Deferasirox (ICL670) is a once-daily oral iron chelator developed for the treatment of chronic iron overload from blood transfusions. A comparative phase 3 trial was conducted to demonstrate the efficacy of deferasirox in regularly transfused patients with β-thalassemia aged 2 years or older. Patients were randomized and received treatment with deferasirox (n = 296) or deferoxamine (n = 290), with dosing of each according to baseline liver iron concentration (LIC). The primary endpoint was maintenance or reduction of LIC; secondary endpoints included safety and tolerability, change in serum ferritin level, and net body iron balance. In both arms, patients with LIC values of 7 mg Fe/g dry weight (dw) or higher had significant and similar dose-dependent reductions in LIC and serum ferritin, and effects on net body iron balance. However, the primary endpoint was not met in the overall population, possibly due to the fact that proportionally lower doses of deferasirox relative to deferoxamine were administered to patients with LIC values less than 7 mg Fe/g dw. The most common adverse events included rash, gastrointestinal disturbances, and mild nonprogressive increases in serum creatinine. No agranulocytosis, arthropathy, or growth failure was associated with deferasirox administration. Deferasirox is a promising once-daily oral therapy for the treatment of transfusional iron overload.
Introduction
Chronic iron overload represents a serious complication of potentially lifesaving blood transfusions. Excess iron deposits in various tissues of the body, particularly the liver, heart, and endocrine organs.1 Once the body's storage capacity is exceeded, free iron catalyzes the formation of highly reactive hydroxyl radicals, which leads to membrane damage and denaturation of proteins. This process leads to tissue damage and ultimately to significant morbidity and mortality.2
Indeed, organ failure due to chronic iron overload represents the major cause of death in patients with β-thalassemia major who receive blood transfusions regularly without appropriate chelation therapy.3-5 Within 1 to 2 years of initiation of regular blood transfusions, evidence of iron overload is manifest as elevated liver iron concentration (LIC) values and elevated serum ferritin levels. An increased risk of iron-induced cardiac disease is observed in thalassemia patients with LIC values above 15 mg Fe/g dry weight (dw), and in patients with serum ferritin values above 2500 μg/L.6 Patients with a number of other congenital and acquired anemias who may receive frequent blood transfusions are also susceptible to the adverse effects of iron loading.7
Deferoxamine mesylate (Desferal; Novartis, Basel, Switzerland) is the current standard for iron chelation therapy. There is clear and consistent evidence in the literature regarding its benefits on both morbidity and mortality and its ability to provide benefit to a variety of organs, such as the liver, heart, and endocrine glands.8 Unfortunately, due to the challenges of administering deferoxamine by slow subcutaneous or intravenous infusion over 8 to 12 hours, 5 to 7 nights per week, compliance with prescribed therapy is often poor, resulting in limited efficacy.9,10
In some countries, marketing authorization has been granted to the oral iron chelator deferiprone (Ferriprox; Apotex Toronto, ON, Canada), which is formulated as solid tablets and administered 3 times a day. Prospective clinical data documenting the efficacy of deferiprone are limited.11,12 In addition, its therapeutic window is narrow, and its safety risks include drug-related agranulocytosis and arthropathy.13
Deferasirox (ICL670, Exjade; Novartis) is a member of a new class of tridentate iron chelators, the N-substituted bis-hydroxyphenyl-triazoles.14 It is orally bioavailable and its terminal elimination half-life (t1/2) is between 8 and 16 hours, allowing for once-daily administration. Metabolism and elimination of deferasirox and the iron chelate (Fe-[deferasirox]2) is primarily by glucuronidation followed by hepatobiliary excretion into the feces. No significant drug-drug interactions have been identified to date. Preclinical studies demonstrated the ability of deferasirox to enter and remove iron from cells.15,16
Previous studies in adult patients with β-thalassemia major revealed that treatment with deferasirox could potentially remove sufficient iron from the body to exceed that administered as part of a chronic transfusion regimen.17 This multinational phase 3 randomized trial comparing deferasirox to deferoxamine over 1 year was initiated in pediatric and adult patients with β-thalassemia receiving regular blood transfusions in order to further evaluate its efficacy in body iron reduction. β-thalassemia was selected as the model disease for demonstration of efficacy across the range of patients at risk of iron overload.
Patients, materials, and methods
Experimental design
This study was undertaken to investigate the hypothesis that deferasirox was noninferior to deferoxamine.
Eligibility
Eligible patients included those at least 2 years of age with a diagnosis of β-thalassemia and chronic iron overload from blood transfusions as indicated by an LIC value of 2 mg Fe/g dw or higher (Table 1). Patients needed to be receiving at least 8 blood transfusions per year, and could be enrolled irrespective of prior chelation therapy. Female patients were required to use double-barrier contraception.
Patients were excluded from this trial if they had one of the following conditions: an alanine aminotransferase (ALT) level greater than 250 U/L during the year prior to enrollment, chronic hepatitis B infection, active hepatitis C infection, a history of a positive HIV test, serum creatinine above the upper limit of normal (ULN), a urinary protein–creatinine ratio of greater than 0.5 mg/mg, nephrotic syndrome, uncontrolled systemic hypertension, a prolonged corrected QT interval, or systemic infection within the 10 days prior to entry. Additionally, patients were excluded if they had gastrointestinal conditions preventing absorption of an oral medication, concomitant conditions preventing therapy with deferasirox or deferoxamine, a history of ocular toxicity related to iron chelation therapy, a poor response to deferoxamine, or noncompliance with prescribed therapy.
Enrollment
Patients providing informed consent entered a screening period during which a variety of baseline evaluations were performed: hematology and chemistry parameters, hepatitis serology, electrocardiogram (ECG; along with echocardiogram and Holter monitoring in patients with a history of heart disease), ophthalmologic examination, auditory evaluation, and LIC determination. The method used for LIC determination in most patients (84%) was liver biopsy. Some patients, mostly children and adults with contraindications to liver biopsy, had LIC measured by the noninvasive technique of magnetic liver susceptometry using a quantum interference device (SQUID).
Liver iron concentration determination
Liver samples for iron concentration determination and pathology studies were obtained by biopsy of the liver using a standardized methodology.18 In order to minimize variation in LIC due to analytical methods, iron quantification was performed using atomic absorption spectrometry (AAS) in a single central laboratory (Clinique des Maladies du Foie [Clinic for Hepatic Illnesses], Centre Hospitalier Universitaire, Rennes, France).19,20 The minimum specimen size acceptable for inclusion in analyses was 0.4 mg dw.
SQUID assessments were performed in 3 centers (Turin, Italy; Hamburg, Germany; and Oakland, CA).21,22 Both baseline and end-of-study (EOS) assessments were always conducted at the same site. An independent calibration study performed between the 3 centers conducting SQUID evaluations indicated that the LIC values at the Turin site were approximately 20% lower than the values obtained at the other 2 sites. A validation substudy comparing LIC determined by liver biopsy and SQUID found that LIC values from SQUID were approximately half those from biopsy. Because these findings were made after initiation of the present study, the LIC values presented were not corrected for such differences.
Dosing of deferasirox and deferoxamine
In order to attempt to appropriately treat patients across the spectrum of iron loading, a dosing algorithm was constructed according to baseline LIC values (Table 2).
A short-term iron balance study indicated that 20 mg/kg deferasirox daily maintained stable iron balance.17 A subsequent phase 2 study, however, indicated that after 1 year a daily dose of 10 mg/kg deferasirox could maintain LIC and that a daily dose of 20 mg/kg could reduce LIC comparably to 40 mg/kg deferoxamine given 5 days a week. Based on these results, and a concern about the potential for excessive iron chelation, a conservative dosing strategy was adopted in which 5- and 10-mg/kg deferasirox doses were selected for maintenance use in patients with lower LIC values. The 20-mg/kg dose was considered appropriate for patients requiring reduction of a moderate iron burden, and a higher dose of 30 mg/kg was felt to be appropriate for patients with high iron burdens requiring major reduction of excess iron. The latter assumptions were based upon data suggesting that the approximate ratio of effective doses of deferasirox to deferoxamine was 1:2 (as a milligram per kilogram–to–milligram per kilogram ratio).
In contrast, the desire to avoid potential safety issues due to inadequate iron chelation in patients who had previously been successfully treated with deferoxamine led to the selection of the 20 to 30 mg/kg and 25 to 35 mg/kg deferoxamine dose ranges for maintenance use in patients with low LIC values. The 35- to 50-mg/kg range and the 50-mg/kg and higher deferoxamine doses were well established as appropriate for patients with moderate or high degrees of iron overload.
Because the dose of deferoxamine taken prior to entry into the study was assumed to have produced clinical efficacy, patients with lower baseline LIC values on the deferoxamine arm were permitted to remain on their higher prestudy doses, even if outside the predefined dosing ranges for the study. As summarized in Table 2, many individuals with LIC values of 2 to 7 mg Fe/g dw or less therefore received significantly lower doses of deferasirox relative to deferoxamine during the study (ratio, approximately 1:4). This contrasted with the groups of patients having LIC values greater than 7 mg Fe/g (ratio, approximately 1:2).
Treatment plan
Patients meeting the eligibility requirements were randomized to receive deferasirox or deferoxamine. Randomization was stratified by age groups: 2 to younger than 12 years, 12 to younger than 18 years, and 18 years or older. After randomization, patients were assigned by the investigator to a dose dependent on their baseline LIC according to the algorithm noted in Table 2. Once-daily treatment with deferasirox at the assigned dose was administered as a suspension in water half an hour prior to breakfast 7 days a week. Deferoxamine was administered as a slow subcutaneous infusion using electronic Microject Chrono infusion pumps (Canè Medical Technology, Torino, Italy) over 8 to 12 hours, 5 days a week. Exceptions were permitted to the number of days of administration, which ranged from 3 to 7 days a week (to facilitate comparison, all deferoxamine doses reported are normalized to administration for 5 days a week; ie, 50 mg/kg administered 7 days a week would be reported as 70 mg/kg).
Treatment with either therapy was continued for 1 year, and was only interrupted at the discretion of the investigator for intercurrent illness or adverse events. Dose modifications were mainly permitted for safety reasons after consultation with Study Monitoring Committee or Program Safety Board members. Drug administration was recorded on the case report form.
Blood transfusions were regularly administered during the study period according to the patients' requirements as judged by the investigators, and the amount of blood (and iron) received by each patient was carefully assessed.
Assessments for safety and efficacy performed at monthly intervals at a central laboratory (B.A.R.C. Laboratories, Gent, Belgium) included complete blood count/differential, electrolytes, liver function tests, trace element analysis, urinary protein/creatinine and serum ferritin, iron, and transferrin. ECGs and ophthalmologic and auditory examinations were performed every 3 months. For patients younger than 16 years of age, additional evaluations included assessment of growth rate and sexual development. Growth was monitored by measuring standing height and sitting height using a Harpenden (Holtain, Crosswell, United Kingdom) stadiometer with an approximation to within 0.1 cm. This assessment was performed every 3 months during the trial in order to calculate growth velocity. At the end of the 1-year period, all patients underwent a repeat LIC determination using the same methodology as the initial determination performed (liver biopsy or SQUID).
Net iron balance (total body iron excretion) was calculated based on the amount of red blood cells (RBCs) transfused (iron intake in milligrams = Kin) and on the changes in total body iron from baseline to the end of the study: net body iron balance = (Kin + [Us(t0) – Us(t)])/(t – t0); iron intake in milligrams of iron was calculated as Kin = (total amount of RBCs transfused) × 1.08. The total amount of RBCs transfused is calculated as the total amount of blood in milliliters multiplied by the hematocrit of each unit in percentage divided by 100. Complete datasets (volume and hematocrit) were available for all transfusions in three quarters of the patients. If an individual hematocrit was missing, the average hematocrit of the blood given as transfusions at the respective center was used, and if this was not available the value was assumed to be 65%. If the amount of blood transfused was given only as units, instead of in milliliters with the hematocrit, the volume of RBCs was assumed to be 185 mL and thus 200 mg iron was assumed to be given per unit. Us(t) is the total body iron extrapolated from the LIC (in mg Fe/g dw) at time t (t0 = 0, for baseline measurement) Us(t) = 10.6 × LIC (× [body weight in kg]).
Both the iron intake Kin and the changes in total body iron Us(to) – Us(t) are expressed in milligrams of iron; therefore, the net body iron balance is expressed in milligrams of iron per day.
Primary response criteria
The primary response criterion for this trial was nonparametric and consisted of maintenance or reduction of LIC (Table 3). As LIC values greater than 7 mg Fe/g dw have been reported in the literature to be associated with an increased morbidity and mortality, maintenance or reduction of LIC values to below this level was desirable as an endpoint. However, because it was considered unrealistic to expect to reach values below 7 mg Fe/g dw after 1 year in heavily iron-overloaded individuals (LIC ≥ 10 mg Fe/g dw), a decrease of at least 3 mg Fe/g dw was selected as a reasonable target to be accomplished in 1 year, since in most patients such an annual reduction would lead to safe body iron levels within a few years. Reduction of LIC to less than 1 mg Fe/g dw was considered undesirable, potentially exposing patients to the risk of overchelation, and such patients were to be considered failures in the analysis of success.
Secondary criteria for response included evaluation of the change in serum ferritin levels over time and evaluation of net body iron balance.
Statistical methods
The percentage of successes for each treatment was calculated in the population representing all patients who completed both LIC assessments or who discontinued due to safety concerns (considered as failures). Deferasirox was to be declared noninferior to deferoxamine if the lower limit of the 95% confidence interval (2-sided) for the difference in the percentage of treatment successes of deferasirox and deferoxamine was above –15%. This margin was chosen based on statistical consideration used for other agents. The sample size was calculated to demonstrate noninferiority at a 2-sided alpha level of 5% if the success rates of deferasirox and deferoxamine were both 50%. Thus, 468 patients (234 per arm) would have been required to achieve a power of 90%. Although intended to recruit only 500 patients, a greater number of patients than expected entered into screening met the enrollment criteria. The confidence intervals for the success rates and differences in success rates were calculated using the normal approximation.
Reported P values for the investigation of secondary endpoints are based on 1- or 2-sided significance tests (Student t test).
Quality control, quality assurance, and monitoring
This trial was conducted in accordance with good clinical practices. Institutional review board or ethics committee approval was obtained at each participating institution and written informed consent was obtained from all patients or their legal guardians prior to participation in any study procedures.
A Study Monitoring Committee composed of 4 investigators supervised the trial conduct and made decisions regarding dose adjustment for individual patients. An independent Program Safety Board composed of 5 experts in the fields of hematology, hepatology, nephrology, and statistics confidentially reviewed clinical and laboratory data on several occasions during this trial.
Results
Baseline demographic characteristics and patient disposition
Between March and November 2003, 586 patients were randomized and started study treatment at 65 centers in 12 countries (296 on deferasirox; 290 on deferoxamine). There were no major differences in the patient populations randomized to receive deferasirox or deferoxamine with regard to baseline demographics, disease characteristics, rate of blood transfusion, or baseline LIC (Table 1). Many (51%) of the patients were younger than 16 years of age; 87.7% of patients were white, and 51.9% of patients were female. Most patients (97.4%) had received prior chelation therapy. The number of blood transfusion episodes was similar across all treatment groups resulting in relatively similar iron intake from blood transfusion. There was no clear correlation between any patient or disease characteristic and baseline LIC. Approximately two thirds of each group was heavily iron overloaded as evidenced by an LIC value of 7 mg Fe/g dw or more at baseline (deferasirox, 69%; deferoxamine, 68%).
Most patients completed 1 year of therapy on this study: 541 (92.3%) of 586 underwent both baseline and 1-year LIC assessments. Discontinuations were relatively similar in the groups receiving deferasirox (n = 17) and deferoxamine (n = 12).
Changes in liver iron concentration
The primary efficacy population in this study consisted of 553 patients with LIC evaluations at baseline and after 52 weeks and those who discontinued due to safety reasons (adverse event, abnormal laboratory value or test procedure result, or iron overload–related death).
The primary objective of noninferiority to deferoxamine across all dose groups based on the success rate analysis was not achieved in the primary efficacy population because the lower limit of the 95% confidence interval was less than –15% as shown in Table 4. This was most likely caused by the disproportionately low dosing of patients with deferasirox at 5 and 10 mg/kg/d relative to deferoxamine and the maintenance of high prestudy deferoxamine doses.
Noninferiority was demonstrated in the group of patients who were allocated to the higher dose groups (deferasirox doses of 20 or 30 mg/kg and deferoxamine doses of ≥ 35 mg/kg) for baseline LIC levels of 7 mg Fe/g dw or higher, where the dose relationship of deferasirox to deferoxamine of 1:2 was maintained. These patients represented most patients enrolled in the primary efficacy population (69%). In these patients, overall success rates as analyzed by biopsy and SQUID were comparable (58.6% vs 58.9%), and the lower limit of the 95% confidence interval (–10.2%) was above the predefined non-inferiority threshold of –15% as shown in Table 4.
Based upon the results of a validation study that was conducted, SQUID underestimated LIC in this study by approximately half (values reported here are uncorrected). If patients with LIC assessments by SQUID are excluded, and the success rates are analyzed only in patients with LIC determinations by liver biopsy, the success rates in patients with LIC values of 7 mg Fe/g dw or higher are comparable (59.7% vs 58.7%) and the lower limit of the 95% confidence interval (–9.2%) is also above the predefined noninferiority threshold of –15%.
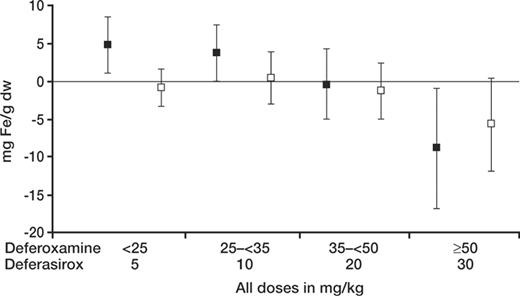
Deferasirox produced a reduction in absolute LIC that was related to the dose administered as shown in Figure 1. A preplanned aim of the secondary efficacy analysis was to test the hypothesis that for deferasirox the absolute reduction in LIC after 1 year in patients with baseline LIC of 7 mg Fe/g dw or higher was statistically significantly greater than 0 (Student t test, 1-sided, alpha = .025). In the 185 patients on deferasirox completing EOS LIC evaluations who received daily doses of 20 or 30 mg/kg deferasirox for LIC of 7 mg Fe/g dw or higher at baseline, a statistically significant reduction in LIC was observed after 1 year (–5.3 ± 8.0 mg Fe/g dw, mean ± SD, P < .001) (Table 5). This reduction was not statistically significantly different from that affected by deferoxamine (–4.3 ± 5.8 mg Fe/g dw, P = .367). This lack of difference with deferoxamine was confirmed in the subgroup of patients assessed by biopsy (deferasirox n = 172, deferoxamine n = 175, P = .325).
Deferasirox produces a reduction in the absolute liver iron concentration. Results are shown according to the actual initial dose administered. Doses of 5 and 10 mg/kg were too low to maintain or reduce absolute LIC, whereas a dose of 20 mg/kg maintained LIC and 30 mg/kg reduced LIC. A similar dose response was not observed in the groups of patients receiving deferoxamine at doses lower than 50 mg/kg, likely because the mean doses of deferoxamine actually administered were within a more narrow range (33.9-42.2 mg/kg versus 5-20 mg/kg). ▪ indicates deferasirox; □, deferoxamine. Mean ± SD.
Deferasirox produces a reduction in the absolute liver iron concentration. Results are shown according to the actual initial dose administered. Doses of 5 and 10 mg/kg were too low to maintain or reduce absolute LIC, whereas a dose of 20 mg/kg maintained LIC and 30 mg/kg reduced LIC. A similar dose response was not observed in the groups of patients receiving deferoxamine at doses lower than 50 mg/kg, likely because the mean doses of deferoxamine actually administered were within a more narrow range (33.9-42.2 mg/kg versus 5-20 mg/kg). ▪ indicates deferasirox; □, deferoxamine. Mean ± SD.
Changes in serum ferritin
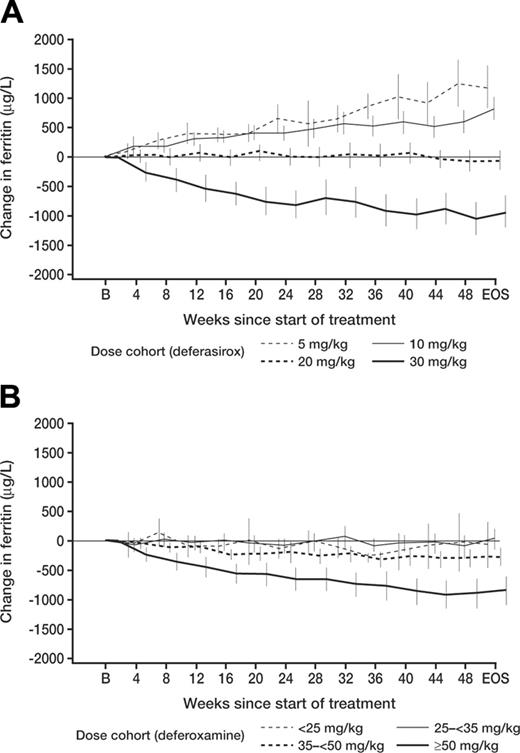
Effects on mean serum ferritin levels over time that paralleled the changes in LIC were observed in both deferasirox- and deferoxamine-treated patients, as noted in Table 6 (representing all available ferritin values in patients who took the study drug). This was true despite the known variability in single measurements of this parameter. With monthly monitoring of serum ferritin values the response to different deferasirox doses could be distinguished after 12 weeks on study (Figure 2). Over this interval, deferasirox doses of 5 and 10 mg/kg led to increasing serum ferritin, 20 mg/kg led to stable ferritin, and 30 mg/kg led to reduced ferritin values. Using linear regression, a correlation was observed between serum ferritin and LIC values (r = .63).
Changes in net body iron balance
Average iron intake per kilogram of body weight during the 1-year period of the study was similar in patients receiving deferasirox and deferoxamine (0.39 ± 0.107 mg/kg/d and 0.41 ± 0.113 mg/kg/d, respectively). In the population of patients in whom LIC was measured by liver biopsy, the mean values for the ratio of iron excretion to iron intake for deferasirox and deferoxamine were 1.21 ± 0.745 and 1.21 ± 0.476, respectively, indicating net iron excretion. Values that are less than 1 indicate iron intake in excess of excretion, and values greater than 1 indicate iron excretion in excess of intake. The ratios were lower in the population of patients in whom LIC was measured by SQUID (0.95 ± 0.24 and 1.05 ± 0.145, respectively). Table 6 illustrates that the changes were related to the dose of deferasirox or deferoxamine administered.
Comparison of results by dose
Changes in serum ferritin, LIC levels, and net body iron balance over a 1-year interval were consistent. For both deferasirox and deferoxamine, these 3 parameters corresponded well with one another. In addition, a relationship between the dose of drug administered and the response observed was apparent for deferasirox (Table 6). In this table, the median dose initially administered during the trial is noted for deferasirox and for deferoxamine. For patients receiving 5 mg/kg deferasirox and the majority of patients receiving 10 mg/kg deferasirox (those with baseline LIC values ≤ 7 mg Fe/g dw), all 3 parameters indicated an increase in iron burden; for those receiving 20 mg/kg deferasirox (corresponding to an LIC between 7 and ≤ 14 mg Fe/g dw), they indicated that iron burden was essentially unchanged; and for those receiving 30 mg/kg (corresponding to an LIC > 14 mg Fe/g dw), they indicated that iron burden was reduced.
The effect of deferasirox on serum ferritin in different dose groups was distinguishable at 3 months. Monthly changes in serum ferritin according to dose are shown, and changes from baseline are plotted at intervals of 4 weeks. Serum ferritin levels were available through EOS for 96% of patients on both deferasirox and deferoxamine arms. Panel A shows deferasirox; panel B, deferoxamine. After 12 weeks, differences between deferasirox dose groups (5 and 10 mg/kg vs 20 mg/kg vs 30 mg/kg) were already apparent. As the actual doses of deferoxamine administered were relatively similar for patients with LIC values lower than 14 mg Fe/g dw, the finding of similar changes in serum ferritin, as observed, would be expected. EOS indicates end of study. Values represent mean ± 2 SEM.
The effect of deferasirox on serum ferritin in different dose groups was distinguishable at 3 months. Monthly changes in serum ferritin according to dose are shown, and changes from baseline are plotted at intervals of 4 weeks. Serum ferritin levels were available through EOS for 96% of patients on both deferasirox and deferoxamine arms. Panel A shows deferasirox; panel B, deferoxamine. After 12 weeks, differences between deferasirox dose groups (5 and 10 mg/kg vs 20 mg/kg vs 30 mg/kg) were already apparent. As the actual doses of deferoxamine administered were relatively similar for patients with LIC values lower than 14 mg Fe/g dw, the finding of similar changes in serum ferritin, as observed, would be expected. EOS indicates end of study. Values represent mean ± 2 SEM.
Safety
Deferasirox was generally well tolerated. Dose adjustments and dose interruptions combined were similar in the deferasirox and deferoxamine groups (36.8% vs 33.1%). Discontinuations during the study were similar in the deferasirox and deferoxamine treatment groups (5.7% vs 4.1%) and consisted of deaths, adverse events, protocol violations, and withdrawal of consent. One asplenic pediatric patient died suddenly of unexplained causes while receiving deferasirox. Three patients died while receiving deferoxamine: 1 each from convulsions, intraventricular thrombus, and sepsis. None of the 4 deaths were felt by the independent Program Safety Board to be related to the administration of study drug.
The most common adverse events with an apparent relationship to deferasirox were transient gastrointestinal events in 15.2% of patients that included abdominal pain, nausea and vomiting, diarrhea, and constipation, as well as skin rash in 10.8% of patients. The gastrointestinal events lasted a median of 8 days or less. These symptoms rarely required dose adjustment or discontinuation of deferasirox.
Mild, dose-dependent increases in serum creatinine were observed in 38% of patients receiving deferasirox, most frequently at doses of 20 and 30 mg/kg in the population of patients having the most dramatic decrease in LIC and serum ferritin. These increases were sometimes transient and generally within the normal range, and they never exceeded 2 times the ULN. Similar increases in serum creatinine occurred in 14% of patients receiving deferoxamine.
A dose reduction of 33% to 50% was undertaken for those 15 years or older with at least 2 consecutive increases in serum creatinine greater than 33% above baseline and for those younger than 15 years with at least 2 consecutive increases in serum creatinine greater than 33% that were also above the upper limit of age-appropriate normal. As the creatinine spontaneously normalized in a number of patients, dose reductions were instituted in only 13% of patients receiving deferasirox. In about 25% of patients the creatinine then returned to baseline, and in the remainder of the patients it remained stable or fluctuated between baseline and the maximum increase observed prior to dose reduction.
Two patients developed elevated ALT values greater than twice the ULN while receiving deferasirox, which was felt by the investigator to be related to its administration. In one case, the ALT values were still elevated to 3 times ULN 4 months after drug discontinuation, and in the other the ALT value returned to baseline within 1 month. In neither case was the alkaline phosphatase or bilirubin elevated significantly above baseline.
Deafness, neurosensory deafness, or hypoacusis were reported as adverse events irrespective of drug relationship in 8 patients on deferasirox and 7 on deferoxamine. These symptoms were considered related to the study drug in 1 patient on deferasirox (0.3%) and 5 patients on deferoxamine (1.7%). Cataracts or lenticular opacities were reported as adverse events regardless of drug relationship in 2 patients on deferasirox and 5 on deferoxamine. These symptoms were considered related to the study drug in 1 (0.3%) patient on deferasirox and 4 (1.4%) patients on deferoxamine.
No drug-related agranulocytosis was observed during this trial. Zinc and copper levels at the end of the study with deferasirox were comparable with those observed in patients receiving deferoxamine. The electrocardiograms performed at baseline and every 3 months during the study were analyzed in a central laboratory for 258 patients receiving deferasirox and 245 patients receiving deferoxamine (86% of the overall population). No cardiac safety concerns specific to deferasirox were identified. A similar percentage of patients receiving deferasirox and deferoxamine experienced cardiac adverse events (deferasirox 5.1%, deferoxamine 6.9%) and serious adverse events (deferasirox 0.7%, deferoxamine 1.0%). Evaluation of pediatric patients revealed that growth and development proceeded normally while patients were receiving deferasirox.
Discussion
Chronic iron overload due to blood transfusions leads to significant morbidity and early mortality unless adequate chelation therapy is administered. Deferoxamine is the reference chelation therapy that has a well-established safety and efficacy profile. Patients who are treated adequately with deferoxamine from early on in life do not develop typical complications of iron overload, including cardiac, endocrine, and hepatic failure.23,24 However, because deferoxamine must be administered by prolonged subcutaneous or intravenous infusion, patient acceptance of, and compliance with, therapy is often poor. So, despite the availability of an effective chelating agent, the compliance issues with deferoxamine mean that many patients still develop clinically significant iron overload, with the related impact on morbidity and mortality.
Deferasirox was developed in response to the need for an oral iron-chelating agent. In particular, it was desirable to have an agent that could be administered conveniently to patients of all ages, and across a range of iron burdens. Previous clinical studies indicated the potential of deferasirox to meet this need, and the current study was performed to compare this agent to deferoxamine.12 Because complications of chronic iron overload have been best studied in β-thalassemia, this population of patients was used for the demonstration of efficacy for deferasirox. However, given that the pathogenesis of chronic iron overload is similar in different underlying types of anemia requiring transfusion, the efficacy results are more broadly applicable.
Methodologic limitations of this study include the fact that although liver biopsies were analyzed at a single central laboratory, there were differences in the techniques used to obtain the specimens. Although biopsy specimens between 0.4 and 1.0 mg dw were considered acceptable in this study, the accuracy of the determinations on these samples may be lower than for those of 1 mg dw or more.20 In addition, the use of SQUID underestimated LIC values, and this affected 14% of patients in the trial. Although this most likely led to underdosing of patients assessed by this methodology, it did not alter the primary outcome of the study.
While the primary statistical endpoint in this study was not met, most probably mainly due to the disproportionately low doses of deferasirox given to regularly transfused patients with mild to moderate iron overload (LIC values of 2 to ≤ 7 mg Fe/g dw), there was clear demonstration of iron excretion related to the dose administered (Table 6). Doses (5 and 10 mg/kg) of deferasirox administered were clearly too low to compensate for ongoing iron loading through transfusion with approximately 0.4 mg/kg/d, particularly relative to deferoxamine (doses of deferasirox relative to deferoxamine were on average half those administered to patients with LIC values > 7 mg Fe/g dw). However, in the group of patients with LIC values greater than 7 mg Fe/g dw to whom doses of deferasirox and deferoxamine were administered in a 1:2 ratio, the success rates were similar. In frequently transfused patients, defined as individuals receiving 2 to 4 units per month (or 7-14 mL/kg/mo) of packed RBCs, oral deferasirox at a once-daily dose of 20 mg/kg led to maintenance of LIC, neutral iron balance, and stable serum ferritin levels. Once-daily doses of 30 mg/kg led to a significant reduction in LIC, negative iron balance, and decreased serum ferritin levels. The results obtained are consistent with a previously published short-term study examining the ability of deferasirox to remove iron from the body.17 In that study, a dose of 20 mg/kg was predicted to be able to maintain stable iron balance in regularly transfused patients, similar to the present study.
The most common symptoms reported with a suspected relationship to repeated administration of deferasirox were abdominal pain, nausea, vomiting, diarrhea, constipation, and skin rash. These symptoms were generally of mild to moderate severity and often resolved even when the drug was continued. Elevations in liver function tests to more than twice the upper limit of normal that were thought to be related to the administration of deferasirox occurred in 2 patients. Mild, nonprogressive increases in serum creatinine, generally within the normal range, were the most common laboratory abnormalities that have occurred in patients receiving deferasirox and were more frequent in patients receiving 20 or 30 mg/kg. Such increases may be related to an overchelation effect in patients receiving less frequent blood transfusions since they were most commonly observed in patients who had the greatest reduction in LIC and serum ferritin. Further work is required, however, to fully understand the underlying mechanism. No agranulocytosis or adverse effects on growth or development in pediatric patients were observed. As the effects of deferasirox on renal and hepatic function as well as growth and development over a number of years are not yet known, many patients have now entered a 3-year noncomparative extension study to investigate longer-term safety and efficacy. Studies are also planned to assess the ability of deferasirox to remove iron from the heart.
In summary, deferasirox represents an important novel once-daily oral agent for the treatment of chronic iron overload due to blood transfusions. Its use has been investigated in a 1-year trial in adults and pediatric patients as young as 2 years of age. The availability of a well-tolerated, effective, and conveniently administered oral iron chelator should permit application of chelation therapy to a larger patient population in need. It should also facilitate patient compliance, a critical factor in effective patient management, and thereby help maintain low iron burdens in patients requiring frequent blood transfusions.
Appendix: participating centers and investigators
Argentina: G. Drelichman, Hospital de Ninos Ricardo Gutierrez, Buenos Aires; N. Watman, Hospital Ramos Mejía, Buenos Aires; and A. Berreta, Hospital Privado de Cordoba, Cordoba.
Belgium: C. Vermylen, Cliniques Universtaires St Luc, Bruxelles; D. Boulet, CHR St Joseph, Mons; A. Ferster, H.U.D.E. Reine Fabiola, Laeken; and M.-F. Dresse, CHR Citadelle, Liege.
Brazil: M. Verissimo and V. Pereira, Centro Infantil de Investigacoes Hematologicas Dr Domingos A. Boldrini, Campinas; and S. Loggetto and M. L. Silva, Centro de Hematologia Sao Paulo, Sao Paulo.
Canada: N. Olivieri, Toronto General Hospital, Toronto, Ontario; and S. Abish, Montreal Children's Hospital, Montreal, Quebec.
France: I. Thuret, Hôpital de la Timone Enfants, Marseille; M. de Montalembert, Hôpital Necker, Paris; D. Bachir, CHU Henri Mondor, Creteil; R. Girot, Hôpital Tenon, Paris; and G. Salles, Centre Hospitalier Lyon-Sud, Pierre-Benite.
Germany: G. Janka-Schaub, Universitaetskrankenhaus Eppendorf, Hamburg; E. Kohne, Universitaetsklinikum Ulm, Ulm; G. Janssen, Universitaetsklinikum Duesseldorf, Duesseldorf; T. Klingbiel, Universitaetsklinikum Frankfurt, Frankfurt; and G. Strauss, Universitaetskinderklinik, Berlin.
Greece: C. Kattamis, V. Ladis, and H. Berdoussi, “Agia Sofia” Children's Hospital, First Department of Pediatrics, Athens University School of Medicine, Athens; A. Koussi and I. Tsatra, “Hippokration” Hospital of Thessaloniki, First Department of Pediatrics, Hematology Division, “Aristotle” University of Thessaloniki, Thessaloniki; N. Zoumbos and I. Constandinidou, University Hospital of Patras, Department of Internal Medicine, Haematology Division, Patras; and K. Bourantas, University Hospital of Ioannina, Haematology Department, Ioannina.
Italy: S. Perrotta, D. Di Pinto, and B. Nobili, Policlinico, II Università di Napoli, Naples; M. D. Cappellini and L. Zanaboni, Ospedale Maggiore—IRCCS, Milan; M. Capra, A. Montesanto, Ospedale Civico e Benefratelli G. di Cristina M. Ascoli, Palermo; R. Origa and A. Zappu, Ospedale Regionale Microcitemie—A.S.L.n. 8, Cagliari; C. Magnano and S. Anastasi, Az. Osp. di Rilievo Nazionale e di Alta Spec. Garibaldi, Catania; A. Mangiagli and S. Campise, Ospedale Umberto I, Siracusa; G. Masera and N. Masera, Ospedale Nuovo San Gerardo Università degli Studi di Milano, Monza; V. De Sanctis and M. R. Gamberini, Az. Osp. Universitaria Sant'Anna, Ferrara; P. Cianciulli and F. Sorrentino, Ospedale S. Eugenio, Rome; D. Gallisai, Istituto di Clinica Pediatrica Amerigo Filia, Sassari; G. Quarta, Ospedale A. Perrino—Az. USL BR1, Brindisi; A. Saviano, Azienda Ospedaliera di Rilievo Nazinale A. Cardarelli, Naples; S. Cossu, Ospedale Civile SS. Annunziata—A.U.S.L. N. 1 Sassari, Sassari; M. E. Lai, Ospedale Regionale Microcitemie—A.S.L.n. 8, Cagliari; A. Maggio, Azienda Ospedaliera V. Cervello, Palermo; R. Malizia, Azienda Ospedaliera Villa Sofia—CTO, Palermo; A. Piga, Ospedale Regina Margherita, Turin; G. L. Forni, Ospedale Galliera, Genoa; F. Locatelli, Policlinico San Matteo—IRCCS, Pavia; and C. Borgna-Pignatti, Az. Osp. Universitaria Sant'Anna—Università degli Studi, Ferrara.
Tunisia: M. Bejaoui, Centre National des Greffes de la Moelle Osseuse, Tunis; S. Fattoum, Hopital d'enfants, Tunis; and B. Meddeb, Hôpital Aziza Othmana, Tunis.
Turkey: L. Agaoglu and Z. Karakas, Istanbul University Istanbul Medical Faculty, Istanbul; Y. Aydinok, Ege University Medical Faculty, Izmir; Y. Kilinc and I. Sasmaz, Cukurova University Medical Faculty, Adana; D. Canatan, Suleyman Demirel University Medical Faculty, Isparta; and Z. Uysal and M. Ertem, Ankara University Medical Faculty, Ankara.
United Kingdom: J. Porter and P. Eleftheriou-Kokkinos, University College Hospital, London.
United States: P. Giardina, New York Presbyterian Hospital, New York, NY; A. Cohen, Children's Hospital of Philadelphia, Philadelphia, PA; T. Coates, Children's Hospital Los Angeles, Los Angeles, CA; A. Thompson, Children's Memorial Hospital, Chicago, IL; E. Vichinsky, Children's Hospital and Research Center at Oakland, Oakland, CA; E. Neufeld, Boston Children's Hospital, Boston, MA; and M. Jeng, Stanford Hospital, Stanford, CA.
Prepublished online as Blood First Edition Paper, December 15, 2005; DOI 10.1182/blood-2005-08-3430.
Supported in part by research funding from Novartis Pharma to Y.A., A.C., S.P., A.P., T.C., A.K., Y.K., G.J.-S., C.M., M.V., A.K.-S., M.D.C., P.G., R.G., G.D., J.P., I.T., C.V., and N.O.
Two authors (P.M. and D.A.) have declared a financial interest in a company whose product was studied in the present work. Several of the authors (H.O., C.R.-D., P.M., D.A.) are employed by a company (Novartis Pharma) whose product was studied in the present work.
M.D.C., A.C., A.P., M.B., S.P., L.A., Y.A., A.K., Y.K., J.P., M.C., R.G., S.F., G.D., C.M., M.V., M.A.-M., P.G., A.K.-S., G.J.-S., T.C., C.V., N.O., and I.T. served as investigators on this trial, enrolling patients. They also reviewed and contributed their comments on this manuscript. M.D.C., A.C., A.P., and A.K. also served as Study Monitoring Committee members overseeing the conduct of the trial. D.A. and H.O. coordinated the design and execution of the trial. P.M. contributed to the analysis of the trial data and drafted the manuscript. C.R.-D. served as the trial statistician.