Griscelli syndrome (GS) was diagnosed in a 2-year-old patient with oculocutaneous albinism and immunodeficiency, but sequencing of RAB27a revealed only a heterozygous mutation. Due to impaired natural killer (NK) and T-cell cytotoxicity implying a high risk of developing hemophagocytic lymphohistiocytosis (HLH), he was prepared for hematopoietic stem cell transplantation (HSCT). Unexpectedly, a severe bleeding episode occurred that led to the demonstration of disturbed platelet aggregation, reduced plateletdense granules, and impaired platelet degranulation. In combination with neutropenia, this suggested the diagnosis of Hermansky-Pudlak syndrome type II (HPSII) and a novel homozygous mutation in AP3B1 was detected. None of the 3 reported HPSII patients had developed HLH, and our patient seroconverted to Epstein-Barr virus (EBV) without clinical symptoms. HSCT was therefore withheld, and granulocyte-colony-stimulating factor (G-CSF) therapy was initiated and prevented further bacterial infections. At 3 years of age, however, the patient developed, without an obvious trigger, fulminant HLH that was resistant to therapy. This patient shows that careful clinical and molecular diagnosis is essential to differentiate the complex disorders of lysosomal trafficking. HPSII belongs to the group of familial hemophagocytic syndromes and may represent an indication for HSCT. (Blood. 2006;108:81-87)
Introduction
Secretory lysosomes are cellular organelles involved in trafficking and exocytosis of intracellular proteins.1 They have important functions in several cell types including melanocytes, neuronal cells, platelets, granulocytes, mast cells, natural killer (NK) cells, and T cells.2 The protein machinery required for adequate biogenesis, transport, and delivery of secretory lysosomes has a variable composition in each cell type. Therefore, the clinical phenotype of diseases due to genetic defects in proteins involved in lysosomal trafficking varies widely despite the similar cell-biologic basis. This has led to their classification as predominantly dermatologic, hematologic, hemostaseologic, or immunologic disorders. While the 7 genetically defined Hermansky-Pudlak syndromes (HPSs) predominantly present as bleeding disorders,3 the most important clinical problem of patients with Chédiak-Higashi syndrome (CHS) and Griscelli syndrome type II (GSII) is immunodeficiency.4 Neurologic defects characterize GSI and CHS,5 while patients with GSIII show only oculocutaneous albinism.6 This latter feature links all of these syndromes. However, studies showing that also some forms of familial hemophagocytic lymphohistiocytosis (FHL) represent lysosomal trafficking disorders7,8 revealed that albinism is not a constant feature in this heterogenous family of diseases.
Lysosomal trafficking disorders associated with immunodeficiency are life-threatening conditions due to the high risk of developing hemophagocytic lymphohistiocytosis (HLH).4 HLH is a systemic inflammatory disorder characterized by uncontrolled CD8+ T-cell and macrophage activation with infiltration of multiple tissues.9 The common defect in most genetic diseases predisposing to HLH is disturbed secretion of lytic granules by cytotoxic T cells (CTLs) and NK cells.2 Regulated secretion of these granules containing the pore-forming protein perforin is required for CTL- and NK-cell-mediated cytotoxicity. Current models suggest that in the context of antimicrobial immune responses, cytotoxicity is required not only to kill infected cells, but also to kill antigen-presenting cells thereby limiting T-cell stimulation.10 A lack of this negative regulation leads to continued T-cell activation with excessive cytokine production and subsequent macrophage activation.10 Patients with genetic deficiency in perforin,11 or in genes absolutely required for lytic granule secretion such as MUNC 13-4,8 develop HLH within the first year of life unless treated by hematopoietic stem cell transplantation (HSCT). In patients with GSII and CHS, the defect in granule mobilization is less severe and HLH usually develops later in life, but GSII is also invariably fatal unless HSCT is performed.12
Recently, disturbed secretion of lytic granules by CTLs has also been described in a patient with HPS type II,13 which is caused by mutations in the gene encoding the β-subunit of the cytosolic adaptor protein AP-3 (AP3B1).14 The clinical presentation of HPSII has so far been described only in 3 patients and included oculocutaneous albinism, bleeding, recurrent infection, and neutropenia.15,16 Mild facial dysmorphia, hepatosplenomegaly, developmental delay, and pulmonary fibrosis have also been reported in one patient,16 but the few reported cases so far preclude a complete definition of the syndrome. Of importance, the clinical consequences of the cytotoxicity defect, in particular the risk of developing HLH, remain unclear. In this report, we present a new patient with HPSII. He is the first patient who developed HLH, indicating that HPSII belongs to the group of familial hemophagocytic syndromes and may represent an indication for HSCT.
Patient, materials, and methods
Informed consent
Informed consent for the performed studies and publication of photographs was obtained from the patient's family in accordance with the guidelines of the local ethics committee. The study protocol was approved by the ethics committee of the University of Freiburg (Freiburg, Germany).
Patient
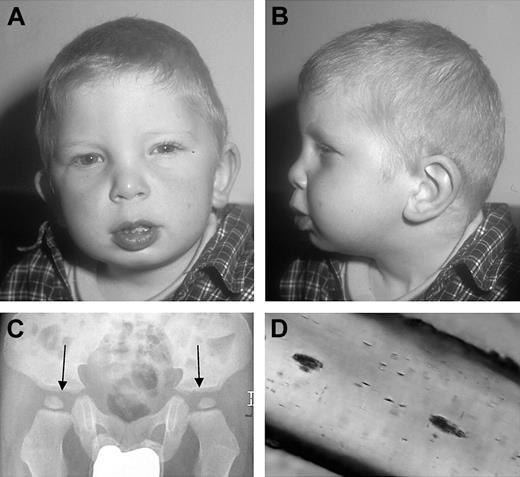
The patient is the third child of healthy first-degree cousins. Both parents and the 2 older siblings are healthy and an extended family history did not reveal the presence of albinism, developmental delay, bleeding, or increased susceptibility to infection. He had connatal nystagmus, blue eyes, bright skin color, silvery-blond hair (Figure 1A-B), and mild facial dysplasia including a broad coarse face, a broad nasal root, mild epicanthal folds, a long philtrum, and low-set posteriorly rotated ears (Figure 1A-B). He had normal skeletal growth, but flat dysplastic acetabulae (Figure 1C). At the age of 2 years, neurophysiologic development was delayed by 8 to 10 months. A magnetic resonance image (MRI) of the brain was normal. Moderate hepatosplenomegaly without signs of liver dysfunction was first noted at the age of 3 months. Neutropenia (0.8 × 109/L [800/μL]) was documented at 8 months of age and subsequently fluctuated. From the age of 6 months, he suffered from more than 10 episodes of otitis media, 3 pneumonias, and recurrent oral thrush. He had self-limiting perinatal cytomegalovirus (CMV) infection, and measles, mumps, and rubella (MMR) live vaccine was well tolerated. No bleeding tendency was reported.
Cytotoxicity assays and flow cytometry
For NK-cell assays, 51Cr-labeled K562 targets were incubated with PBMC effectors directly after Ficoll isolation or after 7-day stimulation with 100 U/mL IL-2 (Chiron, Munich, Germany). CTL cytotoxicity was studied in a redirected lysis assay. For this, PHA blasts cultured for 6 to 8 days (d6-8) were labeled for 2 hours with anti-CD3 (clone UCHT-1; BD Pharmingen, Heidelberg, Germany) and used directly as effector cells on 51Cr-labeled CD95- L1210 target cells. To study CTL degranulation, d2 PHA blasts were stimulated with anti-CD3/CD28 beads (Dynal Biotech, Hamburg, Germany) in the presence of antibodies against CD107a and CD107b (clones H4A3 and H4B4; BD Pharmingen, Heidelberg, Germany). After 1 hour, Monensin A was added (Golgistop; BD Pharmingen) and the cells were incubated for additional 4 to 5 hours.17 After anti-CD3 and anti-CD8 surface and intracellular staining for IFN-γ (clone XMG1.2; BD Pharmingen) using the cytofix/cytoperm kit according to the manufacturer's instructions (BD Pharmingen), cells were analyzed on a FACScan cytometer using Cellquest Pro software (BD Biosciences, Franklin Lakes, NJ).
Dysplastic features of the patient. (A-B) Portrait of the patient showing mild dysplastic features. (C) X-ray of the pelvis with flat, dysplastic acetabulae (arrows). (D) Light microscopy of a representative hair of the head, showing unevenly distributed small clumps of pigment.
Dysplastic features of the patient. (A-B) Portrait of the patient showing mild dysplastic features. (C) X-ray of the pelvis with flat, dysplastic acetabulae (arrows). (D) Light microscopy of a representative hair of the head, showing unevenly distributed small clumps of pigment.
Platelet studies
Blood was taken into 3.8% sodium citrate. PRP was prepared by centrifugation at 80g for 10 minutes, incubated with 50 μM mepacrine (DL Quinacrine; K and K labs, Plain View, NY) at 37°C for 30 minutes, and then centrifuged at 1000g for 10 minutes in the presence of ACD-A (1 vol/9 vol). The platelets resuspended in PBS were examined either by fluorescence microscopy using the Axiapla 2 Imaging microscope coupled with Isis software (Zeiss, Le Pecq, France) or by flow cytometry using the Cytomics FC 500 flow cytometer equipped with CXP software (Beckman-Coulter, Villepinte, France).18 For whole-mount electron microscopy, blood was taken into ACD-A. The platelets were washed as described previously and incubated with 2 mM Ca++ for 30 minutes at room temperature. They were then fixed in 2% (vol/vol) paraformaldehyde (PFA), and a drop was deposited onto collodion-coated nickel grids. They were rinsed twice with PBS and once with distilled water. The samples were then examined with a Jeol JEM-1010 electron microscope (Jeol, Croissy-sur-Seine, France) at 80 kV. To study platelet degranulation, PRP was diluted 1:5 in PBS (pH 7.8) and the peptide GPRP (Bachem Biochemica, Heidelberg, Germany) was added at a final concentration of 1.25 mM. Platelets were then stimulated with different concentrations of thrombin (Behring, Marburg, Germany), stained with anti-CD63 or anti-CD62 (clones CLB-gran12/CLB-thromb/6; Immunotech, Hamburg, Germany), and analyzed by flow cytometry according to published protocols.19
Genetic analysis
Genomic DNA was used to sequence all exons of RAB27A and AP3B1. Primers are available on request (klaus.schwarz@medizin.uni-ulm.de).
Results
Lack of NK-cell cytotoxicity partially restored by IL-2 stimulation
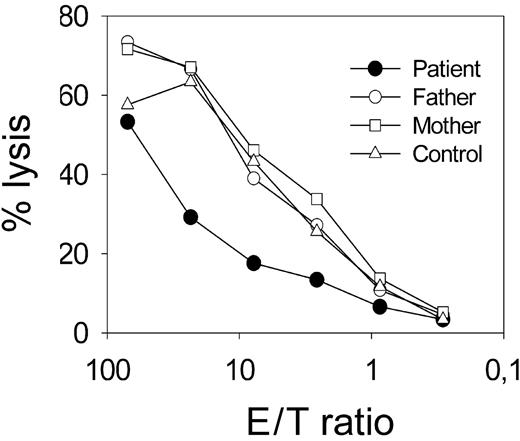
The combination of oculocutaneous albinism (Figure 1A-B) and increased susceptibility to infection suggested a genetic disorder of lysosomal trafficking. Absence of an obvious bleeding tendency and of giant granules in neutrophils excluded CHS. Hair-shaft analysis revealed small clusters of pigment unevenly distributed throughout the hair shaft (Figure 1D). Although lacking the typical large pigmented granules,12 this morphology was interpreted to be compatible with GSII. NK-cell function was studied for further confirmation of this diagnosis. The patient had normal numbers of NK cells, but completely lacked ex vivo cytolytic activity against NK-sensitive K562 target cells (Figure 2A). This was observed in 4 independent experiments, one of which included 2 control patients with HPS of so-far-unknown genetic cause. IL-2 stimulation for 7 days partially restored cytolytic activity against K562 targets (Figure 2B) and against the allogeneic Epstein-Barr virus (EBV)-transformed B-cell line 711 (data not shown), indicating that the defect in cytotoxicity was not absolute.
Impaired NK cytotoxicity. (A) Pooled data from 4 independent NK cytotoxicity assays from the patient (•) and controls (▵) including both parents and 2 patients with HPS of unknown genetic cause (▴). K562 cells were used as targets; spontaneous lysis was less than 18% in all assays. (B) NK cytotoxicity using PBMCs ex vivo or after 7-day stimulation with IL-2 from the patient and mean plus or minus standard deviation of 5 controls, including both parents.
Impaired NK cytotoxicity. (A) Pooled data from 4 independent NK cytotoxicity assays from the patient (•) and controls (▵) including both parents and 2 patients with HPS of unknown genetic cause (▴). K562 cells were used as targets; spontaneous lysis was less than 18% in all assays. (B) NK cytotoxicity using PBMCs ex vivo or after 7-day stimulation with IL-2 from the patient and mean plus or minus standard deviation of 5 controls, including both parents.
Impaired CTL-mediated cytotoxicity and reduced CTL degranulation
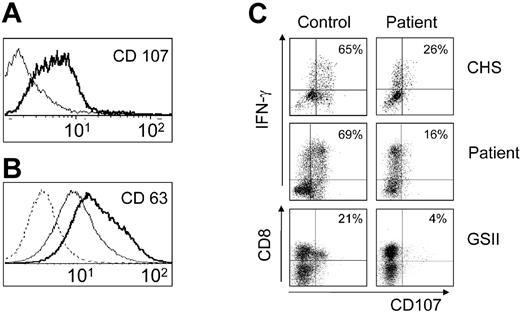
Perforin-mediated CTL activity was studied in an anti-CD3-redirected lysis assay using CD95- L1210 targets. The patient had a normal number of CD8+ T cells with normal intracellular expression of perforin (data not shown). However, lytic activity of d7 PHA blasts was significantly reduced (Figure 3). Expression of the lysosomal marker protein CD107 on activated CTLs has recently been found to indicate degranulation and to correlate with lytic activity.17 Of interest, expression of CD107 and CD63 on unstimulated CTLs was higher in the patient than in controls (Figure 4A-B). By contrast, anti-CD3/anti-CD28 stimulation induced significant CD107 expression in short-term PHA blasts from controls, but not from the patient (Figure 4C). An independent analysis of 2 patients with genetically ascertained GSII or CHS confirmed the validity of this assay (Figure 4C-D). The observed defect in NK and CTL cytotoxicity was consistent with the diagnosis of GSII. Sequencing of the RAB27A gene revealed a paternally inherited mutation (Del381, 382AG, Ins381C) leading to a stop codon at AS 84 (Figure 5A-B). Both truncated and wild-type RNA were present. Although the diagnosis of GSII could not be molecularly ascertained, an unrelated donor search for HSCT was initiated due to the potential risk of developing HLH.
Impaired CTL cytotoxicity. Cytotoxicity of d7 PHA blasts assessed by anti-CD3-mediated redirected lysis on L1210 targets from the patient and 3 controls, including both parents. The percentage of CD8+ T cells among PHA blasts was not determined in this particular experiment, but was similar in patient and controls in 2 other PHA cultures, which were performed for different reasons.
Impaired CTL cytotoxicity. Cytotoxicity of d7 PHA blasts assessed by anti-CD3-mediated redirected lysis on L1210 targets from the patient and 3 controls, including both parents. The percentage of CD8+ T cells among PHA blasts was not determined in this particular experiment, but was similar in patient and controls in 2 other PHA cultures, which were performed for different reasons.
Increased baseline expression of lysosomal marker proteins on T cells and lack of up-regulation upon stimulation. Expression of CD107 (A) and CD63 (B) on resting CD3+CD8+ T cells. Bold lines represent patient; thin lines, control T cells; and dashed line, staining with an isotype control antibody. (C-D) Degranulation of activated T cells assessed by CD107 expression. Short-term PHA blasts (36h) (C) or fresh PBMCs (D) were stimulated with anti-CD3/anti-CD28 beads in the presence of an antibody to CD107 followed by surface staining for CD3 and CD8 and subsequent intracellular staining for IFN-γ. Cells were analyzed by 4-color flow cytometry. Dot plots were gated on CD3+CD8+ cells (C) or on CD3+ cells (D) from healthy control donors (left column), the patient, and 2 patients with genetically confirmed GSII and CHS (right column). The numbers indicate the fraction of degranulating (CD107+) cells among the activated (IFN-γ producing) T cells.
Increased baseline expression of lysosomal marker proteins on T cells and lack of up-regulation upon stimulation. Expression of CD107 (A) and CD63 (B) on resting CD3+CD8+ T cells. Bold lines represent patient; thin lines, control T cells; and dashed line, staining with an isotype control antibody. (C-D) Degranulation of activated T cells assessed by CD107 expression. Short-term PHA blasts (36h) (C) or fresh PBMCs (D) were stimulated with anti-CD3/anti-CD28 beads in the presence of an antibody to CD107 followed by surface staining for CD3 and CD8 and subsequent intracellular staining for IFN-γ. Cells were analyzed by 4-color flow cytometry. Dot plots were gated on CD3+CD8+ cells (C) or on CD3+ cells (D) from healthy control donors (left column), the patient, and 2 patients with genetically confirmed GSII and CHS (right column). The numbers indicate the fraction of degranulating (CD107+) cells among the activated (IFN-γ producing) T cells.
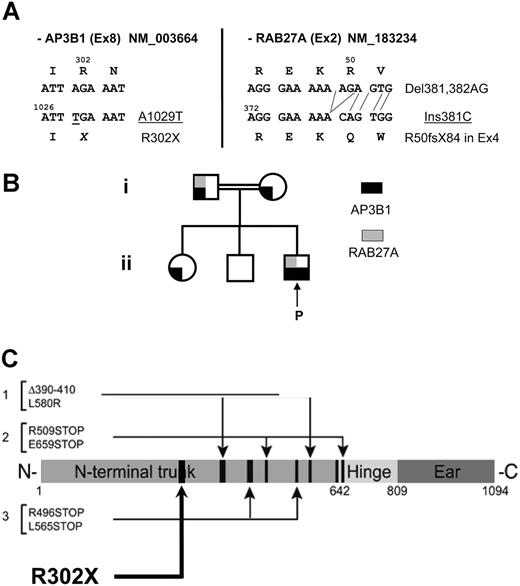
Genetic analysis. (A) DNA mutations and consequences on the protein level for the AP3B1 mutation identified in exon 8 and the RAB27a mutation in exon 2. NM indicates the reference sequences. (B) Pedigree of the family illustrating the genetic status for the RAB27a and the AP3B1 mutations. (C) Location of the new mutation relative to the previously identified mutations.
Genetic analysis. (A) DNA mutations and consequences on the protein level for the AP3B1 mutation identified in exon 8 and the RAB27a mutation in exon 2. NM indicates the reference sequences. (B) Pedigree of the family illustrating the genetic status for the RAB27a and the AP3B1 mutations. (C) Location of the new mutation relative to the previously identified mutations.
Abnormal number, size, and distribution of dense granules associated with impaired platelet aggregation
Unexpectedly, the patient developed severe bleeding after a tooth extraction in preparation for HSCT. PT, aPTT, and platelets were normal, but the bleeding time was prolonged (15 minutes). Analysis of platelet function revealed almost no platelet aggregation after stimulation with epinephrine, collagen, and arachidonic acid. Aggregation after ADP stimulation (2 μM) was severely reduced (22% maximum response; Figure 6A), while ristocetin (1.6 mg/mL) induced a normal maximal response with early disaggregation. Platelet degranulation was quantified by measuring expression of the lysosomal marker protein CD63 in response to thrombin stimulation. Similar to the CTL studies, CD63 expression was already increased on resting platelets, but there was only little increase after thrombin stimulation (Figure 6B). By contrast, expression of CD62, which is up-regulated independently of secretory lysosomes, was similar in the patient and a control subject both at baseline and after stimulation (Figure 6C).
Impaired platelet aggregation and degranulation. (A) Platelet aggregation after stimulation with ADP from the patient (bottom line) and 2 healthy controls (top lines). (B) Degranulation of activated platelets assessed by CD63 expression. The data show the mean fluorescence intensity (MFI) of anti-CD63-stained unstimulated or thrombin-stimulated platelets from the patient and 3 controls, including both parents. (C) Baseline expression of CD62 and up-regulation upon activation with the indicated concentrations of thrombin.
Impaired platelet aggregation and degranulation. (A) Platelet aggregation after stimulation with ADP from the patient (bottom line) and 2 healthy controls (top lines). (B) Degranulation of activated platelets assessed by CD63 expression. The data show the mean fluorescence intensity (MFI) of anti-CD63-stained unstimulated or thrombin-stimulated platelets from the patient and 3 controls, including both parents. (C) Baseline expression of CD62 and up-regulation upon activation with the indicated concentrations of thrombin.
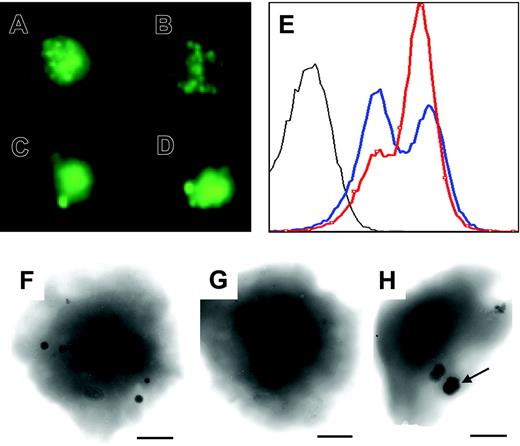
Characterization of the platelets was extended to mepacrine staining and whole-mount electron microscopy. When mepacrineloaded platelets were observed by fluorescence microscopy, fewer dense bodies were seen in the patient compared with healthy controls. Those that were present were often of increased size (Figure 7A-B, control; 7C-D, patient) and did not flash normally. Yet, when identical samples were analyzed by flow cytometry, the fluorescence histograms were similar for patient and control, suggesting that overall mepacrine uptake by the platelets was similar (Figure 7E). Analysis of PFA-fixed platelets loaded with calcium by electron microscopy showed a large reduction in the number of dense granules (mean numbers of 1 per patient platelet versus 3.55 per control platelet [n = 100 platelets]; Figure 7F-H). Nevertheless, granules were present in 41% of the patient's platelets. Significantly, many of the residual dense bodies were of abnormal shape and of increased size as illustrated in Figure 7C, D, and H.
Reduction, abnormal size, and distribution of platelet-dense granules. (A-D) Mepacrine staining of platelets from a healthy control (A-B) and the patient (C-D). Representative staining patterns are shown. (E) Fluorescence histograms of mepacrine-stained platelets from the patient (blue) and a healthy control (red). Unstained platelets from the patient are shown in gray. (F-H) Whole-mount electron microscopy of representative platelets from a control (F) and the patient (G-H). Granules were present in 41% of the patient's platelets.
Reduction, abnormal size, and distribution of platelet-dense granules. (A-D) Mepacrine staining of platelets from a healthy control (A-B) and the patient (C-D). Representative staining patterns are shown. (E) Fluorescence histograms of mepacrine-stained platelets from the patient (blue) and a healthy control (red). Unstained platelets from the patient are shown in gray. (F-H) Whole-mount electron microscopy of representative platelets from a control (F) and the patient (G-H). Granules were present in 41% of the patient's platelets.
G-CSF-responsive neutropenia due to an incomplete maturational arrest in the bone marrow
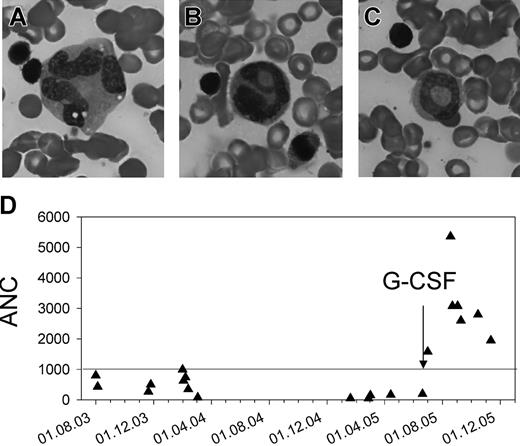
The neutropenia was initially thought to be transient and caused by infections, but an absolute neutrophil count (ANC) persistently lower than 1.0 × 109/L (1000/μL) prompted further investigations. A bone marrow aspirate revealed an incomplete maturation arrest of the myeloid lineage at the stage of promyelocytes and myelocytes. Among the few cells that matured beyond that stage, there was moderate granulocytic dysplasia (Figure 8A-C). There was a good response to granulocyte-colony-stimulating factor (G-CSF) therapy, and stable ANCs higher than 1.5 × 109/L (1500/μL) were obtained with 7.5 μg/kg per day (Figure 8D).
A novel homozygous mutation in the AP3B1 gene
In the context of neutropenia and bleeding, HPSII was suspected and the AP3B1 gene was sequenced. The patient is homozygous for a novel mutation (A1029T) in exon 8 leading to a stop codon at position 302 (Figure 5A-C). Both parents are heterozygous for the mutation (Figure 5B). The father is also heterozygous for the RAB27a mutation (Figure 5A-B), but does not show clinical signs and was normal in all functional CTL and platelet assays.
Impaired myeloid maturation and response to G-CSF treatment. (A-C) Bone marrow smear. Multinucleality and vacuolization of a metamyelocyte (A), abnormal nuclear segmentation (B), and a ring nucleus (C) in neutrophils. (D) Absolute neutrophil counts (ANCs) before and after treatment with G-CSF.
Impaired myeloid maturation and response to G-CSF treatment. (A-C) Bone marrow smear. Multinucleality and vacuolization of a metamyelocyte (A), abnormal nuclear segmentation (B), and a ring nucleus (C) in neutrophils. (D) Absolute neutrophil counts (ANCs) before and after treatment with G-CSF.
Development of lethal hemophagocytic syndrome
None of the 3 published HPSII patients aged 7,15 20,16 and 25 years (and Charles C. Scher [Tulane University, Covington, LA], personal e-mail communication, December 2005) nor the 2 additional teenage patients (Christoph Klein [University of Hannover, Germany], personal phone communication, February 2006) have developed HLH. Moreover, our patient had asymptomatically seroconverted to EBV, a known strong trigger for HLH in patients with familial hemophagocytic syndromes. In the absence of an HLA-identical sibling, we therefore decided to withhold HSCT despite the clearly demonstrated cytotoxicity defect. After initiation of G-CSF therapy, the clinical status of the patient significantly improved and he remained free of infections. However, 8 months later, he presented with an upper-airway infection that rapidly led to respiratory failure. He developed high fever and hepatosplenomegaly, severe cytopenia, and elevated levels of triglycerides, LDH, ferritin, and sCD25, leading to the clinical diagnosis of HLH. Bone marrow analysis showed signs of hemophagocytosis. An extensive search for pathogens revealed a positive polymerase chain reaction (PCR) for parainfluenza virus in the broncho-alveolar lavage (BAL), human herpesvirus 6 (HHV-6), and parvovirus as well as a low copy number (< 1000/μg DNA) for EBV in peripheral blood. Chemotherapy according to the HLH-2004 protocol induced transient remission allowing extubation, but he relapsed on day 10 of the protocol. Rescue therapy with monoclonal antibodies (anti-CD52, anti-TNF, and IL-1 receptor antagonist) was unsuccessful. Conditioning with fludarabine, thiotepa, and ATG was initiated in preparation of a haploidentical bone marrow transplantation, but the child died before transplantation due to pulmonary failure and lung bleeding.
Discussion
HPSII is caused by mutations in the AP3B1 gene encoding the β3A subunit of the AP-3 complex.14,15 Clinical details are so far available for 3 patients (Table 1). A pair of siblings was reported at 20 and 24 years of age with albinism, bleeding, neutropenia, recurrent upper respiratory tract infections during childhood, poor balance, and pulmonary fibrosis.14,15 They were compound heterozy-gous for a 60-bp deletion (del AA390-410) and a nonconservative point mutation (L580R) leading to instability of the protein. A more severely affected child was reported at 5 years of age with similar symptoms, but in addition showed mild facial dysplasia, hepatosplenomegaly, and developmental delay.16 That child was compound heterozygous for 2 nonsense mutations (R509X and E659X), leading to nonsense-mediated mRNA decay and lack of detectable protein. The clinical presentation of our patient was similar to this latter patient. The homozygous R302X mutation predicts to produce no residual protein, but this was not formally analyzed.
The AP-3 complex consists of 4 different subunits and is important for the formation of vesicles at the early endosomes.20 It is ubiquitously expressed and functions to recruit cargo proteins to the newly formed vesicles and transport them to late endocytic compartments.21,22 Defects in the β3A subunit disrupt the complex and all subunits are rapidly degraded. The absence of the AP-3 complex leads to missorting of various proteins from specialized intracellular compartments to the general exocytotic pathway.23 Protein missorting may explain several features of the HPSII phenotype. Aberrant subcellular targeting of neutrophil elastase likely contributes to the observed neutropenia,24,25 while missorting of tyrosinase contributes to the pigmentation disorder.26,27 Misrouting of lysosomal proteins such as CD107 or CD63 to the cell membrane has also been found on fibroblasts14 and CTL clones.13 In this study, we demonstrate increased baseline expression of CD63 also on resting platelets and of CD63 and CD107 on resting CTLs. However, in contrast to platelets and CTLs from healthy controls, activation did not further increase surface expression of these proteins. This correlates with previously demonstrated impaired secretion of platelet-dense granules28 and lytic granules,13 leading to severe impairment of platelet aggregation and of lytic CTL- and NK-cell activity. Our findings indicate that studies of expression of these lysosomal proteins on the surface of resting and activated cells will be useful for the diagnosis of HPSII. The CD107 CTL degranulation assay17 will also be a useful adjunct for the detection of degranulation defects in the differential diagnosis of HLH.29
Two additional cell-biologic consequences of AP-3 deficiency are disturbed vesicle biogenesis and impaired polarization of secretory lysosomes.13,30 It is plausible to assume that these features are due to missorting of structural components of lysosomes and/or of proteins required for lysosomal polarization,1 but this remains to be proved. Malformed and enlarged secretory lysosomes have been demonstrated in AP-3-deficient mouse melanocytes30 and platelets28 and in human CTL clones.13 We show that human AP-3-deficient platelets show a characteristic pattern in size and distribution of dense bodies. Of note, in contrast to most other variants of HPS,31 dense bodies are reduced, but present and overall mepacrine uptake is similar. Furthermore, from a clinical perspective, the bleeding disorder in our and the previously reported patients15,16 was mild and did not dominate the initial clinical presentation. We therefore suggest that a bleeding time should be performed in any patient presenting with albinism and immunodeficiency.
HPSII is distinguished from the other forms of HPS by an increased susceptibility to infections. There are various factors that may contribute to the immunodeficiency of AP-3-deficient patients. First, the degranulation defect of CTLs and NK cells impairs lysis of infected target cells.13 Second, presentation of lipid antigens from bacteria is reduced due to less efficient sorting of CD1b to the lysosome.32 Third, all reported patients had severe neutropenia.15,16 Since G-CSF therapy controlled the increased incidence of infections in our and in one previously reported patient,16 it appears that neutropenia and not impaired antigen presentation, the cytotoxicity defect, or other unidentified factors mainly determine the increased susceptibility to infection in these patients.
A key clinical question is whether the cytotoxicity defect in HPSII predisposes to HLH and therefore represents an indication for preemptive bone marrow transplantation. AP-3 deficiency causes loss of microtubule-mediated movement of lytic granules toward the immunologic synapse in NK cells and CTLs.13 Impaired movement of lytic granules is also observed in CTLs from patients with GSII lacking Rab27a.33 GSII patients invariably develop HLH within the first 10 years of life.12 Similar to observations in GSII patients, our patient had complete loss of ex vivo NK-cell cytotoxicity, which could partly be restored by stimulation with IL-2.34 The basis for this compensation is unexplained, but it is possible that perforin-independent pathways contribute. CTL cytotoxicity was also significantly impaired in the absence of AP-3. A previously published comparison of lytic activity of CTL clones derived from HPSII and GSII patients suggested a similar degree of impairment.13 Unfortunately, we did not have access to fresh cells from a GSII patient who had not yet undergone transplantation for a direct comparison. However, the data obtained with the CD107 degranulation assays also suggest that the impairment of CTL degranulation is in a similar range in both disorders. Overall, these immunologic data would predict a significant risk of HLH also in patients with HPSII. However, this has not been reported in the 3 published patients15,16 and in 2 unpublished teenage patients (C. Klein, personal communication). Asymptomatic seroconversion to EBV further encouraged us to withhold HSCT. Tragically, our patient then developed lethal HLH associated with a parainfluenza infection at 3 years of age. It is unclear whether the additional heterozygous RAB27a mutation contributed to this complication. Although the patient's father, heterozygous for both mutations, is clinically healthy and shows normal results in all functional assays, it cannot be excluded that under conditions of homozygous AP3B1 deficiency, a heterozygous RAB27a mutation can contribute to the phenotype. Thus, the important question of whether preemptive bone marrow transplantation in HPSII patients is justified currently remains incompletely answered.
Finally, the observations of our patient suggest that mild facial dysmorphia, dysplasia of the hip, hepatosplenomegaly, and developmental delay are also part of this complex clinical syndrome.15,16 Although not observed in our young patient, HPSII probably also predisposes to pulmonary fibrosis as reported previously.15,16 Some of these features are probably explained by the fact that osteoclast granules and lamellar bodies in pulmonary epithelial cells also belong to the family of lysosome-related organelles.35 A full definition of HPSII and a better answer to the important question of prognosis and therapy await detailed characterization of further patients. With respect to the wide range of organ systems potentially affected by disturbed lysosomal trafficking, it may certainly be anticipated that more variants of these fascinating disorders will be identified.
Prepublished online as Blood First Edition Paper, March 21, 2006; DOI 10.1182/blood-2005-11-4413.
Supported by the German Research Foundation (SFB 620: A4 to S.E.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very grateful to Geneviève de Saint-Basile for a helpful discussion of this patient and several aspects of the experimental analysis. We thank Andrea Superti-Furga for continuous support and discussion. The technical help of Sylvia Braun, Tatjana Kersten, and Annette Schult is kindly appreciated.