Popat and colleagues and Passamonti and colleagues provide additional evidence that the constitutive mobilization of CD34+ cells into the peripheral blood (PB) of patients with chronic myeloproliferative disorders (MPDs) is not merely due to the physical disruption of the bone marrow (BM) microenvironment by fibrosis, but rather is a consequence of the continuous biologic processes provided by the cellular progeny of the malignant clone.
Both chronic idiopathic myelofibrosis (CIMF) and myelofibrosis (MF) that occurs during the terminal phases of polycythemia vera (post-PV MF) are accompanied by constitutive mobilization of CD34+ hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) into the peripheral blood (PB).1 This mobilization of CD34+ cells and the resultant extramedullary hematopoiesis have previously been thought to be a consequence of a fibrous distortion of the BM microenvironment, leading to the forced egress of CD34+ cells from the BM. Recently, this dogma has been challenged by data that suggest that the abnormal trafficking of CD34+ cells in CIMF is due to the release of proteases by the cellular progeny of HSCs/HPCs. These proteases have been shown to lead to the disruption of adhesive interactions that normally result in the retention of HSCs/HPCs within the BM.1 Popat and colleagues show that patients with pulmonary arterial hypertension (PAH) and secondary BM fibrosis equivalent to a control population with CIMF do not have high levels of CD34+ cells in their PB. The authors conclude that BM fibrosis alone cannot account for the abnormal HSC/HPC trafficking observed in CIMF and post-PV MF.
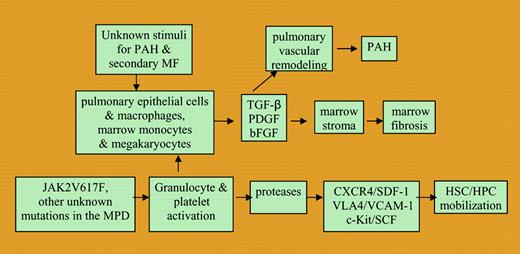
Chronic myeloproliferative disorders (MPDs) have previously been reported to be associated with an unusually high incidence of PAH.2 TGF-β, PDGF, and basic FGF have all been implicated in the development of not only PAH but also primary and secondary forms of MF.2,3 PAH occurring in patients with MPDs has been reported to improve with treatment of underlying chronic MPDs.2 Schermuly et al3 have recently reported that the PDGF receptor antagonist imatinib mesylate, which is routinely used effectively to treat chronic MPDs, reverses pulmonary vascular remodeling in 2 different animal models of PAH. A pilot trial evaluating the safety and efficacy of imatinib in patients with PAH is currently in progress.3 The clinical observations of the PAH's association with secondary BM fibrosis and with chronic MPDs suggest that there are common mediators of disease in these 2 diverse settings (see figure).FIG1
A proposed schema of common pathobiological events leading to bone marrow fibrosis and pulmonary vascular remodeling in such diseases as pulmonary arterial hypertension, secondary myelofibrosis, and chronic myeloproliferative disorders.
A proposed schema of common pathobiological events leading to bone marrow fibrosis and pulmonary vascular remodeling in such diseases as pulmonary arterial hypertension, secondary myelofibrosis, and chronic myeloproliferative disorders.
Passamonti and colleagues provide additional insight into the origins of abnormal CD34+ cell trafficking in chronic MPDs. They studied the relationship between the JAK2 (V617F) mutational status of patients with PV and the degree of CD34+ cell mobilization into the PB. The authors demonstrated that PV patients with fibrotic BMs and abnormal CD34+ cell trafficking had a higher percentage of mutant alleles than PV patients without BM fibrosis. Several groups (Popat et al, Passamonti et al, and Tefferi et al4 ) have now shown that most patients with post-PV MF are homozygous for the JAK2 (V617F) mutation, while the mutant allele percentage is less than 50% in many PV patients without BM fibrosis. Two of those groups, who studied PV patients sequentially during their clinical course, were able to show that patients had an increase in the mutant allele percentage over time (Passamonti et al and Tefferi et al4 ). Passamonti et al conclude that the significant mobilization of CD34+ cells into the PB of PV patients is a consequence of the dose of the mutant gene present in PV, with the transition from heterozygosity to homozygosity for JAK2 (V617F). The gene dosage of JAK2 (V617F) in PV or other genetic mutations yet to be defined in CIMF appears likely to generate signals that lead to the creation of a proteolytic environment and a cytokine milieu within the BM that results in abnormal CD34+ cell trafficking and BM fibrosis (see figure). Passamonti et al have reported that high doses of mutant JAK2 might lead to abnormal CD34+ cell trafficking by creating intracellular signals that result in activation of granulocytes. Such activation of granulocytes would be expected to result in the elaboration of proteases within the BM. Similarly, Falanga et al5,6 have suggested that activation of granulocytes and platelets is an important mediator of thrombosis, the most common cause of morbidity and mortality in chronic MPDs.
Further understanding of the link between the underlying genetic events that lead to chronic MPDs and the activation of platelets, granulocytes, and possibly endothelial cells is likely to lead to new insights into causes of abnormal CD34+ cellular trafficking as well as the increased thrombotic risk that characterizes these disorders (see figure). It is to be hoped that interruption of these chains of events by small molecule inhibitors will generate novel treatments for chronic MPDs, and that such treatments might also be applicable for the treatment of PAH. ▪