Abstract
The Ets transcription factors regulate a wide variety of biologic processes. Several members have been shown to play a role in regulating angiogenesis and vascular development. For example, the Ets factor ELF-1 is enriched in the developing vasculature of the embryo, where it regulates the expression of the Tie2 gene. We have determined that ELF-1 and Tie2 expression is also enriched in tumor blood vessels, and have identified a short peptide, 34 amino acids in length, corresponding to the terminal portion of the highly conserved ETS domain that potently blocks the function of ELF-1. A tailored ELF-1 blocking peptide, containing a 12–amino acid HIV-1 TAT protein, readily crosses the cell membrane and enters into the nucleus of endothelial cells, leading to a marked reduction in the expression of ELF-1 gene targets including Tie2 and endothelial nitric oxide synthase. Furthermore, the ELF-1 blocking peptide potently inhibits angiopoietin-1–mediated endothelial cell migration. Systemic administration of this peptide markedly attenuates B16 melanoma tumor growth and tumor-associated angiogenesis in nude mice. These results support the function of ELF-1 in the regulation of Tie2 gene expression during the development of tumor angiogenesis.
Introduction
The Tie receptors are vascular endothelial–specific receptor tyrosine kinases that are required for normal vascular development. Targeted disruption of both of the Tie receptors in mice results in severe vascular defects and death by embryonic day 10.5.1,2 The major function of the Tie receptors is to promote the later stages of blood vessel development. Whereas the natural ligand for the Tie2 receptor is angiopoietin-1 (Ang-1), the ligand for the Tie1 receptor remains unknown. In addition to their role during vascular development, the Tie receptors have also been shown to be up-regulated in angiogenic blood vessels associated with tumors or inflammation.3-5 Ang-1 has been shown to be up-regulated at sites of angiogenesis associated with inflammation and may function to promote the migration of Tie2-positive endothelial cells.6,7
We recently identified a role for selected members of the ETS transcription factor family in the regulation of the Tie2 gene.8-10 There are several conserved ETS binding sites in the promoters of both of these genes. Among the ETS family members, ELF-1 regulates the Tie2 gene in large and small blood vessels during chicken vascular development.10 The regulatory elements for both of these genes have been shown to be sufficient to direct the expression of marker genes such as LacZ in an endothelial-specific fashion. Mutations in the conserved ETS binding sites lead to marked reductions in the vascular-specific expression of these genes in transgenic animals.
The overall goal of this study was to evaluate the role of ELF-1 in the regulation of endothelial function and angiogenesis, and to determine the effect of selectively blocking ELF-1 using tailored membrane-permeable peptides. The results of our study demonstrate that synthetic peptides can be engineered to block DNA binding and transactivation of selected ETS factors via the addition of a protein-transducing domain (PTD) that facilitates entry across the cell membrane of endothelial cells, leading to inhibition of angiogenesis and certain aspects of endothelial function. Furthermore, by inhibiting angiogenesis in vivo, these peptides inhibit melanoma tumor growth.
Materials and methods
Tissue culture and reagents
The cell line HEK293 (human embryonic kidney) was grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum. Primary human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics (San Diego, CA) and grown as recommended by the manufacturer. Angiopoietin-1 and basic fibroblast growth factor were obtained from R&D Systems (Minneapolis, MN).
DNA transfection assays and electrophoretic mobility shift assay
Cotransfections of 1.5 × 105 HEK293 cells were carried out as previously described.8 DNA gel mobility shift assays were performed as previously described.8 Oligonucleotides used as probes are as follows: human Tie2 promoter, 5′-GTTAAGTTCCTTTTTCCTGTTTCCTTTGCA-3′ and 3′-TGCAAAGGAAACAGGAAAAAGGAACTTAAC-5′. The Ets-1/Ets-2 consensus binding oligonucleotide sequence was Ets-1 consensus oligo sequence: 5′-CGGCCAACCGGAAGCATGTGC-3′.
Peptide synthesis and intracellular localization
All peptides were synthesized at the Tufts University Core Facility (Boston, MA). The HIV-1 TAT peptide (TyrGlyArgLysLysArgArgGlnArgArgArgGly) was added to the carboxyl terminus to facilitate intracellular delivery. The amino terminus was biotinylated. Synthetic peptides were added to HUVECs at a concentration of 2.5 μM. Cells were fixed at different time points with 4% paraformaldehyde and treated with 95% ethanol for 5 minutes to permeabilize the cell membrane. Detection of the biotinylated peptides by immunofluorescence was performed using streptavidin-594 (Molecular Probes, Eugene, OR). Nuclear staining was performed using DAPI (Molecular Probes).
Microscopy and digital images
Slides were examined and photographed with transmitted light or fluorescence using a Nikon Eclipse E800 microscope (Nikon, Tokyo, Japan) equipped with a 40 ×/0.75 numeric aperture objective lens as well as a Zeiss AxioCam digital camera (Carl Zeiss International, Heidelberg, Germany). Images were analyzed using Openlab imaging software version 3.0.4 (Improvision, Lexington, MA). Gross images of the Matrigel plugs were photographed using a Zeiss Stemi SV6 Stereo Zoom microscope equipped with a 0.63 × objective lens.
Cell proliferation
B16 melanoma tumor cells were grown in 10% FBS, DMEM. Proliferation assays were performed by placing 10 000 cells into 24-well dishes, and counting the number of cells 24 and 48 hours later in the presence or absence of peptide A1, or the mutant peptide A1. Cells were trypsinized at different time points and the total number of cells was counted using a Coulter counter (Birmingham, United Kingdom). All experiments were performed in triplicate.
Tube formation assays on Matrigel
Matrigel (200 μL; Becton Dickinson, San Jose, CA) supplemented with 20 ng/mL bFGF (R & D Systems) was added to each well of a 24-well tissue-culture plate and allowed to solidify at 37°C for at least 30 minutes. The cultures were incubated at 37°C, 5% CO2, and observed at 8 and 24 hours for rearrangement of cells into capillary-like structures. Individual experiments were performed in triplicate and representative wells recorded by photomicrography. Quantitative analysis of tube formation in Matrigel plugs was measured by counting the cycles among tubes manually.
Cell migration assays
Cell migration in response to Ang-1 was performed as previously described, with slight modifications.11 The lower surface of a Transwell filter (3-μm pores; BD Bioscience, San Jose, CA) was coated with fibronectin (10 μg/mL). HUVECs were incubated overnight in serum-free media. Transwell plates (Corning Laboratories, Corning, NY) were blocked with 1% BSA/PBS for 2 hours. HUVECs (8 × 104) were first added to the upper chamber; then peptide A1 and mutant peptide A1 were added to the upper chamber. The concentration of Ang-1 in the lower chamber was 100 ng/mL. After 22 hours at 37°C, transwell filters are fixed in 2% paraformaldehyde and stained with hematoxylin. Cells migrating to the lower side of the filter were counted in 4 random high-power fields (× 100).
In vivo angiogenesis assays
Matrigel (Becton Dickinson) containing 80 ng/mL bFGF was mixed with medium alone, peptide A1, or mutant peptide A1 at final concentrations of 5 to 10 μM, and was injected subcutaneously in the abdominal region of C57BL/6 mice (n = 5 per group). After 7 days, the animals were killed and the Matrigel plugs were harvested, fixed in 4% neutral buffered paraformaldehyde solution (Sigma Chemical, St Louis, MO), and then embedded in paraffin. All tissues were sectioned (5-μm thickness) and mounted onto slides for further staining. Histologic sections from Matrigel plugs were stained with hematoxylin and eosin. Immunostaining was performed using a CD31 antibody. Quantitative analysis of angiogenesis in Matrigel plugs used a computerized semiautomated digital analyzer (Image-Pro Plus, Media Cybernetics, Silver Spring, MD). For each plug, 15 separate fields were evaluated.
Real-time RT-PCR analysis
Synthesis of complementary DNA (cDNA) was performed using 1 μg total RNA from HUVECs. We did real-time reverse-transcription–polymerase chain reaction (RT-PCR) using specific primers on an Opticon Monitor apparatus (MJ Research, Bio-Rad Laboratories, Hercules, CA). Housekeeping GAPDH transcript was used to normalize the amount and quality of RNA. Subsequent amplifications of the partial cDNA encoding Tie2, Tie1, eNOS, Flk1, Flt-1, and ELF-1 were performed as templates with specific oligonucleotide primers as follows: Tie2: sense, 5′-CCAGTATTTAGGACGCGGTCC-3′ and antisense, 5′-AAGTTCTTCAAGTAGGCCTGCG-3′; eNOS: sense, 5′-CAGTGTCCAACATGCTGCTGGAAATTG-3′ and antisense, 5′-TAAAGGTCTTCTTCCTGGTGATGC-3′; Flk1: sense, 5′-CATGTACGGTCTATGCCATTCCT-3′ and antisense, 5′-GCTCGTTGGCGCACTCTT-3′; ELF-1: sense, 5′-CCAGTATTTAGGACGCGGTCC-3′ and antisense, 5′-AAGTTCTTCAAGTAGGCCTGCG-3′; Flt-1: sense, 5′-TTAACGAGTAGCCACGAGTCAAAT-3′ and antisense, 5′-CCCTCGCCGGAAGTTGTA-3′.
Western blot analysis
HUVECs were lysed, and Western blot analysis was performed using polyclonal antibodies to Tie2, eNOS, and Flk1 (Santa Cruz Biotechnology, Santa Cruz, CA), followed by rabbit antibody to horseradish peroxidase (Santa Cruz Biotechnology), as previously described.12 β-Actin was used as a control.
Mouse tumor models
C57BL/6 nu/nu mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). For the mouse tumor model, 5 × 106 B16 melanoma cells in PBS were injected subcutaneously on the back. At the same time, an Alzet minipump (Duret, Cupertino, CA) was embedded subcutaneously in the back. Female nu/nu mice were divided into 3 treatment groups (n = 6 per group), in which the minipumps were filled with saline, peptide A1, or mutant peptide A1, at a final concentration of 10 mg/kg per day. Tumor size was assessed every other day using calipers. Tumor masses were surgically removed after 14 days. Tumor volumes were calculated by the formula width2 × length × 0.52, as previously described.13 Statistical analysis of the tumor volume was performed using a Student 2-sided t test (Excel software, Placitas, NM). Tumor weight was measured in grams. Tumors were fixed with 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with either hematoxylin or eosin. Tumors were stained for CD31 and Tie2 using a monoclonal antibody to mouse CD31 and Tie2 (PharMingen, San Diego, CA). ELF-1 antibody was from Santa Cruz Biotechnology. Specific binding of the primary antibody was visualized with Alexa Fluor-488–labeled anti–rat IgG antibody (Molecular Probes). After CD31 staining, vessel density and vessel area were determined in 6 high-powered fields for each tumor using Image-Pro Plus software.
Serum measurement of peptide levels and liver function tests
After implantation of the Alzet minipumps containing the peptides, blood samples were obtained via the tail vein on days 1 and 3, and at the time the mice were killed at day 10. Serum biotinylated peptide A1 was measured by an enzyme-linked immunosorbent assay (ELISA)–based assay. To generate a standard concentration curve, serum containing different concentrations of peptide A1 was added to 96-well streptavidin-coated plate (Pierce Biotechnology, Rockford, IL) and incubated at room temperature for 1 hour, followed by incubation with goat antibiotin antibody (1:1000; Cell Signaling Technology, Beverly, MA) and horseradish peroxidase–conjugated donkey anti–goat IgG (1:1000; Santa Cruz Biotechnology). Plates were read after development with HRP substrate TMB(3,3′,5,5′-tetramentylbenzidine) (Pierce Biotechnology) at 450 nm. Serum ALT(GPT) on day 10 was measured with Infinity ALT/GPT diagnostic kit (Thermo Electron, Waltham, MA). Units per liter (U/L) of ALT/GPT activity is defined as the amount of enzyme that oxidases 1 μM NADH per minute according to the manufacturer's instruction (normal range, 38-66 U/L).14
Statistical analysis
Comparisons between groups were analyzed by one-way ANOVA or 2-sided t tests for experiments with more than 2 subgroups. (Excel software; Microsoft, Redmond, WA). Results are presented as means ± standard error.
Results
ELF-1 is expressed during vascular development and in tumor blood vessels
We have recently identified ELF-1 as a transcriptional mediator of the Tie2 gene during vascular development in the chicken.10 We similarly observed increased expression of Tie2 and ELF-1 in the developing dorsal aorta of the mouse (Figure 1). Tie2 expression is also enriched in the vasculature of a number of tumors.4,15 We observed strong Tie2 expression in blood vessels within B16 melanoma tumors. Furthermore, ELF-1 was highly expressed within these vessels and colocalized to sites of tumor angiogenesis. Expression of the related Ets factor ELF-2 was also evaluated by immunohistochemistry and was not detected in these tumors or angiogenic vessels (data not shown). We therefore postulated that ELF-1 might serve as a therapeutic target to inhibit tumor growth by inhibiting Tie2 expression and thereby blocking tumor angiogenesis.
Design of synthetic peptides and functional blocking studies
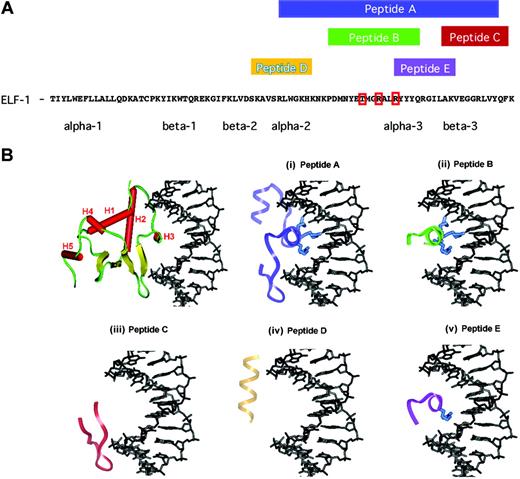
It has previously been shown that truncated forms of the ETS-1 transcription factor, encoding the ETS DNA binding domain, can function as potent inhibitors of the DNA binding and transactivation.16 Analysis of the crystal structure of certain ETS family members has resulted in the identification of selected conserved amino acids within the terminal portion of the DNA binding domain that mediate the interaction of the ETS domain with DNA.17 To further evaluate whether shorter fragments of the ETS domain that include critical, previously established DNA contact residues that are localized to the C-terminus of this domain could also abrogate DNA binding and transactivation, we chemically synthesized several peptides. These peptides are 10 to 34 amino acids in length and correspond to sequentially juxtaposed regions within the ETS domain of the Ets factor ELF-1 (Figure 2A). As is illustrated using the crystal structure of the Ets-1 DNA binding domain in complex with DNA as a model of the interaction of the ETS domain with DNA, these peptides correspond to very unique 3-dimensional structural elements (Figure 2B).18 Figure 2B (top left) illustrates the orientation of the ETS domain of Ets-1 in complex with the DNA duplex. This structure is essentially identical to that observed in other ETS domain crystal structures with 3 primary α-helices (H1, H2, and H3), 2 short α-helices (H4 and H5) separated by a turn, and a 4-stranded antiparallel β sheet illustrated in yellow.17-20
Expression of ELF-1 during vascular development and tumor angiogenesis. (A) Immunofluorescence staining in the developing dorsal aorta and B16 melanoma tumors for Tie2 (green) and ELF-1 (red), and colocalization (yellow) with DAPI staining (blue) for detection of cell nuclei. (B) Immunostaining for ELF-1 within endothelial cells of a Matrigel plug, supplemented with bFGF (80 ng/mL), injected subcutaneously into C57BL/6 nu/nu mice with an isotype-matched control.
Expression of ELF-1 during vascular development and tumor angiogenesis. (A) Immunofluorescence staining in the developing dorsal aorta and B16 melanoma tumors for Tie2 (green) and ELF-1 (red), and colocalization (yellow) with DAPI staining (blue) for detection of cell nuclei. (B) Immunostaining for ELF-1 within endothelial cells of a Matrigel plug, supplemented with bFGF (80 ng/mL), injected subcutaneously into C57BL/6 nu/nu mice with an isotype-matched control.
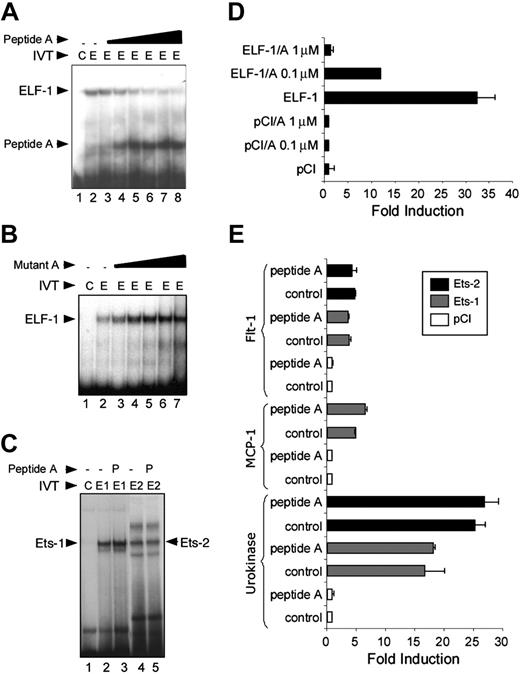
We evaluated the ability of synthetic peptides composed of various ETS sequences to compete with and/or block DNA binding in gel mobility shift assays. None of the peptides B through E, which are all shorter than 20 amino acids in length, could prevent ELF-1 from binding their requisite DNA as determined using gel mobility shift assays (data not shown). However, peptide A, which is 34 amino acids in length and includes the recognition helix (H3), a portion of H2, and the first 2 strands of the β sheet, was able to block ELF-1 binding to the ETS binding sites in the Tie2 promoter in a dose-dependent manner (Figure 3A).21 To verify the specificity of this interaction, we synthesized a peptide variant in which 3 amino acids (rendered and illustrated in blue, Figure 1B) that are required for mediating the major groove interaction of the ETS domain with its DNA were mutated (Figure 1A). Mutation of these amino acids to glycine resulted in a mutant variant of peptide A that was unable to prevent DNA binding to ELF-1 (Figure 3B). We also evaluated the ability of peptide A to interfere with both Ets-1 and Ets-2 binding to DNA. Peptide A had no effect on Ets-1 or Ets-2 binding in gel mobility shift assays at the same concentration of the peptide (1.2 μM) that potently blocks the binding of ELF-1 to DNA (Figure 3C).
We next tested the ability of peptide A to inhibit transactivation. Peptide A potently blocks transactivation of the Tie2 promoter by ELF-1 in a dose-dependent manner with nearly complete inhibition of transactivation at a concentration of 1 μM (Figure 3D). We have previously shown the specificity of different Ets transcription factors in selectively regulating different promoters. For example, Ets-1 and Ets-2 transactivate the promoter of the Flt-1 gene but not the Tie2 gene. Of interest, there was no effect of peptide A on the transactivation of the Flt-1 promoter by Ets-1 or Ets-2 with similar concentrations of the peptide (Figure 3E). Finally, we tested the ability of peptide A to inhibit transactivation of 2 other promoters, MCP-1 and urokinase, that we have previously shown are targets of Ets-1 and Ets-2.8,22 These results provide additional support of the specificity of peptide A with regard to its ability to block only the function of ELF-1.
Sequence and structure of ELF-1 dominant-negative peptides. (A) Protein sequence of the Ets domain of ELF-1. Red boxes demonstrate amino acids altered in mutant peptide to block binding. Amino acid sequences spanned by peptides A through E are highlighted above the sequence and alpha and beta helical structural components of the DNA binding domain are denoted below the sequence. (B) Structural model illustrating the critical secondary structural elements of ETS domains, using the crystal structure of the Ets-1 DNA binding domain in complex with duplex DNA, 5′-GGAA-3′ solved to a resolution of 0.24 nm (2.4 Å). The 5 α-helices are colored red and labeled H1 to H5, the turns and loops of the ETS domain are green, the antiparallel β sheet is colored yellow, and the DNA is rendered in black using InsightII (Accelrys, San Diego, CA). (i) Peptide A is 34 amino acids in length and is composed of the H3 recognition helix, which possesses 3 amino acids that contact the core of the DNA duplex with a set of highly conserved base contacts illustrated in blue. (ii) Peptide B includes most of the recognition helix and encompasses these 3 critical residues but does not include the remainder of the canonical ETS anchor that is composed of several loops or “wings” that are responsible for minor groove DNA contacts. (iii-v) Peptides C through E are composed of other ETS structural elements but lack the H3 recognition helix and/or the 3 critical residues that are responsible for anchoring the ETS domain within the major groove.
Sequence and structure of ELF-1 dominant-negative peptides. (A) Protein sequence of the Ets domain of ELF-1. Red boxes demonstrate amino acids altered in mutant peptide to block binding. Amino acid sequences spanned by peptides A through E are highlighted above the sequence and alpha and beta helical structural components of the DNA binding domain are denoted below the sequence. (B) Structural model illustrating the critical secondary structural elements of ETS domains, using the crystal structure of the Ets-1 DNA binding domain in complex with duplex DNA, 5′-GGAA-3′ solved to a resolution of 0.24 nm (2.4 Å). The 5 α-helices are colored red and labeled H1 to H5, the turns and loops of the ETS domain are green, the antiparallel β sheet is colored yellow, and the DNA is rendered in black using InsightII (Accelrys, San Diego, CA). (i) Peptide A is 34 amino acids in length and is composed of the H3 recognition helix, which possesses 3 amino acids that contact the core of the DNA duplex with a set of highly conserved base contacts illustrated in blue. (ii) Peptide B includes most of the recognition helix and encompasses these 3 critical residues but does not include the remainder of the canonical ETS anchor that is composed of several loops or “wings” that are responsible for minor groove DNA contacts. (iii-v) Peptides C through E are composed of other ETS structural elements but lack the H3 recognition helix and/or the 3 critical residues that are responsible for anchoring the ETS domain within the major groove.
Evaluating the effect of dominant-negative peptides on endothelial function
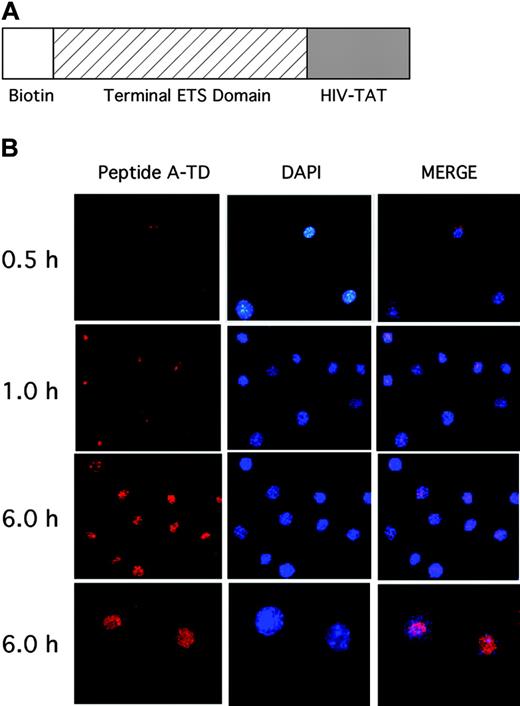
To evaluate the ability of these peptides to alter gene expression of ELF-1 gene targets and to examine the effect of selectively blocking these ETS proteins in endothelial cells, we synthesized a peptide containing peptide A in tandem with a 12–amino acid HIV-TAT protein-transducing membrane peptide (peptide A1) that has previously been shown to facilitate the transfer of peptides across the cell membrane and into the nucleus (Figure 4A).23 The synthetic peptides were also biotinylated at the amino terminus to facilitate tracking in cells. To further define the biologic effects of blocking selective ETS factors, we first wanted to evaluate the ability of this peptide to cross the cell membrane and enter into the nucleus (Figure 4B). The dominant-negative peptide was added to HUVECs at a concentration of 2.5 μM. Cells were stained with DAPI and fixed at different time points. Immunohistochemical analysis was performed to detect the biotinylated peptides. As is shown in Figure 4B, the peptides begin to penetrate the cell and enter the nucleus within 30 minutes. There is continued rapid uptake of the peptide by the cells and into the nuclei by 6 hours. These results suggest that the HIV-TAT–like protein-transducing domain (PTD) greatly facilitates uptake of this peptide into endothelial cells.
Effect of Elf-1 dominant-negative peptides on the expression of endothelial-specific genes
To evaluate the effect of the ELF-1 inhibitory peptides upon endothelial gene expression, we examined the expression of several endothelial-specific genes by quantitative RT-PCR before and after administration of peptide A1 at increasing concentrations, and in comparison with the mutant peptide. In addition to Tie2, 2 other known targets of ELF-1 are Tie1 and endothelial nitric oxide synthase (eNOS).9,24 We observed significant reductions in Tie1, Tie2, and eNOS, whereas no change was observed in the expression of the VEGF receptors Flt-1 or Flk-1 (Figure 5A). Furthermore, the mutant peptide had no effect on the expression of these genes. A similar reduction in Tie2 and eNOS expression was observed at the level of protein, with increasing concentrations of peptide A1, whereas there was no change in the expression of Flk-1 (Figure 5B). The mutant peptide had no effect on the expression of any of the genes.
ELF-1 dominant-negative peptides block endothelial migration and tube formation
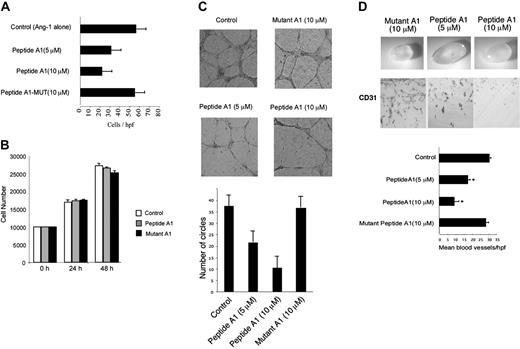
We next evaluated the biologic effect of adding the peptide to the cells by examining several aspects of endothelial function. Because one of the main targets of ELF-1 is the Tie2 gene, we predicted that the blocking peptides would reduce Ang-1–mediated endothelial cell migration.8,9 As is shown in Figure 6A, the addition of increasing concentrations of peptide A1 (5-10 μm) led to a marked reduction in cell migration of HUVECs in a dose-dependent manner. However, no reduction was observed with the mutant peptide. Of interest, neither the wild-type peptide A1 nor mutant A1 had any effect on B16 melanoma tumor cell growth in culture at a peptide concentration of 10 μm.
To further define the therapeutic potential of these peptides with regard to blocking angiogenesis, we tested their ability to inhibit tube cell formation of endothelial cells plated on Matrigel. Endothelial tube–like structures develop within a few hours. Over a 24-hour period, the vessel-like structures contain larger number of cells with a greater distance in length of the individual tubes, between branch points. Peptide A1 markedly reduced the formation of endothelial tube–like structures on Matrigel (Figure 6C). This effect was evident as early as 8 hours (data not shown) and at later (24 hours) time points. In contrast, the mutant peptide A1 had no effect at maximal concentrations.
Effect of dominant-negative ETS domain peptide A on DNA binding and transactivation. (A) Gel mobility shift assay, demonstrating binding of in vitro–translated (IVT) ELF-1 (arrow) to oligonucleotide probe encoding the conserved ETS binding site in the Tie2 promoter (lane 2), compared with control extract (lane 1). The effect of increasing concentrations of peptide A (0.1, 0.5, 0.8, 1.0, 1.1, and 1.2 μm) upon the binding of ELF-1 to the DNA are shown (lanes 3-8). The binding of peptide A to the oligonucleotide probe is shown (arrow). (B) Competition of binding of ELF-1 to oligonucleotide probe with increasing concentrations of a mutant peptide. (C) Competition of binding of Ets-1 and Ets-2 to an Ets-1/Ets-2 consensus oligonucleotide probe with and without 1.2 μm peptide A1 (P), using in vitro–translated Ets-1 (E1), Ets-2 (E2), versus control (C) extracts. (D) Transactivation of the Tie2 promoter by ELF-1. Addition of 0.1- and 1-μM concentrations of peptide A leads to marked inhibition of transactivation. (E) Transactivation of the Flt-1, MCP-1, and urokinase promoters by ETS factors Ets-1 and Ets-2, or the empty mammalian expression plasmid (pCI; Promega, Madison, WI) in the presence or absence of 1 μM peptide A. Results represent mean ± SD. All experiments were performed 3 times in duplicate.
Effect of dominant-negative ETS domain peptide A on DNA binding and transactivation. (A) Gel mobility shift assay, demonstrating binding of in vitro–translated (IVT) ELF-1 (arrow) to oligonucleotide probe encoding the conserved ETS binding site in the Tie2 promoter (lane 2), compared with control extract (lane 1). The effect of increasing concentrations of peptide A (0.1, 0.5, 0.8, 1.0, 1.1, and 1.2 μm) upon the binding of ELF-1 to the DNA are shown (lanes 3-8). The binding of peptide A to the oligonucleotide probe is shown (arrow). (B) Competition of binding of ELF-1 to oligonucleotide probe with increasing concentrations of a mutant peptide. (C) Competition of binding of Ets-1 and Ets-2 to an Ets-1/Ets-2 consensus oligonucleotide probe with and without 1.2 μm peptide A1 (P), using in vitro–translated Ets-1 (E1), Ets-2 (E2), versus control (C) extracts. (D) Transactivation of the Tie2 promoter by ELF-1. Addition of 0.1- and 1-μM concentrations of peptide A leads to marked inhibition of transactivation. (E) Transactivation of the Flt-1, MCP-1, and urokinase promoters by ETS factors Ets-1 and Ets-2, or the empty mammalian expression plasmid (pCI; Promega, Madison, WI) in the presence or absence of 1 μM peptide A. Results represent mean ± SD. All experiments were performed 3 times in duplicate.
In addition to evaluating the effects of the blocking peptides on in vitro tube formation, we were also interested in determining whether the peptides might also block the development of angiogenesis in vivo. We therefore used the Matrigel plug assay of angiogenesis, in which liquid Matrigel is injected subcutaneously into mice. After several days, angiogenic vessels can be observed migrating into the gel. Prior to injection, we mixed the Matrigel with similar concentrations of wild-type and mutant peptide A1. The Matrigel plugs were harvested after 1 week, fixed, and embedded in paraffin. Capillary density was measured by immunostaining with an antibody against the endothelial-specific marker CD31 (Figure 6C). Incorporation of the peptides into the Matrigel resulted in marked inhibition of angiogenesis with a 30% to 50% reduction in capillary density compared with that observed in the control experiments (P < .05). No significant change was observed with the mutant peptides.
Dominant-negative ELF-1 peptides block B16 melanoma growth and tumor angiogenesis
To extend these studies we tested the ability of the peptides to inhibit the tumor growth of B16 melanoma cells. The melanoma cells were injected subcutaneously and measurements of the size of the tumor were made every other day. Osmotic minipumps were embedded in the mice at the time of tumor implantation containing PBS, PBS and the A1 peptide, or PBS and the mutant A1 peptide. Steady-state serum levels of the peptide were approximately 12 to 17 ng/mL, and the peptide administration was not associated with abnormalities in liver function (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). The tumors were excised after 14 days. Exponential growth was observed for the control tumors or those treated with the mutant peptide, whereas treatment with peptide A1 led to a marked reduction in tumor growth (Figure 7A). The average volume (mm3) of the tumors was 3951 ± 146, with 3711 ± 345 for the mutant peptide A1–treated mice and 592 ± 63 for the peptide A1–treated mice (P < .001 compared with control mice). This was associated with marked reductions in blood vessel density as measured by CD31 staining (Figure 7B).
Localization of dominant-negative ETS protein in human endothelial cells. (A) Structure of the dominant-negative peptide A1 used to block ELF-1 function, including a biotinylated amino terminus, the terminal portion of the ETS domain corresponding to ETS peptide A, and a 12–amino acid HIV-TAT protein membrane–transducing domain (see “Materials and methods” for the sequence). (B) Time-dependent entry of the dominant-negative ETS peptide into the nucleus of HUVECs at different time points (0.5, 1, and 6 hours) is shown. Cells were fixed at various time points and immunostaining was performed to detect the biotinylated peptide. Nuclear localization was performed using the DAPI stain, and evidence of colocalization was demonstrated by merging the 2 images. Images were taken at × 20 magnification for 0.5-, 1-, and 6-hour time points, and also at × 40 for the 6-hour time point (bottom panel).
Localization of dominant-negative ETS protein in human endothelial cells. (A) Structure of the dominant-negative peptide A1 used to block ELF-1 function, including a biotinylated amino terminus, the terminal portion of the ETS domain corresponding to ETS peptide A, and a 12–amino acid HIV-TAT protein membrane–transducing domain (see “Materials and methods” for the sequence). (B) Time-dependent entry of the dominant-negative ETS peptide into the nucleus of HUVECs at different time points (0.5, 1, and 6 hours) is shown. Cells were fixed at various time points and immunostaining was performed to detect the biotinylated peptide. Nuclear localization was performed using the DAPI stain, and evidence of colocalization was demonstrated by merging the 2 images. Images were taken at × 20 magnification for 0.5-, 1-, and 6-hour time points, and also at × 40 for the 6-hour time point (bottom panel).
Effect of dominant-negative ELF-1 peptide on endothelial gene expression. (A) Quantitative RT-PCR of Tie2, Tie1, eNOS, Flk1, and Flt1 at baseline in HUVECs, and after administration of peptide A1 and mutant peptide A1. Experiments were performed in triplicate and represent means ± SE. (B) Western blot analysis of Tie2, eNOS, and Flk1 expression in HUVECs at baseline (control), at different concentrations (μM) of the mutant peptide A1 (Mut A1) and peptide A1.
Effect of dominant-negative ELF-1 peptide on endothelial gene expression. (A) Quantitative RT-PCR of Tie2, Tie1, eNOS, Flk1, and Flt1 at baseline in HUVECs, and after administration of peptide A1 and mutant peptide A1. Experiments were performed in triplicate and represent means ± SE. (B) Western blot analysis of Tie2, eNOS, and Flk1 expression in HUVECs at baseline (control), at different concentrations (μM) of the mutant peptide A1 (Mut A1) and peptide A1.
Effect of dominant-negative ELF-1 peptide on endothelial migration, tube formation, and in vivo angiogenesis. (A) Effect of peptide A1 on Ang-1–dependent endothelial migration compared with the mutant peptide (Mutant A1). The concentration of Ang-1 was 100 ng/mL (*P < .05 compared with control). (B) Effect of peptide A1 (10 μM) or mutant A1 (10 μM) on cell growth. (C) Endothelial tube formation on Matrigel supplemented with bFGF (20 ng/mL) at 8 hours and 24 hours in the presence of the mutant peptide A1 (10 μm) or peptide A1 (5, 10 μm) compared with no peptide (control). Quantitation of endothelial tube formation at 24 hours (*P < .05 compared with control). (D) Effect of peptide A1 and mutant peptide A1 on angiogenesis in vivo using the Matrigel plug assay (supplemented with bFGF; 80 ng/mL). The dissected Matrigel plug and accompanying immunostaining for CD31 (PECAM-1) are shown. Determination of vessel density within the Matrigel plug per high-power field (hpf) (*P < .05 compared with peptide A1). Data are presented as means ± SE.
Effect of dominant-negative ELF-1 peptide on endothelial migration, tube formation, and in vivo angiogenesis. (A) Effect of peptide A1 on Ang-1–dependent endothelial migration compared with the mutant peptide (Mutant A1). The concentration of Ang-1 was 100 ng/mL (*P < .05 compared with control). (B) Effect of peptide A1 (10 μM) or mutant A1 (10 μM) on cell growth. (C) Endothelial tube formation on Matrigel supplemented with bFGF (20 ng/mL) at 8 hours and 24 hours in the presence of the mutant peptide A1 (10 μm) or peptide A1 (5, 10 μm) compared with no peptide (control). Quantitation of endothelial tube formation at 24 hours (*P < .05 compared with control). (D) Effect of peptide A1 and mutant peptide A1 on angiogenesis in vivo using the Matrigel plug assay (supplemented with bFGF; 80 ng/mL). The dissected Matrigel plug and accompanying immunostaining for CD31 (PECAM-1) are shown. Determination of vessel density within the Matrigel plug per high-power field (hpf) (*P < .05 compared with peptide A1). Data are presented as means ± SE.
Discussion
Several studies have demonstrated a role for ETS transcription factors in the regulation of endothelial-specific genes including Flk-1, Flt-1, Tie1, and Tie2. Whereas the ETS factors ETS-1 and ETS-2 potently transactivate the Flt-1 gene promoter, they do not appear to regulate the Tie1 and Tie2 gene promoters.8,25 In contrast, the ETS factor ELF-1 is a potent transactivator of the Tie1 and Tie2 genes.8,25 ELF-1 has been shown to regulate other genes involved in angiogenesis including angiopoietin-2 and endothelial nitric oxide synthase.24,26 The ability of the different ETS factors to transactivate the selective gene targets also correlates with their ability to bind to specific conserved ETS binding sites within these genes. This suggests that different subsets of the ETS factors may regulate different vascular-specific genes.
Expression of selected ETS factors is enriched at sites of active blood vessel development. The ETS transcription factor Fli-1 is enriched in the developing blood vessels of zebra fish embryos.27 ELF-1 is highly expressed in extraembryonic and embryonic blood vessels of the developing chicken embryo.9 The ETS factor ETS-1 has also been shown to be enriched in the developing blood vessels of the chicken, and antisense oligonucleotides have been shown to inhibit angiogenesis when delivered to the chicken chorioallantoic membrane.28
Expression of selected ETS factors is also enriched at sites of angiogenesis, where they are involved in regulating the expression of angiogenic growth factors. In situ hybridization studies of the developing mouse have also demonstrated that ETS-1 is expressed in developing blood vessels associated with tumor angiogenesis.29 ETS-1 has been shown to cooperate with HIF-2α in the setting of hypoxia to regulate the expression of the VEGF receptor 2 (Flk-1).30,31
Effect of dominant-negative peptide on B16-F1 melanoma tumor growth. (A) B16 tumor cell growth (mm3) in nude mice (control), in the presence of peptide A1, or mutant peptide A1 over time using calipers (left panel). Tumors were excised at 14 days and tumor volume was determined (*P < .001 versus control). Values represent means ± SE; n = 6 in each group. (B) Immunofluorescence staining for CD31, in tumors grown as described in “Mouse tumor models,” and assessment of vessel density (*P < .001 compared with control).
Effect of dominant-negative peptide on B16-F1 melanoma tumor growth. (A) B16 tumor cell growth (mm3) in nude mice (control), in the presence of peptide A1, or mutant peptide A1 over time using calipers (left panel). Tumors were excised at 14 days and tumor volume was determined (*P < .001 versus control). Values represent means ± SE; n = 6 in each group. (B) Immunofluorescence staining for CD31, in tumors grown as described in “Mouse tumor models,” and assessment of vessel density (*P < .001 compared with control).
The DNA binding domain of the ETS family of proteins is a highly conserved region of approximately 85 residues that shares a strong sequence homology and 3-dimensional structural scaffold that closely mimics the α + β helix-turn-helix family of DNA binding proteins.17,18 Previous investigations have clearly established that several highly conserved residues that are localized within the H3 recognition helix are responsible for anchoring the ETS domain through requisite DNA contacts within the major groove (Figure 2A). However, residues within the turns separating helices H2 and H3 and the first 2 β-strands are also involved in critical phosphate backbone contacts within the DNA minor groove. In the crystal structures of both Ets-1 and PU.1 in complex with their high-affinity DNA sequence, the highly conserved arginine residues (red box, Figure 1A; and rendered in blue, Figure 2A), which are mutated to glycine in the dominant-negative peptide (Figure 1B), are responsible for critical hydrogen and van der Waals interactions with the core DNA sequence. Similarly, lysine388 (Ets-1 nomenclature) or lysine229 (PU.1 nomenclature), which is a threonine in ELF-1 (additional red box in Figure 1A), is within the H3 recognition helix and positionally juxtaposed to the DNA surface, facilitating phosphate backbone contacts to the major groove of the core DNA also. If the H3 recognition helix were simply enough to stabilize the ETS domain interaction with its high-affinity DNA sequence, we would have expected peptide B (Figure 2B), which is composed of the majority of the H3 sequence, to similarly prevent DNA binding to ELF-1 (Figure 3). The inability of this peptide to prevent ELF-1 DNA binding as monitored in gel mobility shift assays supports the accessory role of the previously mentioned loop residues that facilitate additional minor groove DNA interactions that have been identified for both Ets-1 and PU.1, permitting the H3 helix to be anchored within the DNA major groove. Taken together, these data help us better understand why synthetic peptide A was the only peptide capable of preventing ELF-1 DNA binding. This is the first study demonstrating the ability of short synthetic peptides, significantly shorter than the DNA binding domain, to specifically interfere with DNA binding. Defining these critical ETS domain epitopes will allow us to further refine our peptide therapy strategies and permit elucidation of localized pharmacophores that could be used to mimic these functional interactions.
The use of membrane-permeable peptides to block the function of other transcription factors in cells has recently been demonstrated. A peptide with a membrane-permeable sequence and the nuclear localization signal sequence of the transcription factor NF-κB inhibited nuclear import of this transcription factor upon activation.32 This action of this peptide, however, was not specific to NF-κB and also inhibited other transcription factors such as AP-1, NFAT, and STAT1 from entering the nucleus.33 Another membrane-permeable peptide with the ability to interfere with DNA binding of a transcription factor was recently demonstrated using the Tet repressor (TetR) of Escherichia coli synthesized in tandem with a cell membrane–transducing peptide. This peptide was able to repress the expression of a tetracycline-responsive reporter unit stably transfected into the genome of HeLa cells.34 The results of our study confirm earlier reports that the HIV-1 TAT protein (48-60) is sufficient not only to promote transport of a peptide across the cell membrane but also led to their accumulation in the cell nucleus.35 This is the first study to demonstrate the use of membrane-permeable peptides to block the transcription of specific target genes in mammalian cells. Furthermore, the identification of relatively short peptides of 30 to 40 amino acids long that can selectively block the DNA binding of specific transcription factors, or subsets of transcription factors, suggests that this may be a very potent novel approach toward targeting transcription factors as a therapeutic modality to inhibit angiogenesis.
It has recently been shown that hematopoietic progenitor cells are mobilized from the bone marrow and home to the periphery of growing tumors where they act to promote neovascularization by releasing angiogenic growth factors.13 Systemic administration of an antibody directed against c-Kit, one of the main surface antigens on these cells, is associated with a marked reduction in tumor angiogenesis. Most of the recruited bone marrow–derived stem cells do not function as endothelial progenitors, but instead surround angiogenic blood vessels and secrete growth factors.36 One of the major mechanisms by which this recruitment may occur is via the expression of Tie2 on the surface of the hematopoietic cells. These cells infiltrate the tumor by chemotaxis in response to expression of their cognate ligands, the angiopoietins. More recently, genetically modified hematopoietic stem cells expressing Tie2 have been used to target tumors, by delivering “suicide” genes, resulting in significant reductions in tumor growth.37 The down-regulation of the expression of Tie2 through inhibition of the expression of ELF-1 may support an additional mechanism by which tumor angiogenesis could be diminished.
In summary, our results support a role of ELF-1 in the development of tumor angiogenesis and that targeting of ELF-1 in melanoma tumors results in decreased angiogenesis, which may occur through a reduction in the expression of Tie2, eNOS, and other ELF-1 target genes in endothelial cells.
Prepublished online as Blood First Edition Paper, December 13, 2005; DOI 10.1182/blood-2005-08-3206.
Supported by National Institutes of Health grants HL-67219 (P.O.), P01 HL76540-01 (P.O.), and a Focused Giving Grant from Johnson and Johnson (P.O.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.