .965, respectively). Seventeen patients (2 FLT3/ITD, 2 FLT3/ALM, 13 FLT3/WT) withdrew from the study without a response evaluation or did not have data to determine response. PFS at 4 years from study entry for the patients with FLT3/ITD was 31% ± 12% compared with 55% ± 5% for the FLT3/WT population (P < .001; Figure 2). Patients with FLT3/ALM had a PFS of 51% ± 19%, no different than those without FLT3 mutations. Corresponding actuarial OS at 4 years from study entry for patients with FLT3/ITD and FLT3/WT was 33% ± 13% versus 54% ± 5%, respectively (P = .018). Again, overall survival of patients with FLT3/ALM did not differ from that of patients with FLT3/WT (59% ± 19% versus 54% ± 5%; P = .677). Of the 533 patients who achieved a CR, relapse rate (cumulative incidence) at 4 years from remission for patients with FLT3/ITD was 65% ± 14% compared with 43% ± 6% for the FLT3/WT population (P = .007). Corresponding OS from CR for patients with FLT3/ITD and FLT3/WT was 44% ± 16% and 61% ± 6%, respectively (P = .113).
Prevalence of FLT3/ITD and FLT3/ALM by age categories and by karyotype. (A) Prevalence of FLT3 mutations is presented for different age categories. Bars inside the box represent correspondence to the prevalence of FLT3 mutations in adults 18 to 55 years7 and older than 55 years5 as previously published. (B) Prevalence of FLT3 mutations is presented for each cytogenetic category. *Statistically significant difference compared with FLT3/WT.
Prevalence of FLT3/ITD and FLT3/ALM by age categories and by karyotype. (A) Prevalence of FLT3 mutations is presented for different age categories. Bars inside the box represent correspondence to the prevalence of FLT3 mutations in adults 18 to 55 years7 and older than 55 years5 as previously published. (B) Prevalence of FLT3 mutations is presented for each cytogenetic category. *Statistically significant difference compared with FLT3/WT.
Clinical significance of FLT3/ITD allelic ratio
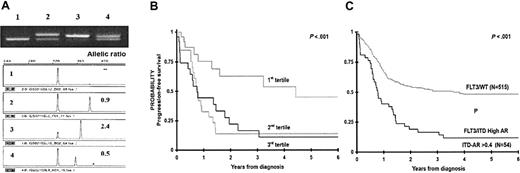
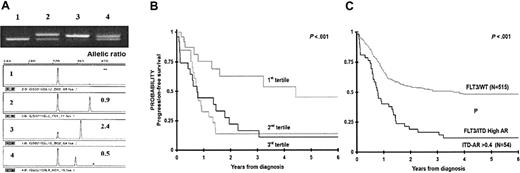
ITD-AR was defined for each patient with FLT3/ITD. ITD-AR varied from 0.01 to 7.5, with a median of 0.53, with 4 patients having ITD-AR greater than 1.0. To determine the clinical significance of ITD-AR, we initially evaluated the ITD-AR as a continuous variable for PFS from study entry to determine whether increasing ITD-AR correlates with worsening outcome. Base 2 log-transformed ITD-AR was analyzed, whereby the hazard ratio (HR) corresponds to a doubling of the ITD-AR. Over the range of ITD-AR values in the study, increasing ITD-AR corresponded to worsening PFS from study entry with an HR of 1.41 (P = .049; ie, a doubling of the allelic ratio correlates with 41% decrease in PFS). Subsequently, we aimed to determine whether we might be able to use allelic ratio to establish a prognostic threshold to separate the FLT3/ITD population into those who are at high risk of treatment failure from those who are not. To this end, we divided the FLT3/ITD-positive population into 3 equal groups (tertiles) based on their ITD-AR, wherein the first tertile included patients with the lowest ITD-AR (0.01-0.42), and the third tertile included patients with the highest ITD-AR (0.65-7.5), and PFS was evaluated for each of the 3 cohorts. PFS at 4 years from study entry for the patients with lowest allelic ratio (n = 26) was 64% ± 25%, whereas PFS for the patients in the second and third tertiles was 15% ± 16% and 18% ± 16%, respectively (P = .014; Figure 3B). To more accurately identify an ITD-AR threshold that can distinguish patients with FLT3/ITD at high risk from the low-risk patients, we compared the significance of ITD-AR thresholds of 0.4, 0.5, and 0.6. Compared with the patients with FLT3/WT, those with ITD-AR higher than 0.4, 0.5, and 0.6 had a corresponding HR for disease progression of 2.5 (P < .001), 2.5 (P < .001), and 2.1 (P = .001), respectively. As a result, ITD-AR of 0.4 was chosen as the prognostic threshold for further comparisons. In comparisons of the patients with FLT3/WT with patients with high ITD-AR (ITD-AR > 0.4), patients with high ITD-AR had a PFS from study entry of 16% ± 11%, compared with 55% ± 5% for the patients with FLT3/WT (P < .001; Figure 3C). Actuarial PFS at 4 years from study entry for the patients with low ITD-AR was similar to those with FLT3/WT (72% ± 22% versus 55% ± 5%, P = .420). Corresponding OS at 4 years from study entry for patients with high ITD-AR was 19% ± 13%, compared with 54% ± 5% for the patients with FLT3/WT (P = .001). Relapse risk at 4 years from remission for patients with high ITD-AR was 83% ± 13%, compared with 43% ± 6% for those with FLT3/WT (P < .001) with a corresponding OS at 4 years from remission of 27% ± 17% and 61% ± 6% for the those with high ITD-AR and FLT3/WT, respectively (P = .009).
Actuarial progression-free survival from study entry for patients with FLT3/ITD, FLT3/ALM, or FLT3/WT.
Actuarial progression-free survival from study entry for patients with FLT3/ITD, FLT3/ALM, or FLT3/WT.
Clinical significance of FLT3/ITD by FLT3/ITD-AR. (A) Example of ITD-AR determination by Genescan analysis. The top panel is the agarose gel resolution of PCR product from a normal marrow (lane 1) and specimens from 3 patients with FLT3/ITD (lanes 2-4). The lower panels show the result of the Genescan analysis and ITD-AR determination. (B) Actuarial progression-free survival from study entry for patients with FLT3/ITD based on allelic ratio by tertiles. (C) Actuarial PFS from study entry for patients with high ITD-AR (ITD-AR > 0.4) compared with those with FLT3/WT.
Clinical significance of FLT3/ITD by FLT3/ITD-AR. (A) Example of ITD-AR determination by Genescan analysis. The top panel is the agarose gel resolution of PCR product from a normal marrow (lane 1) and specimens from 3 patients with FLT3/ITD (lanes 2-4). The lower panels show the result of the Genescan analysis and ITD-AR determination. (B) Actuarial progression-free survival from study entry for patients with FLT3/ITD based on allelic ratio by tertiles. (C) Actuarial PFS from study entry for patients with high ITD-AR (ITD-AR > 0.4) compared with those with FLT3/WT.
The laboratory and clinical characteristics of those with high versus low ITD-AR were compared (Table 2). Those with high ITD-AR appeared to have a higher diagnostic WBC count, although this difference did not reach statistical significance. Those with high ITD-AR had a CR rate of 77% versus 91% for those with low ITD-AR (P = .203). Otherwise, besides the differences in clinical outcome demonstrated in Figure 3, there were no other differences between those with high and low ITD-AR (Table 2).
Prognostic factors
Prognostic factors were evaluated for PFS from study entry. We used Cox regression analysis to evaluate high FLT3/ITD-AR (AR > 0.4), diagnostic WBC count, high-risk cytogenetics (–7, –5/del5q), and race as predictor of disease progression in a univariate model (Table 3). In the univariate model, the presence of the FLT3/ITD was a significant prognostic factor for induction failure or relapse with an HR of 1.9 (P < .001), and HR increased to 2.6 for patients with high ITD-AR compared with low ITD-AR, FLT3/ALM, or FLT3/WT (P < .001). Univariately, diagnostic WBC count greater than 100 × 109/L (HR, 1.7; P = .002), as well as high-risk cytogenetics (HR, 2.6; P = .023) were associated with increased risk of induction failure or relapse. Black race was not associated with clinical outcome in the univariate model. In a multivariate model that included FLT3/ITD-AR (high ITD-AR versus low ITD-AR, FLT3/ALM, and FLT3/WT), cytogenetics (high-risk versus others), diagnostic WBC count (> 100 × 109/L versus ≤ 100 × 109/L), and race (black versus nonblack), high ITD-AR remained an independent prognostic factor for leukemic progression with an HR of 2.6 compared with other patients with low ITD-AR, FLT3/ALM, or FLT3/WT (P < .001). In this multivariate analysis, in addition to high ITD-AR, high-risk cytogenetics was an independent prognostic factor for progressive disease (HR, 2.4; P = .034) (Table 3), whereas diagnostic WBC count greater than 100 × 109/L, which was a significant factor for poor outcome in the univariate model, lost its significance in the multivariate analysis (HR, 1.5; P = .073).
Independent validation of ITD-AR threshold
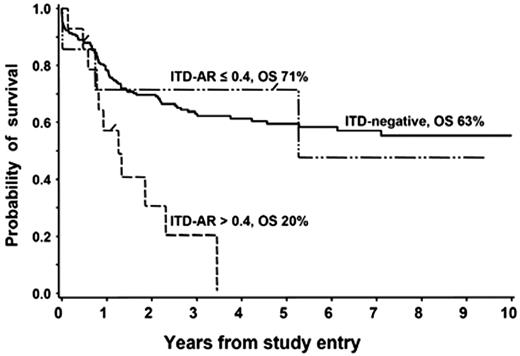
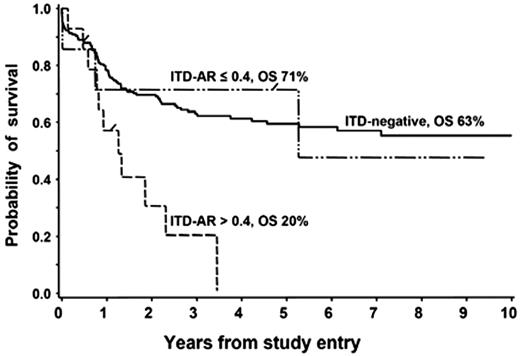
Clinical utility of the ITD-AR threshold of 0.4 was validated in a European cooperative protocol, German AML-BFM SG and the Dutch Childhood Oncology Group (DCOG) study. In a collaborative effort that evaluated the prognostic significance of FLT3/ITD in 234 children treated on the European cooperative studies, Zwaan et al19 showed that patients with FLT3/ITD had an overall and event-free survival of 32% and 29%, compared with 58% and 46% in patients without FLT3/ITD (P = .037 and P = .006, respectively). We reanalyzed the available data from patients with FLT3/ITD to define the clinical outcome for those with ITD-AR greater than 0.4 to those with 0.4 or less. Of the 21 patients with available ITD-AR, 7 patients (33%) had ITD-AR 0.4 or less, and the remaining 14 patients (67%) had ITD-AR greater than 0.4. Overall survival at 3 years from diagnosis for patients with high ITD-AR, low ITD-AR, and FLT3/WT was 20%, 71%, and 63%, respectively (Figure 4; P < .001 for high ITD-AR versus FLT3/WT). Corresponding EFS at 3 years from study entry for those with high ITD-AR, low ITD-AR, and FLT3/WT was 21%, 61%, and 57%, respectively (P = .004 for high ITD-AR versus FLT3/WT). This study validates our findings that ITD-AR of 0.4 is a clinically useful threshold for risk identification within the FLT3/ITD cohort.
Efficacy of allogeneic stem cell transplantation in FLT3/ITD-positive AML
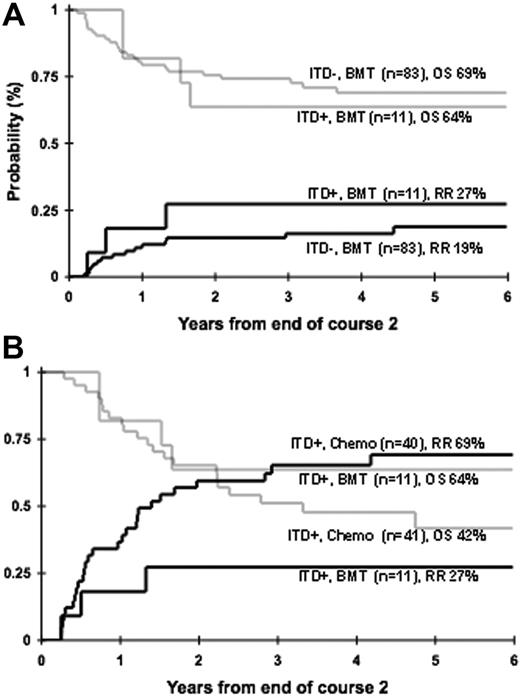
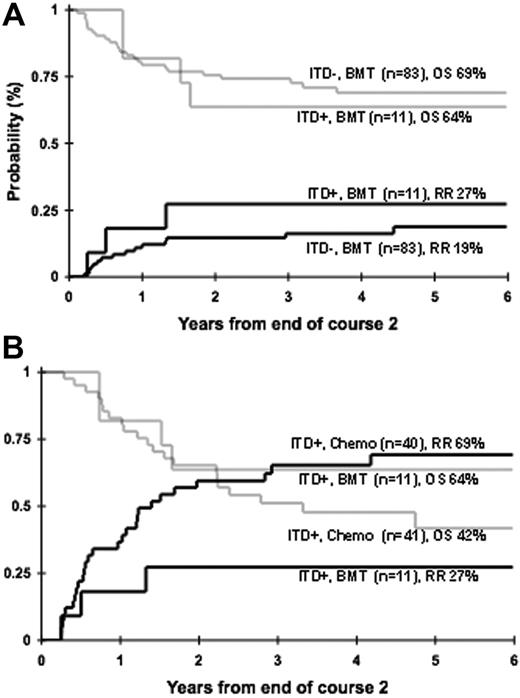
Patients who had a matched family donor were censored prior to transplantation to provide a uniformly treated population for the prognostic evaluation of FLT3 mutations. Therefore, we evaluated the efficacy of allogeneic stem cell transplantation (SCT) in patients with FLT3/ITD. We initially inquired whether FLT3/ITD maintains its prognostic significance in the setting of allogeneic SCT. Of the 116 patients with MFDs, 94 patients went on to receive a MFD SC transplant. Of the 94 SC transplant recipients, 11 (12%) had FLT3/ITD and the remaining 83 (88%) did not. Relapse risk at 4 years from end of course 2 for those with and without FLT3/ITD was 27% ± 27% and 16% ± 8%, respectively (P = .513; Figure 5A). Corresponding OS at 4 years from end of course 2 for those with and without FLT3/ITD was 64% ± 29% and 69% ± 11%, respectively (P = .751; Figure 5A). Of the 11 patients with FLT3/ITD, 6 had high ITD-AR. Four of the 6 patients with high ITD-AR are alive and relapse-free after SCT at 4 years from end of course 2, no different than the patients without FLT3/ITD (P = .292 for RR; P = .326 for OS, respectively).
We subsequently directly compared the outcome of patients with FLT3/ITD who received an MFD SC transplant (n = 11) to those treated with chemotherapy only (n = 40). In patients with FLT3/ITD, RR (cumulative incidence) at 4 years from end of course 2 was 27% ± 27% for the recipients of SC transplants versus 65% ± 15% for those who were treated with chemotherapy only (Figure 5B; P = .050). Corresponding OS at 4 years from end of course 2 was 64% ± 29% versus 48% ± 17% for SC transplant and chemotherapy recipients, respectively (Figure 5B; P = .401). Of the 11 patients with FLT3/ITD who received an SC transplant, 6 had high and 5 had low ITD-AR, whereas of the 40 patients with FLT3/ITD who received conventional chemotherapy 28 had high ITD-AR and 12 had low ITD-AR. In patients with high ITD-AR who received chemotherapy, RR was 81% ± 15%, compared with 33% ± 38% in SC transplant recipients (P = .113). Four of the 6 patients with high ITD-AR who received an SC transplant in first CR are alive with an OS of 50% ± 41% at 4 years from end of course 2, compared with 26% ± 18% for the chemotherapy recipients (P = .529).
Evaluation of prognostic significance of ITD-AR threshold of 0.4 in BFM SG and Dutch DCOG cohort. Overall survival for patients with FLT3/ITD with ITD-AR of greater than 0.4 versus 0.4 is compared with those without FL3/ITD.
Evaluation of prognostic significance of ITD-AR threshold of 0.4 in BFM SG and Dutch DCOG cohort. Overall survival for patients with FLT3/ITD with ITD-AR of greater than 0.4 versus 0.4 is compared with those without FL3/ITD.
Discussion
Despite growing data on the clinical significance of FLT3 mutations, difference in prognostic significance of FLT3/ITD and FLT3/ALM as well as different prognostic subpopulations within patients with FLT3/ITD have precluded its use in risk-based therapy. In this large pediatric study, we show that FLT3 mutations are present in nearly 20% of the patients with pediatric AML. We also show that patients with a FLT3/ITD and an ITD-AR greater than 0.4 have a poor prognosis compared with patients with wild-type FLT3. There was no difference in outcome for patients with FLT3/ITD and AR less than 0.4, patients with FLT3/ALM, and patients with wild-type FLT3.
The prevalence of FLT3/ITD in pediatric AML was 12% in this study. In fact, the actual prevalence in the overall pediatric AML population may indeed be lower, given the fact that a lower proportion of samples from younger patients and those with FAB M7, where FLT3/ITD is rare, were available for testing. However, FLT3/ITD prevalence increased from 1.5% in infants to nearly 20% in teenage patients. Such an age-associated increase in prevalence may offer clues to the pathology of FLT3/ITD in AML. FLT3/ITD is considered a cooperating event in the evolution of AML, and such an age-associated increase fits this model, in which evolution of FLT3/ITD in the background of a previously acquired, early “preleukemic” hit leads to AML. In this model, an early molecular event (eg, translocation) may occur in a minor clone leading to maturation arrest. This subpopulation may remain quiescent until such a time when FLT3/ITD is acquired. Such a time-dependent process provides a proliferative advantage and subsequent evolution of AML in the preleukemic clone.
In contrast to FLT3/ITD, FLT3/ALM appears to evolve in patients with a different profile, in which it is seen in younger patients, those with 11q23 abnormalities, and does not appear to be associated with leukocytosis or poor prognosis. In addition to the difference in clinical outcome, other biologic differences between FLT3/ITD and FLT3/ALM have been defined. We have shown that patient samples with FLT3/ITD have a distinctly different gene expression profile than those with FLT3/ALM.24 Other studies have defined signal transduction differences in in vitro and in vivo model systems,25,26 substantiating that biologic differences underlie the difference in clinical outcome in patients with the 2 different FLT3 mutations. Thus, in comparing the clinical outcome data from patients with FLT3/ALM, FLT3/WT, and FLT3/ITD with varying ITD-AR, it is clear that it is not the mere presence of activating FLT3 mutation but the allelic imbalance that defines the prognosis in FLT3-mutant AML, as the outcome for those with FLT3/ALM, FLT3/WT, and FLT3/ITD with low ITD-AR are quite similar but vastly different than those with high ITD-AR.
This study defines the clinical significance of the ITD-AR in risk identification within patients with FLT3/ITD. Although significance of ITD-AR had been previously suggested,14,19 there has not been a clinically useful threshold established that identifies patients with FLT3/ITD who are at high risk of treatment failure from those who are not. This large cohort allowed us to perform subclass analyses to evaluate different risk classes within the FLT3/ITD patient population and define such a threshold. We further validated this threshold in an independent European cohort, showing that this threshold can be reliably used for risk identification across different populations. This threshold identified nearly 70% of the patients with FLT3/ITD at extremely high risk of treatment failure whereby nearly 90% of such patients either failed induction or relapsed following an initial CR. In contrast, relapse risk for the patients with ITD-AR of 0.4 or less was no higher than that of patients with FLT3/WT.
Although ITD-AR provides a simple means of risk identification in the patients with FLT3/ITD, its underlying biology is not understood. Variation in FLT3/ITD-AR represents allelic imbalance in FLT3 gene at the genomic level, and clarification of the mechanism and biology underlying the evolution of this allelic imbalance may enable more accurate risk assessment in patients with FLT3/ITD, whereby instead of testing for a surrogate marker (variation in ITD-AR), the central event causing the variation can be directly tested. We have shown that some patients with high ITD-AR have loss of heterozygosity (LOH) in 13q12.10 However, LOH of 13q12 has not been a uniform finding in all patients with high ITD-AR.14 Presence of multiple leukemic clones with varying FLT3/ITD status would also contribute to variation in ITD-AR, and, indeed, the presence of multiple FLT3/ITD clones with different ITD-AR in AML patients has been demonstrated, suggesting that leukemic cells with FLT3/ITD which may be a minor clone at the time of diagnosis may re-emergence at relapse. Amplification of the allele containing the ITD may also contribute to allelic imbalance, and high ITD-AR; however, such an amplification has not been shown in patients with high ITD-AR.14 Interrogation of the FLT3 gene with more sensitive means of identifying gene amplification in the region of interest is ongoing and may provide more insight into the cause of ITD-AR. Until the exact biologic basis of ITD-AR variation is determined and specific tests for its evaluation developed, ITD-AR, despite its limitations, provides the best means of not only identifying patients with FLT3/ITD who are at the highest risk of relapse but also, and just as importantly, identifying those who are expected to do well.
Efficacy of allogeneic SCT in FLT3/ITD-positive AML. (A) Relapse risk (solid lines) and overall survival (shaded lines) in recipients of MFD SC transplant with and without FLT3/ITD. (B) Relapse risk (solid lines) and overall survival (shaded lines) in patients with FLT3/ITD treated with consolidation chemotherapy versus MFD SCT.
Efficacy of allogeneic SCT in FLT3/ITD-positive AML. (A) Relapse risk (solid lines) and overall survival (shaded lines) in recipients of MFD SC transplant with and without FLT3/ITD. (B) Relapse risk (solid lines) and overall survival (shaded lines) in patients with FLT3/ITD treated with consolidation chemotherapy versus MFD SCT.
The question of therapeutic options for such high-risk patients remains to be answered. Traditional approaches to high-risk patients with AML have been to refer them for allogeneic SCT with the aim that intensive conditioning combined with graft-versus-leukemia effect would improve their outcome. We provide data that suggest that, although the FLT3/ITD is a poor prognostic indicator for patients with AML receiving conventional chemotherapy, it does not seem to influence outcome after allogeneic SCT. This is similar to the situation of Philadelphia chromosome–positive acute lymphoblastic leukemia, a terrible prognostic factor with chemotherapy but not so with SCT. There is also a suggestion that allogeneic SCT may improve the outcome in patients with high ITD-AR, who are at extremely high risk of relapse. There are emerging corroborating data about the efficacy of SCT in patients with FLT3/ITD,27-29 suggesting that patients with FLT3/ITD may benefit from this approach. To fully evaluate the role of SCT in high-risk FLT3/ITD-positive AML, we are pursuing a collaborative effort to study the role of allogeneic SCT in a larger number of children with FLT3/ITD-positive AML. This collaboration will combine data from patients treated from multiple cooperative groups, whereby the outcome for patients with FLT3/ITD who received chemotherapy will be compared with those who underwent allogeneic SCT. In addition, novel targeted agents are emerging as possible therapeutic options in patients with FLT3 mutations at high risk of relapse.30-32 Identification of patients with FLT3 mutations at high risk of relapse as well as appropriate therapeutic intervention may improve outcome in this high-risk population.
Authorship
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 15, 2006; DOI 10.1182/blood-2006-03-009233.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Ms Cristina Galer and Ms Kristen Miller for excellent technical contribution to this work. We also thank Ms Mariko Kawabori in the COG reference laboratory for assistance in specimen identification. We are grateful to the patients and families of patients who consented to the use of biologic specimens in this trial.
This work was supported by the National Institutes of Health (grants R21 CA10262-01, K23 CA092405, and R01 CA114563-01).