Comment on Buffet et al, page 3745
Over the years, physiological studies have provided many insights into the function of the spleen, and now, in this issue of Blood, Buffet and colleagues describe significant new mechanistic insights into clearance of parasitized human red cells by the spleen.
In ancient times the spleen was an organ of mystery, held to be the seat of ill humors. Two millennia after Aristotle and Hippocrates, in 1885, a physiologic function was finally ascribed to the spleen: it was a filter, which eliminated unwanted particles from the blood.1
It has long been established that the spleen is a highly efficient device for sequestering abnormal red cells, such as hereditary spherocytes or elliptocytes. Splenectomy, in consequence, prolongs the life of such cells and is an indicated measure in the treatment of patients suffering from such conditions. In malarial infections, on the other hand, the spleen serves the useful function of ridding the bloodstream of parasitized cells,2 and if it is removed, parasitemia levels are apt to rise and the symptoms worsen.3 But to what extent and in what circumstances the spleen exercises its beneficial function have been far from clear. Our ignorance has been compounded by the profound differences in structure and physiology between the human spleen and that of laboratory animals, such as mice. An ingenious study with wide implications, reported in this issue of the journal, now sheds new light on the subject. Buffet and colleagues have devised an ex vivo experimental system using human spleens that allows them to follow the time course of clearance of parasite-infected red cells. The authors show that 95% of Plasmodium falciparum–infected cells, preexposed to the antimalarial drug artesunate, are cleared from the perfusate in 2 hours and that more than 90% of these cells are retained and processed in the red pulp of the spleen.
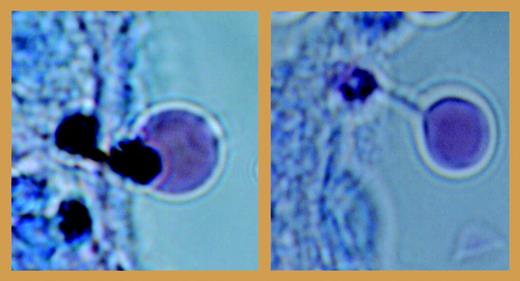
What are the implications of these findings? One is that the “pitting” action of the spleen in clearing the intracellular parasites from the infected red cells is now unequivocally established (see figure). But in the broader context, the new experimental system affords a general route to the study of physiologic and pathologic mechanisms of human spleen function in the remodeling of reticulocytes and in clearing types of abnormal red cells from the circulation. “You shall,” as Shakespeare has it, “digest the venom of your spleen” (Julius Caesar, act 4, scene 3, line 53). ▪FIG1
Pitting of dead parasite remnants from the infected red cells during passage from the splenic cord to sinus lumen. See the complete figure in the article beginning on page 3745.
Pitting of dead parasite remnants from the infected red cells during passage from the splenic cord to sinus lumen. See the complete figure in the article beginning on page 3745.