Comment on Attal et al, page 3289
The InterGroupe Francophone du Myélome (IFM) reports the important finding that maintenance treatment with thalidomide, after double autologous stem cell transplantation (ASCT), significantly improved overall survival in multiple myeloma patients.
The role of maintenance therapy in myeloma is controversial. The IFM designed a randomized trial to evaluate the role of thalidomide and pamidronate as maintenance treatment in patients without progressive disease after double ASCT. Thalidomide improved the quality of response, and extended event-free survival and overall survival compared with no maintenance therapy. Patients who benefited were those who did not achieve a complete or very good partial response 2 months after ASCT and who did not have a chromosome 13 deletion. Maintenance treatment with pamidronate in this context had no effect on the incidence of bone events. This finding supports the conservative use of bisphosphonates.FIG1
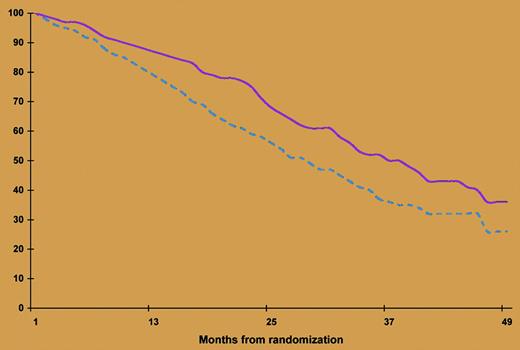
Event-free survival according to treatment arm. The broken line indicates without thalidomide; the solid line, with thalidomide. See the complete figure in the article beginning on page 3289.
Event-free survival according to treatment arm. The broken line indicates without thalidomide; the solid line, with thalidomide. See the complete figure in the article beginning on page 3289.
The IFM previously reported that double ASCT significantly prolonged both overall and event-free survival, particularly among patients who did not achieve remission after the first transplantation.1 The present report shows that the benefit of this approach can be extended by giving thalidomide to a further subgroup of patients who did not enter remission after the second transplantation. However, the absence of a plateau in the event-free survival curve (see figure) calls for improvement of the present strategy. New classes of agents, including the potent thalidomide analogue lenalidomide and the proteasome inhibitor bortezomib, have proven effectiveness in relapsed and refractory myeloma as well as in newly diagnosed myeloma patients. However, these agents can cause irreversible and disabling chronic adverse effects.
The main clinical challenge now is how to integrate ASCT and new agents, minimizing toxicity and optimizing anticlone activity. Two strategies are being investigated. The first is combining new agents and ASCT to enhance cytotoxicity and avoid drug resistance with the aim of annihilating the tumor. Such an approach was explored in a large controlled trial by Barlogie et al,2 who added thalidomide throughout an intensive combined therapy including double ASCT. Thalidomide increased the frequency of complete response and extended event-free survival, but the drug was associated with considerable toxicity and failed to prolong overall survival owing, in part, to the higher drug resistance that translated into shorter survival after relapse. The second strategy is sequentially using the new agents to decrease the tumor mass before3 and after transplantation. The outcome of the IFM trial supports this second strategy, since thalidomide further decreased the tumor mass in patients who did not achieve at least a very good partial response after double ASCT without drug resistance at the time of relapse. As noted by the authors, there is room for improving the response rate by adding dexamethasone to thalidomide and for decreasing toxicity by reducing the dosage of thalidomide and limiting its use until achievement of a better-quality response. In patients with chromosome 13 deletion, maintenance could be performed with bortezomib or lenalidomide, as their activity does not correlate with the deletion. The sequential use of ASCT and the new agents, including those under development targeting other sensitive mechanisms of myeloma survival and progression, such as growth factor signaling cascades,4 may lead to a sustained plateau phase. High-resolution genomic profiling5 has disclosed high molecular heterogeneity of multiple myeloma and will direct the use of these new agents, reducing the toxicity and achieving lasting control. ▪
Dr. Merlini is also affiliated with the Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo.