Hematopoietic stem cell (HSC) mobilization is still not well understood. Accumulating evidence suggests that protease activation is an important step: proteases release cytokines or chemokines from the extracellular matrix, thereby modulating biological responses.1,2 The urokinase-type plasminogen activator receptor (uPAR) is the receptor for urokinase-type plasminogen activator (uPA). The ligand uPA cleaves uPAR between domains I and II, liberating domain I—uPAR(I)—while uPAR(II-III) remains on the cell surface.3 Proteases cleave uPAR(I-III) and soluble urokinase plasminogen activator receptor (suPAR) (I-III) in vitro,3-5 unmasking a region with chemotactic properties.6 Recently, a role of suPAR for granulocyte colony-stimulating factor (G-CSF)–mediated HSC mobilization in allogeneic donors has been proposed in this journal.7 Results we obtained in autologous patients receiving chemotherapy prior to G-CSF, however, challenge the proposed role for suPAR in certain settings of G-CSF–mediated HSC mobilization.
Serum samples were collected at our center before G-CSF and/or prior to leukapheresis from healthy allogeneic donors and autologous patients, most of whom were suffering from lymphoid malignancies in remission of the disease. The G-CSF dose for donors was 2 × 5 or 2 × 8 μg/kg body weight per day (BW/d) subcutaneously. Leukapheresis was performed on day 5 of G-CSF. Autologous patients were mobilized with chemotherapy chosen for optimal stem cell mobilization and disease control. G-CSF (5 or 10 μg/kg BW/d subcutaneously) was started 6 days before scheduled leukapheresis.
Blinded serum samples were analyzed with time-resolved fluoroimmunoassays by measuring uPAR variants concentrations before and after uPAR depletion.8 Accuracy was determined by measuring recoveries of spiked standards in 20% (vol/vol) serum pool in buffer.
Progenitor cell mobilization was similar in autologous patients (mean CD34+ cell count on the day of leukapheresis, 84 128 ± 15 893/mL [SEM] blood; n = 66) and allogeneic donors (79 470 ± 10 710/mL; n = 21). However, leukocyte counts on the day of leukapheresis were inferior for autologous pts (20.89 ± 1.46/nL, n = 66, compared with 49.6 ± 2.5/nL in allogeneic donors; n = 28, P < .001, unpaired t test).
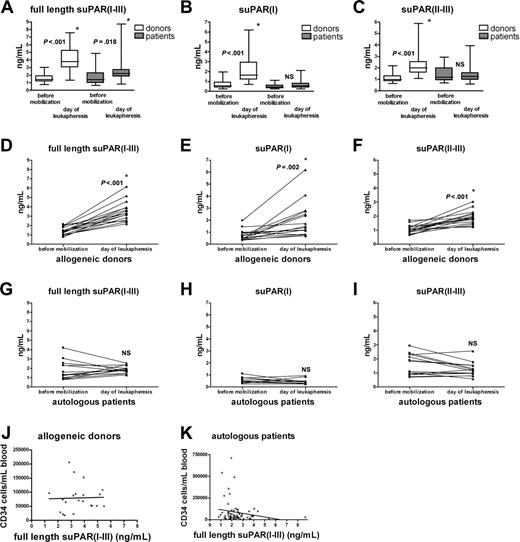
Increased suPAR serum levels for donors and patients on the day of leukapheresis after G-CSF administration. Serum samples of allogeneic donors and autologous patients were obtained before G-CSF administration (35 and 22, respectively) and on the day of leukapheresis (34 and 66 patients, respectively) (A-C); suPAR(I-III), suPAR(I), and suPAR(II-III) levels were measured. The boxes extend from the 25th to the 75th percentile with a line at the median. For the paired analysis serum levels of suPAR(I-III), suPAR(I), and suPAR(II-III) from individual allogeneic donors (n = 17) (D-F) and autologous patients (n = 14) (G-I) were plotted before mobilization and on the day of leukapheresis. Number of CD34+ progenitor cells as determined by flow cytometry plotted against full-length suPAR serum levels in allogeneic donors (n = 21; panel J, NS) and autologous patients (n = 66; panel K, NS). *Statistically significant increase (unpaired t test for panels A-C; paired t test for panels D-I). NS indicates not significant.
Increased suPAR serum levels for donors and patients on the day of leukapheresis after G-CSF administration. Serum samples of allogeneic donors and autologous patients were obtained before G-CSF administration (35 and 22, respectively) and on the day of leukapheresis (34 and 66 patients, respectively) (A-C); suPAR(I-III), suPAR(I), and suPAR(II-III) levels were measured. The boxes extend from the 25th to the 75th percentile with a line at the median. For the paired analysis serum levels of suPAR(I-III), suPAR(I), and suPAR(II-III) from individual allogeneic donors (n = 17) (D-F) and autologous patients (n = 14) (G-I) were plotted before mobilization and on the day of leukapheresis. Number of CD34+ progenitor cells as determined by flow cytometry plotted against full-length suPAR serum levels in allogeneic donors (n = 21; panel J, NS) and autologous patients (n = 66; panel K, NS). *Statistically significant increase (unpaired t test for panels A-C; paired t test for panels D-I). NS indicates not significant.
Before G-CSF administration, suPAR(I-III), suPAR(I), or suPAR(II-III) levels were identical in both groups (Figure 1A-C). Serum levels for full-length suPAR(I-III) significantly increased in the pooled allogeneic and autologous group after G-CSF administration (Figure 1A), but mean suPAR(I-III) levels were lower in autologous patients than in allogeneic donors. An increase in suPAR(II-III) and suPAR(I) levels was found in serum from allogeneic but not from autologous patients after G-CSF (Figure 1B-C). In paired samples, G-CSF significantly augmented serum levels of the different suPAR forms in allogeneic donors, confirming recently published results (Figure 1D-F).7 In contrast, however, in serum from the autologous group, neither full-length suPAR(I-III) and suPAR(II-III) levels, nor suPAR(I) levels, increased after chemotherapy plus G-CSF treatment (Figure 1G-I). Most importantly, serum levels for full-length (Figure 1J-K) or cleaved suPAR (data not shown) after G-CSF administration did not correlate with CD34+ counts in both groups, but did correlate with leukocyte counts (data not shown).
On the basis of our data, we propose that the leukocyte increase after G-CSF is responsible for higher full-length suPAR serum levels, possibly through G-CSF–mediated induction of proteases in neutrophils.8 A successful progenitor cell mobilization after G-CSF treatment, as observed in the patient group receiving chemotherapy, does not necessarily correlate with increased suPAR serum levels.
Supported by grants from the Leukemia & Lymphoma Foundation (B.H.) and Grants-in-Aid from the Ministry of Education, Science, Technology, Sports, and Culture, Japan (K.H.). We thank Jesper Pass and Gunilla Høyer-Hansen (grant support, European Union Framework Program 6 project LSHC-CT-2003-503297) for their continuous support, for providing vital analytical tools, and for performing the suPAR analysis, as well as the Finsen laboratory, Copenhagen, Denmark (laboratory head, Keld Dano).
T.F. designed and performed research, analyzed data, and wrote the letter; K.H. designed research; E.T. designed research and reviewed the letter; and B.H. designed research, analyzed data, and wrote the letter.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal