Treatment for chronic hepatitis C is based on combination therapy with pegylated interferon and ribavirin (RBV).1,2 One of the most important side effects of RBV is hemolytic anemia, which may require RBV dose reduction or discontinuation3 with a significant decrease in the rate of sustained virologic response (SVR).4 Little is known about the safety of RBV treatment in patients with concomitant glucose-6-phosphate dehydrogenase (G6PD) deficiency, who are inherently prone to hemolysis.5
We prospectively studied changes in hemoglobin (Hb) levels in 112 patients with chronic hepatitis C, associated or not with G6PD deficiency, during and after combination therapy. G6PD activity was tested by a spectrophotometric method.6,7 Twenty-six (23.2%) patients (6 women, 20 men) had G6PD deficiency; 4 (3.6%) women had partial G6PD deficiency. Peginterferon alfa-2a or alfa-2b were administered subcutaneously once weekly at 180 μg and 1.5 μg/kg, respectively; RBV was administered orally daily at 800 to 1200 mg according to body weight. Sixty-nine patients with genotype 1 or 4 were treated for 48 weeks and 43 patients with genotype 2 or 3 for 24 weeks. The RBV dose was reduced if Hb fell below 10 g/dL and discontinued with Hb values below 8.5 g/dL. The mean dose of RBV received by patients with and without G6PD deficiency was similar.
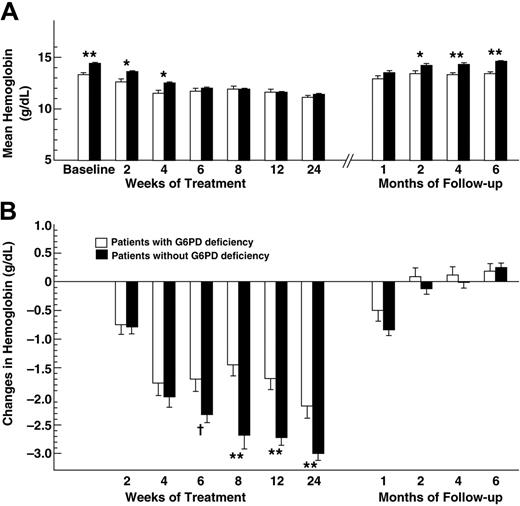
The baseline characteristics of the 2 groups of patients were similar, except for the mean Hb levels that were significantly lower (P < .001) in patients with G6PD deficiency (Table 1). This difference persisted at weeks 2 and 4 of therapy (Figure 1A). Subsequently, however, the Hb levels were no longer different between the 2 groups. One month after completion of therapy, Hb returned to baseline levels in patients with G6PD deficiency, while it was still significantly lower than baseline in those without G6PD deficiency (P < .001). The mean changes in Hb levels from baseline to week 2 or 4 of therapy were similar in patients with and without G6PD deficiency, then became significantly greater in patients without G6PD deficiency until week 24 of therapy (Figure 1B). None of the patients prematurely discontinued therapy because of anemia. Adjustments of the RBV dose were required in 11.5% and 9.8% of patients with and without G6PD deficiency, respectively (P = .724). Thus, the presence of G6PD deficiency was not associated with a significant risk of RBV discontinuation or dose adjustments. The rate of SVR, defined as undetectable hepatitis C virus (HCV) viremia 24 weeks after cessation of therapy, was 61% in patients with G6PD deficiency and 71% in those without G6PD deficiency (P = .336), indicating that G6PD deficiency was not associated with a significantly lower rate of SVR.
Although patients with G6PD deficiency had lower Hb levels at baseline, most likely due to chronic hemolysis, they failed to show a more profound Hb decrease during treatment. Moreover, they showed an earlier achievement of pretreatment levels after therapy, suggesting a better fitness of G6PD-deficient erytrhocytes under RBV stress. Our findings have important clinical implications, as they provide evidence that patients with chronic hepatitis C and G6PD deficiency can be successfully treated with peginterferon-alfa and RBV without an increased risk of RBV-induced anemia.
Hemoglobin values during and after treatment with pegylated interferon and ribavirin in patients with chronic hepatitis C with and without G6PD deficiency. (A) Mean absolute hemoglobin values (Hb). □, patients with G6PD deficiency; ▪, patients without G6PD deficiency. Mann-Whitney tests were performed. *P < .005 and **P < .001 for the comparison between patients with and without G6PD deficiency. (B) Mean changes in hemoglobin levels from baseline. Mann-Whitney tests were performed. †P = .026 and **P < .001 for the comparison between patients with and without G6PD deficiency. Data are expressed as means ± SE.
Hemoglobin values during and after treatment with pegylated interferon and ribavirin in patients with chronic hepatitis C with and without G6PD deficiency. (A) Mean absolute hemoglobin values (Hb). □, patients with G6PD deficiency; ▪, patients without G6PD deficiency. Mann-Whitney tests were performed. *P < .005 and **P < .001 for the comparison between patients with and without G6PD deficiency. (B) Mean changes in hemoglobin levels from baseline. Mann-Whitney tests were performed. †P = .026 and **P < .001 for the comparison between patients with and without G6PD deficiency. Data are expressed as means ± SE.