Abstract
Non-Hodgkin lymphomas develop from nodal and extranodal lymphoid tissues. A distinct subset of extranodal lymphomas arising from B cells of the marginal zone (MZ) of mucosa-associated lymphoid tissue (MALT) or spleen has been individualized. Growing evidence indicates that MZ lymphomas are associated with chronic antigenic stimulation by microbial pathogens and/or autoantigens. The list of microbial species associated with MZ lymphoproliferations has grown longer with molecular investigations and now comprises at least 5 distinct members: H pylori, C jejuni, B burgdorferi, C psittaci, and hepatitis C virus (HCV), which have been associated with gastric lymphoma, immunoproliferative small intestinal disease, cutaneous lymphoma, ocular lymphoma, and spleen lymphoma, respectively. A pathophysiologic scenario involving chronic and sustained stimulation of the immune system leading to lymphoid transformation has emerged. It defines a distinct category of infection-associated lymphoid malignancies, in which the infectious agent does not directly infect and transform lymphoid cells, as do the lymphotropic oncogenic viruses Epstein-Barr virus (EBV), human herpesvirus 8 (HHV8), and human T-lymphotropic virus 1 (HTLV-1), but rather indirectly increases the probability of lymphoid transformation by chronically stimulating the immune system to maintain a protracted proliferative state.
Introduction
The geographic heterogeneity in the incidence of B-cell non-Hodgkin lymphomas (NHLs) suggests that environmental factors such as infections might have a role in lymphomagenesis.1,2
Because of the inherent genetic instability of lymphocytes, lymphoid proliferation increases the risk of transformation, and sustained activation of the lymphoid system, which can be observed during chronic infection, immunodeficiency, and autoimmunity, constitutes a risk factor for lymphomas.3-5 Congenital and acquired immunodeficiencies associated with HIV infection and solid organ or hematopoietic transplantation increase the risk of developing B-cell NHLs.6,7 Similarly, Sjögren syndrome and other autoimmune conditions are also associated with an increased risk of lymphomas.8,9
Certain types of lymphomas are associated with specific microbial infections, and infection-associated lymphomas currently fall in diverse histopathologic categories (Table 1).10 Infections may contribute to lymphomagenesis by promoting favorable conditions for lymphocyte transformation, such a increased proliferation or decreased apoptosis of lymphoid cells.11
Direct lymphocyte transformation by a given microbial agent is the simplest scenario accounting for infection-associated lymphomas. Lymphotropic transforming viruses such as Epstein-Barr virus (EBV), human herpesvirus 8 (HHV8), and human T-lymphotropic virus 1 (HTLV-1) directly infect a subset of lymphoid cells in which they express viral oncogenes.12-14
An alternative scenario to direct transformation of lymphocytes has more recently emerged for microbial species associated with lymphomas but that do not directly infect or transform lymphoid cells. They have in common the ability to persist chronically in host tissues and trigger a sustained lymphoid proliferation, giving a selective advantage to lymphoid clones that still remain dependent upon antigen stimulation.15,16 According to this model, the microbial pathogen is neither intrinsically transforming nor oncogenic, but can be viewed as a chronic source of antigens increasing the proliferative rate of lymphoid effectors, hence fueling the transformation process.
This model has emerged with the description of several lymphomas developing in the context of chronic antigen-dependent immune stimulation, among which H pylori–associated gastric mucosa-associated lymphoid tissue (MALT) lymphoma is the best characterized15,17,18 (Figure 1). Precise elucidation of the mechanisms underlying this “indirect” lymphomagenesis as well as completion of the inventory of the microbial species driving these antigen-dependent lymphoproliferations may provide important clues for their early diagnosis and the rationalization of the therapeutic interventions for this subtype of lymphomas.
This group of lymphomas often involves extranodal sites—normally devoid of organized lymphoid tissue—and manifests initially as indolent low-grade proliferations, reminiscent of the normal lymphoid hyperplasia driven by a physiologic antigenic stimulation.19-21 With the progression of the disease, additional oncogenic events may occur—such as constitutive activation of signaling pathways following chromosomal translocations or inactivation of tumor suppressor genes by hypermethylation or mutations—leading the lymphoproliferation to become independent of antigenic stimulation.22
H pylori and gastric MALT lymphomagenesis paradigm for infection-associated indirect lymphoid transformation. (A) Persisting antigens (Ag's) elicit a polyclonal B-cell response. Costimulation is provided by cytokines and members of the tumor necrosis factor superfamily (CD40-CD40L in T-cell–dependent responses and B-cell activating factor [BAFF]/B-lymphocyte stimulator [BLyS] or a proliferation-inducing ligand [APRIL] produced by dendritic cells in T-cell–independent responses). In the case of H pylori, T cells specific for H pylori epitopes provide help to B cells that recognize cross-reactive autoantigens present in the gastric mucosa such as fucosylated sialyl–Lewis X through CD40-CD40L costimulation. (B) Occurrence of genetic events such as p15 and p16 hypermethylation provide a selective advantage leading to the outgrowth of an antigen-responsive clone. Antigen dependence reflects the requirement for BCR signals such as NF-κB activation (see also Figure 5). (C) Progression toward antigen-independent (therefore antimicrobial-insensitive) MALT lymphoma is associated with the occurrence of additional genetic events. Chromosomal translocations involving MALT1 and Bcl-10 lead to a constitutive activation of NF-κB, bypassing the requirement for BCR-dependent signals (Figure 5). The t(11;18) translocation occurs early during B-cell proliferation and accelerates the transformation process in an antigen-independent fashion. The t(1;14) and t(14;18) translocations also lead to BCR-dependent NF-κB activation, but occur later after an antigen-dependent phase and can be associated with additional cytogenetic abnormalities. The t(3;14) translocation produces an IgH-FoxP1 transcript in 10% of MALT lymphomas that do not harbor other translocations involving the MALT1/Bcl-10 pathway. The precise role of t(3;14) is not known. P53 mutation is associated with transformation to high-grade lymphoma.
H pylori and gastric MALT lymphomagenesis paradigm for infection-associated indirect lymphoid transformation. (A) Persisting antigens (Ag's) elicit a polyclonal B-cell response. Costimulation is provided by cytokines and members of the tumor necrosis factor superfamily (CD40-CD40L in T-cell–dependent responses and B-cell activating factor [BAFF]/B-lymphocyte stimulator [BLyS] or a proliferation-inducing ligand [APRIL] produced by dendritic cells in T-cell–independent responses). In the case of H pylori, T cells specific for H pylori epitopes provide help to B cells that recognize cross-reactive autoantigens present in the gastric mucosa such as fucosylated sialyl–Lewis X through CD40-CD40L costimulation. (B) Occurrence of genetic events such as p15 and p16 hypermethylation provide a selective advantage leading to the outgrowth of an antigen-responsive clone. Antigen dependence reflects the requirement for BCR signals such as NF-κB activation (see also Figure 5). (C) Progression toward antigen-independent (therefore antimicrobial-insensitive) MALT lymphoma is associated with the occurrence of additional genetic events. Chromosomal translocations involving MALT1 and Bcl-10 lead to a constitutive activation of NF-κB, bypassing the requirement for BCR-dependent signals (Figure 5). The t(11;18) translocation occurs early during B-cell proliferation and accelerates the transformation process in an antigen-independent fashion. The t(1;14) and t(14;18) translocations also lead to BCR-dependent NF-κB activation, but occur later after an antigen-dependent phase and can be associated with additional cytogenetic abnormalities. The t(3;14) translocation produces an IgH-FoxP1 transcript in 10% of MALT lymphomas that do not harbor other translocations involving the MALT1/Bcl-10 pathway. The precise role of t(3;14) is not known. P53 mutation is associated with transformation to high-grade lymphoma.
Although these lymphomas are associated with diverse microbial species, they all appear to originate from “marginal zone” (MZ) lymphocytes. These cells are anatomically positioned in the lymphoid organs (spleen and lymph nodes) and in the MALT to function as a first line of defense against invading pathogens.23,24 Furthermore, the low activation threshold of these cells may predispose them to neoplastic transformation.16,25
Different B-cell subsets participating in the antigenic response: germinal center (GC) versus marginal zone (MZ) B cells
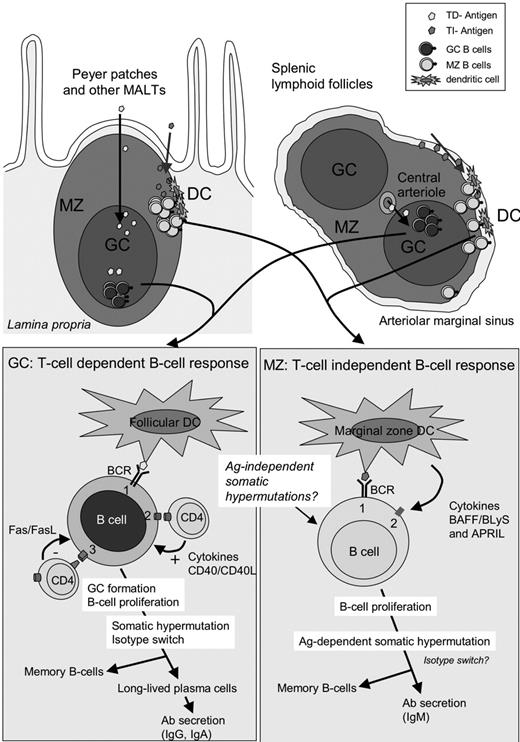
Mature B cells are heterogeneous with respect to their microanatomic location in the lymphoid organs and functional properties (Figure 2). Follicular (FO) B cells (IgMlowIgDhighCD21interCD23high) constitute the major subset of B cells and participate in T-cell–dependent (TD) immune responses in the germinal center (GC), where they receive help from antigen-specific T cells (ie, cognate interactions) through CD40-CD40L engagement. The GC reaction leads to isotype class-switched memory B cells (IgM–IgD–CD27+) with somatic hypermutations (SHMs) in their immunoglobulin gene segments. Upon antigenic rechallenge, they rapidly undergo terminal differentiation into plasma cells producing large amounts of high-affinity antibodies.
Organization of the lymphoid organs and B-cell responses. Top: Schematic view of a Peyer patch (left) and lymphoid follicles in the spleen (right) surrounded by the arteriolar marginal sinus. MZ indicates marginal zone; GC, germinal center; DC, dendritic cell; and FDC, follicular dendritic cell. The marginal zone is less conspicuous in the lymph nodes. Bottom left: Antigens having access to B-cell follicles are presented to B cells by FDCs. Antigen-specific B cells are activated by their BCR (signal 1) and proliferate, leading to the formation of the GC. Costimulation (signal 2) is provided by T-cell–derived cytokines and CD40L-CD40 interactions. GC T cells also express FasL, and autoreactive B cells (expressing Fas) are deleted by FasL-Fas interactions. Bottom right: Antigens captured in the blood by dendritic cells or directly accessing the MZ are presented to MZ B cells. MZ B cells are activated by BCR signal (signal 1). Costimulation (signal 2) is provided by DC-derived cytokines and the tumor necrosis factor superfamily member BAFF/BLyS or APRIL. Activated MZ B cells rapidly differentiate into plasma cells that secrete large amounts of IgM. In MZ B cells, mutations in immunoglobulin genes are acquired either during ontogeny in an antigen-independent fashion26 or during T-cell–dependent responses occurring in the GC.27
Organization of the lymphoid organs and B-cell responses. Top: Schematic view of a Peyer patch (left) and lymphoid follicles in the spleen (right) surrounded by the arteriolar marginal sinus. MZ indicates marginal zone; GC, germinal center; DC, dendritic cell; and FDC, follicular dendritic cell. The marginal zone is less conspicuous in the lymph nodes. Bottom left: Antigens having access to B-cell follicles are presented to B cells by FDCs. Antigen-specific B cells are activated by their BCR (signal 1) and proliferate, leading to the formation of the GC. Costimulation (signal 2) is provided by T-cell–derived cytokines and CD40L-CD40 interactions. GC T cells also express FasL, and autoreactive B cells (expressing Fas) are deleted by FasL-Fas interactions. Bottom right: Antigens captured in the blood by dendritic cells or directly accessing the MZ are presented to MZ B cells. MZ B cells are activated by BCR signal (signal 1). Costimulation (signal 2) is provided by DC-derived cytokines and the tumor necrosis factor superfamily member BAFF/BLyS or APRIL. Activated MZ B cells rapidly differentiate into plasma cells that secrete large amounts of IgM. In MZ B cells, mutations in immunoglobulin genes are acquired either during ontogeny in an antigen-independent fashion26 or during T-cell–dependent responses occurring in the GC.27
The marginal zone (MZ), which surrounds B-cell follicles in the spleen and in extranodal lymphoid tissue, contains a distinct subset of B cells, the MZ B cells (IgMhighIgDlowCD21highCD23low/–).28 MZ B cells participate in T-cell–independent (TI) “innatelike” immune responses to microbial pathogens,29 and can rapidly proliferate and differentiate into IgM or even switch to other isotype-secreting plasma cells, producing the bulk of the primary antibody response.30 TI responses do not generate memory B cells, consistent with a relatively short-lived antibody production. MZ B cells can be viewed as a bridge between the innate and adaptive immune responses to pathogens invading the host. In contrast to rodents, most human MZ B cells are somatically mutated (IgM+IgD+CD27+).26 It is still debated if these mutated MZ B cells in humans are in fact memory B cells originating from TD responses occurring in the GC and later homing to the MZ,27,31 or whether they are generated through a preimmune, antigen-independent diversification pathway.26 There is evidence that these mutated MZ B cells could be positively selected by autoantigens during B-cell ontogeny.32 Autoantigens or commensal bacterial antigens could be candidates for such selection processes occurring outside of the GC, and possibly involving CD40-CD40L cognate help by natural killer T (NKT) cells30 or BAFF-mediated noncognate help by MZ-resident macrophages or dendritic cells.28,33 Both B-cell subsets cooperate during the immune response to microbial polysaccharides: MZ B cells constitute the initial rapid response and can efficiently prime CD4 T cells that will subsequently provide costimulation to GC B cells.28 MZ B cells can also participate, though less efficiently than FO B cells, in TD antibody responses.28 Not surprisingly, owing to their frequent autoreactive and cross-reactive repertoire and to their relative hyperreactivity to activation, these cells are found in various pathologic conditions involving autoimmunity and infection. Lymphomas arising from MZ B cells can thus be expected to retain some of their seminal features, as explained in “Indirect transformation of lymphoid cells by microbial pathogens.”
Direct transformation of lymphoid cells by a microbial pathogen
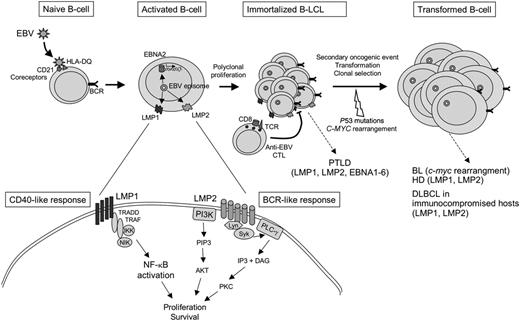
EBV is associated with a number of B-cell malignancies, including Burkitt lymphoma (BL), Hodgkin disease (HD), posttransplantation lymphoproliferative disorder (PTLD), as well as a subset of T- and NK-cell malignant proliferations,12 and frequently arises in the course of an underlying immunodeficiency. EBV infects, immortalizes, and transforms B cells in vitro and establishes a persistent latent infection34,35 (Figure 3). Viral genes expressed during latency subvert normal proliferation and survival pathways.36 One of the major oncogenes, the latent membrane protein 1 (LMP1), functions as a constitutively active member of the TNF-R family, closely related to CD40, a receptor whose engagement on normal B cells leads to B-cell activation.37 LMP1 expression is required for proliferation and transformation and is critical for in vitro immortalization of B cells.38,39 LMP2A is another EBV latent protein that can mimic survival signals from the B-cell receptor and rescue B cells lacking surface immunoglobulin.40,41 In HD, the malignant Reed-Sternberg cells are thought to derive from GC B cells that have undergone extensive crippling mutations of their immunoglobulin genes, precluding the expression of a functional surface receptor. Normal GC B cells, in this situation, would be eliminated by apoptosis, and one possible role for EBV in the pathogenesis of HD would be to provide proliferative and antiapoptotic signals (through LMP1 and LMP2) to “crippled” GC B cells, enabling them to escape apoptosis.42
EBV infection and direct transformation of B cells. EBV infects naive B cells through 2 surface receptors, CD21 and the class II MHC, HLA-DQ. During latency, the virus is maintained as an episome in the nucleus of infected cells, and viral genes are expressed, in the absence of lytic replication of the virus. EBV subverts normal B-cell differentiation, notably by the expression of LMP1, a viral latent protein expressed at the surface of infected cells. LMP1 associates with transduction molecules such as TRADD and TRAF and activates the NF-κB pathway in a CD40-like manner. LMP1 is required for the activation and immortalization of B cells. LMP2 is a viral transmembrane protein that associates with Lyn/Syk kinase and PI3 kinase, leading to the activation of PKC and AKT, respectively. LMP2 can substitute for signals emanating from the BCR. Both LMP1 and LMP2 converge to activate proliferation and survival pathways in EBV latently infected cells. EBNA2 transactivates LMP1 and a number of cellular genes involved in activation and proliferation. Polyclonal infected B cells proliferate and produce immortalized lymphoblastoid cell lines in vitro. In vivo, EBV-infected B cells are negatively controlled by anti-EBV cytotoxic T lymphocytes (CTLs). Failure to control EBV-infected B cells may lead to the development of posttransplantation lymphoproliferative disorder (PTLD). Additional oncogenic mutations lead to clonal selection and evolution toward monoclonal tumors such as Burkitt lymphoma (BL), Hodgkin disease (HD), and diffuse large B-cell lymphomas (DLBCLs) in immunocompromised patients.
EBV infection and direct transformation of B cells. EBV infects naive B cells through 2 surface receptors, CD21 and the class II MHC, HLA-DQ. During latency, the virus is maintained as an episome in the nucleus of infected cells, and viral genes are expressed, in the absence of lytic replication of the virus. EBV subverts normal B-cell differentiation, notably by the expression of LMP1, a viral latent protein expressed at the surface of infected cells. LMP1 associates with transduction molecules such as TRADD and TRAF and activates the NF-κB pathway in a CD40-like manner. LMP1 is required for the activation and immortalization of B cells. LMP2 is a viral transmembrane protein that associates with Lyn/Syk kinase and PI3 kinase, leading to the activation of PKC and AKT, respectively. LMP2 can substitute for signals emanating from the BCR. Both LMP1 and LMP2 converge to activate proliferation and survival pathways in EBV latently infected cells. EBNA2 transactivates LMP1 and a number of cellular genes involved in activation and proliferation. Polyclonal infected B cells proliferate and produce immortalized lymphoblastoid cell lines in vitro. In vivo, EBV-infected B cells are negatively controlled by anti-EBV cytotoxic T lymphocytes (CTLs). Failure to control EBV-infected B cells may lead to the development of posttransplantation lymphoproliferative disorder (PTLD). Additional oncogenic mutations lead to clonal selection and evolution toward monoclonal tumors such as Burkitt lymphoma (BL), Hodgkin disease (HD), and diffuse large B-cell lymphomas (DLBCLs) in immunocompromised patients.
HHV8 is closely related to EBV and is also associated with a number of B-cell lymphoproliferative disorders including multicentric Castleman disease and primary effusion and plasmablastic lymphomas.43 As does EBV, HHV8 encodes several genes that interfere with cell-signaling pathways involved in proliferation and survival, and that may play a role in cellular transformation (for review see Damania41 ).
The human oncogenic retrovirus HTLV-1 infects and immortalizes CD4+ T cells. The Tax oncoprotein interferes with numerous cell-signaling pathways and is thought to play a major role in immortalization.14,44
In general, the relationship between viral cycle and viral oncogenesis is complex. As a retrovirus, HTLV-1 integrates in the genome of infected cells. Although HTLV-1 replication is present in infected individuals, leukemic cells in adult T-cell leukemia (ATL) harbor latent integrated virus. The genomes of EBV and HHV8 are maintained as episomes in latently infected cells, and viral replication is not required for B-cell transformation of EBV-infected cells. Although HHV8 also establishes a latent infection in B cells, there is evidence that lytic replication is implicated in the early steps of oncogenesis.43 Since antiviral drugs target replication, they are mostly ineffective against virus-associated lymphoproliferations. HTLV-1, however, stands out as an exception because antiretroviral therapy with azidothymidine and high-dose interferon alfa has shown to be effective, at least in previously untreated ATL,14 although these drugs may also act at different levels by modulating cellular and viral gene expression such as inhibition of NF-κB and up-regulation of viral genes.45
Indirect transformation of lymphoid cells by a microbial pathogen
Since the identification of the role of Helicobacter pylori in the pathogenesis of gastric MALT lymphoma, several other low-grade B-cell lymphomas have been associated with chronic infections. Strikingly, most if not all of these lymphomas are derived from MZ B cells.
The International Lymphoma Study Group now recognizes 3 distinct lymphoma entities deriving from MZ B lymphocytes10,46 : (1) splenic MZ lymphomas, (2) extranodal MZ lymphoma of MALT-type, and (3) nodal MZ lymphomas. Although many aspects of their histology and molecular pathogenesis are distinct, these entities share a number of common features, the most striking being their possible association with chronic antigenic stimulation by microbial and/or autoantigens.
MALT lymphomas
Low-grade lymphomas originating from the MALT were originally described in the stomach and small intestine.47,48 They may also develop in other mucosal sites such as the salivary and lacrimal glands, the thyroid, and the bronchi. They have now all been classified in the same nosologic entity generically called “MALT lymphoma.”15,49,50 The majority of the organs in which MALT lymphomas develop are normally devoid of lymphoid tissue, and, in most cases, MALT acquisition is induced prior to the development of lymphoma, as a response to a persistent antigenic stimulation.16
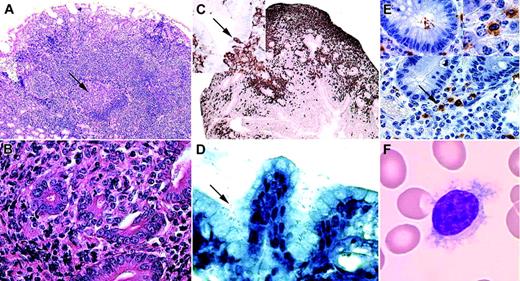
Neoplastic cells in MALT lymphomas exhibit features of MZ B cells from which they are thought to derive within the MALT.19,20,51-54 In MALT lymphomas, the MZ is expanded and surrounds residual GCs (Figure 4A). The neoplastic cells are small and resemble centrocytes, and hence are often called “centrocyte-like cells” (CCLs) (Figure 4B). They extend in the adjacent epithelial mucosa and invade the glandular epithelium, producing pathognomonic lympho-epithelial lesions (LELs) (Figure 4B-C).18 A prominent plasmacytic differentiation is common.
Histopathologic illustration of MALT/MZ lymphomas. (A) Low-power view (× 50) of the lymphoid infiltration in a gastric biopsy sample tissue section stained with hematoxylin and eosin from a patient with H pylori–associated gastric MALT lymphoma. The arrow shows a germinal center surrounded by an enlarged marginal zone infiltrating the gastric lamina propria. (B) High-power view (× 400) of an intestinal biopsy sample tissue section stained with hematoxylin and eosin from a patient with C jejuni–associated IPSID, revealing centrocyte-like cells (CCLs) infiltrating the crypt epithelium and forming lymphoepithelial lesions (LELs). (C) Sections of jejunum (× 100) stained with primary antibodies directed against the B-cell marker CD20 (appears brown when stained with enzyme-linked secondary antibodies) and counterstained with hematoxylin show CD20+ centrocyte-like lymphocytes pervading the lamina propria surrounding crypts. (The top inset shows a higher power view of the epithelium.) CD20+ CCLs infiltrate the crypt epithelium and produce characteristic LELs (arrow). (D) High-power view (× 400) of a gastric biopsy sample tissue section stained according to the Giemsa technique from a patient with H pylori –associated gastric MALT lymphoma. The gastric mucosa is heavily infected by H pylori (arrow). (E) Immunohistochemical analysis of a jejunal section (× 400) from a patient with IPSID and stained with an anti–C jejuni monoclonal antibody (brown) and hematoxylin. The arrows point to immunolabeled material shown at a higher magnification in the top-right inset. The top-left inset shows a crypt section with intraluminal immunolabeled bacteria. (F) A blood smear (× 1000) colored according to the May-Grünwald-Giemsa technique showing a typical villous lymphocyte from a patient with HCV-associated splenic lymphoma with villous lymphocytes. Sections were viewed using a Zeiss Axiophot microscope (Carl Zeiss, Thornwood, NY) and Olympus UPlan F1 5 ×/0.12 numeric aperture (NA) (A), 40 ×/0.7 NA (B, D, E), 10 ×/0.30 (C), and 100 ×/0.30 NA (F; oil immersion) objectives (Olympus, Melville, NY), and were photographed using a Spot RT digital camera and Spot 3 software (both from Diagnostic Instruments, Sterling Heights, MI).
Histopathologic illustration of MALT/MZ lymphomas. (A) Low-power view (× 50) of the lymphoid infiltration in a gastric biopsy sample tissue section stained with hematoxylin and eosin from a patient with H pylori–associated gastric MALT lymphoma. The arrow shows a germinal center surrounded by an enlarged marginal zone infiltrating the gastric lamina propria. (B) High-power view (× 400) of an intestinal biopsy sample tissue section stained with hematoxylin and eosin from a patient with C jejuni–associated IPSID, revealing centrocyte-like cells (CCLs) infiltrating the crypt epithelium and forming lymphoepithelial lesions (LELs). (C) Sections of jejunum (× 100) stained with primary antibodies directed against the B-cell marker CD20 (appears brown when stained with enzyme-linked secondary antibodies) and counterstained with hematoxylin show CD20+ centrocyte-like lymphocytes pervading the lamina propria surrounding crypts. (The top inset shows a higher power view of the epithelium.) CD20+ CCLs infiltrate the crypt epithelium and produce characteristic LELs (arrow). (D) High-power view (× 400) of a gastric biopsy sample tissue section stained according to the Giemsa technique from a patient with H pylori –associated gastric MALT lymphoma. The gastric mucosa is heavily infected by H pylori (arrow). (E) Immunohistochemical analysis of a jejunal section (× 400) from a patient with IPSID and stained with an anti–C jejuni monoclonal antibody (brown) and hematoxylin. The arrows point to immunolabeled material shown at a higher magnification in the top-right inset. The top-left inset shows a crypt section with intraluminal immunolabeled bacteria. (F) A blood smear (× 1000) colored according to the May-Grünwald-Giemsa technique showing a typical villous lymphocyte from a patient with HCV-associated splenic lymphoma with villous lymphocytes. Sections were viewed using a Zeiss Axiophot microscope (Carl Zeiss, Thornwood, NY) and Olympus UPlan F1 5 ×/0.12 numeric aperture (NA) (A), 40 ×/0.7 NA (B, D, E), 10 ×/0.30 (C), and 100 ×/0.30 NA (F; oil immersion) objectives (Olympus, Melville, NY), and were photographed using a Spot RT digital camera and Spot 3 software (both from Diagnostic Instruments, Sterling Heights, MI).
Gastric MALT lymphoma and Helicobacter pylori: a model for infection-associated MALT lymphomas. Gastric MALT lymphoma is the most common MALT lymphoma and represents the majority of lymphomas involving the stomach.15,17,18 Gastric MALT lymphoma develops on a background of chronic inflammation and lymphoid infiltration displaying features of classic MALT architecture, of which the gastric mucosa is physiologically devoid.17
Gastroduodenal Helicobacter pylori infection is strongly associated with gastric MALT lymphoma18 (Figure 4D). H pylori is a Gram-negative bacterium that colonizes the gastric mucosa and is associated with peptic ulcers and gastric adenocarcinomas.55-57 Hpylori is present in gastric biopsy samples of the majority of patients with gastric MALT lymphoma.58 The incidence of gastric MALT lymphoma is the highest in regions where H pylori infection is endemic,59 and the seroprevalence of H pylori is higher in patients with gastric MALT lymphomas60 than in control patients without MALT lymphomas. Eradication of H pylori leads to complete regression of the lymphoma during the early stages of the disease in nearly 80% of the cases.61-64 Low-grade gastric MALT lymphoma may evolve into aggressive large-cell gastric lymphoma, which is typically refractory to antibiotics.17,65-67 Retrospective analyses have shown that clonal B cells were already present in the gastritis, years before the clinical emergence of the lymphoma.68
Immunoproliferative small intestinal disease: the quest for a microbial association. Immunoproliferative small intestinal disease (IPSID), also called alpha heavy-chain (αHC) disease or Mediterranean lymphoma, is a MALT lymphoma arising in the small intestine. The malignant cells have a distinctive lymphoplasmacytic phenotype and secrete a monotypic, truncated immunoglobulin α-heavy chain lacking an associated light chain, which can be detected as a paraprotein in the serum of patients.69-71 Histologic features of IPSID range from early lymphoplasmacytic intestinal infiltration to overt malignant diffuse large B-cell lymphoma. IPSID shares all the histologic features of MALT lymphomas, namely the presence of CCL, LEL, and plasma-cell differentiation.47,72 IPSID is chiefly observed in young adults originating from the Mediterranean basin, the Middle East, the Far East, and Africa.71 Antimicrobial therapy with tetracycline, ampicillin, or metronidazole is effective in early-stage IPSID,71 a finding that led the first investigators to hypothesize (years before H pylori was identified and associated with gastric MALT lymphoma) that a microbial species might play an etiologic role in IPSID development.71 Nevertheless, despite repeated attempts using classic culture-based approaches, the efforts made toward the identification of a microbial species associated with IPSID remained vain. Although H pylori was recently proposed to be involved in IPSID, this proposition was not corroborated in a subsequent retrospective analysis of more than 20 cases.73,74
Applying an unbiased molecular approach previously used with success to identify the bacterial species associated with bacillary angiomatosis (Bartonella henselae)75 and Whipple disease (Tropheryma whipplei),76 we have demonstrated the presence of C jejuni–specific sequences in intestinal tissue samples of an IPSID patient with a spectacular response to antimicrobial therapy. These results were confirmed by in situ hybridization and immunohistochemistry (Figure 4E) on this index case and 4 of 6 archival additional cases.77 Identification of C jejuni in 5 of 7 patients with IPSID and the dramatic response observed in the index patient after microbial eradication make for a strong argument for the association of C jejuni with IPSID.78 However, association is not proof for causation, although it is usually the first step in proving the microbial etiology of a disease. To definitively demonstrate that C jejuni infection causes IPSID, the Koch postulate would need to be fulfilled (ie, Is C jejuni detectable in the host's intestine in early stages of the disease? Can C jejuni be cultivated from the diseased tissue? Can C jejuni trigger the disease in an animal model? Can C jejuni be isolated from the diseased animal?). Note that recent advances in molecular diagnostic tools have led to the restatement of new criteria for this postulate that take into account the putative uncultivability and host specificity of microbial species identified by molecular techniques.79,80 Demonstration of long-term C jejuni intestinal persistence is important for incriminating this bacterial species in IPSID development. The epidemiology of C jejuni in developing countries, in which IPSID is exclusively observed, sharply contrasts with that reported in developed countries. Up to 15% of asymptomatic children in developing countries carry Campylobacter organisms in their stools, whereas in developed countries, fecal Campylobacter is present in less than 0.5%.81 It is not known whether C jejuni can persistently colonize the small intestinal mucosa without concomitant detectable fecal shedding. Future studies need to focus on the extent of the asymptomatic and long-term intestinal carriage, its putative correlation with lamina propria lymphoid infiltration that might precede the emergence of lymphoma, and ultimately its causal relationship with IPSID. The absence of a functional B-cell receptor in IPSID raises the question as to how antigens might persistently stimulate these cells. In the early stages of infection, antigen-specific B cells in the lamina propria responding to microbial antigens (and possibly cross-reacting with autoantigens) would be stimulated and proliferate. During persistent stimulation, mutations would accumulate, leading to the selection of a clone having lost the ability to express a complete immunoglobulin and thus rendered insensitive to a negative feedback loop.71 According to this model, the subsequent proliferation would depend on survival factors (eg, BAFF) induced in the inflamed mucosa by persisting infection, as well as a proliferative advantage of the clone due to the loss of the negative regulation of normal IgA synthesis.82
Extraintestinal MALT-type lymphomas may also be associated with chronic infections due to so-far-unidentified bacterial species. A similar approach to that used for IPSID could show promises in identifying such bacterial species.
Other bacteria-associated MALT lymphomas. The presence of Borrelia burgdorferi has been reported in primary cutaneous B-cell lymphoma (PCBCL) tissue.83-85 Despite contradictory reports from investigators from other geographic areas,86,87 there is significant support for the hypothesis that B burgdorferi infection might be associated with chronic antigen-driven lymphomagenesis in the skin, a tissue in which B burgdorferi is known to establish a persistent infection.85,88 Skin is the portal of entry of B burgdorferi and is the most commonly affected tissue in Lyme borreliosis.88 Late in the disease, lymphocytes may infiltrate the dermis and produce the characteristic borrelial “lymphocytoma.” B burgdorferi is present within the early skin lesions of erythema migrans and later in lymphocytoma, and its DNA can be readily amplified from biopsies of diseased skin. PCBCL is a rare entity that commonly displays the histologic features of MZ lymphoma.89 The incidence of PCBCL is higher in areas endemic for Lyme disease, and borrelial DNA has been amplified from skin biopsies.85,90 Regression of the lymphoma after antimicrobial therapy has been reported.84,91,92 Histologically, borrelial lymphocytoma can be difficult to distinguish from PCBCL, and has led to the improper term of “Borrelia-associated pseudolymphoma.”93 Having in mind the H pylori model of gastric MALT lymphomagenesis, the cutaneous manifestations of Lyme borreliosis could be viewed as a multistep progression from lymphocytoma to “pseudolymphoma” eventually leading to PCBCL.90,93 Evidence of B-cell monoclonality may help distinguish between the different stages of the disease,90,93 although it does not constitute a definitive proof of malignancy.
Recently, Chlamydia psittaci infection has also been associated with ocular adnexal MALT lymphomas.94,95 Adnexal MALT lymphomas have been described in the context of chronic conjunctivitis, and particularly “inclusion conjunctivitis,” which can be associated with Chlamydia infection.96,97 The presence of Chlamydia psittaci DNA in biopsy material and peripheral-blood mononuclear cells from patients with ocular adnexal lymphomas94 was demonstrated by targeted polymerase chain reaction (PCR), and C psittaci DNA was detected in 80% of ocular adnexal lymphoma samples. In some patients, antimicrobial treatment with doxycycline was associated with a clinical response.94 Together, these data argue for a putative role for C psittaci in ocular adnexal MALT lymphomagenesis.
Splenic marginal zone lymphoma (SMZL) and splenic lymphoma with villous lymphocytes (SLVL)
SMZL is a rare low-grade B-cell lymphoma involving predominantly the spleen.54 A leukemic phase with cytologically distinct lymphocytes defines the SLVL variant of SMZL.98 SMZL usually presents as an indolent lymphoma, and autoimmune manifestations such as serum rheumatoid factor (RF) are frequently associated. Histologically, the marginal zone surrounding the follicular areas is expanded and neoplastic cells have cytologic and phenotypical features of marginal zone lymphocytes,98,99 clearly distinguishing them from the lymphocytes present in the follicular center or the mantle area.
Hepatitis C virus (HCV) infection and MZ lymphomas: a correlation between lymphoid proliferation and viral load. We have reported the association of a subset of SLVL with chronic HCV infection,100,101 a finding that has now been confirmed by other investigators.102 HCV is an RNA virus associated with extrahepatic manifestations, such as essential mixed cryoglobulinemia (EMC) and B-cell lymphoproliferations.103 HCV-associated SLVL is indistinguishable from classic SLVL, except for the presence of HCV viral replication.100 EMC is consistently present in HCV-associated SLVL.101 Antiviral treatment with interferon alfa with or without ribavirin results in a marked reduction of lymphocytosis and splenomegaly in HCV-associated SLVL, whereas it is ineffective in HCV-negative SLVL.100,101 Complete virologic response correlates with sustained hematologic response, and virologic relapse is associated with re-emergence of circulating villous lymphocytes and splenomegaly. Reduction in HCV viral load after restarting the antiviral treatment correlates with hematologic remissions. Overall, these data indicate a strong correlation between serum viral load and tumor burden in HCV-associated SLVL, and support the existence of a causal relationship between HCV chronic antigenic stimulation and the MZ lymphomatous process.104
Other HCV-associated lymphomas. EMC is considered a nonmalignant B-cell lymphoproliferation characterized by the synthesis of a monoclonal IgM with RF activity against immune complexes containing HCV proteins.105-107 Several epidemiologic studies have reported an association between HCV infection and B-cell lymphomas,108-112 and most cases of these HCV-associated lymphomas are low-grade MZ lymphomas.53,109,112 MALT lymphomas of the salivary glands may be associated with HCV infection.113 Monoclonal B cells can be detected during chronic HCV infection, especially in patients with HCV-associated ECM.103 Furthermore, the existence of a cryoglobulinemia is an independent risk factor for lymphomas in HCV-infected patients112 and may thus be considered as an early marker of HCV-associated lymphoproliferation.
MZ lymphomas and autoimmunity
Sjögren syndrome (SS) and autoimmune Hashimoto thyroiditis (HT) are characterized by autoreactive T- and B-lymphocyte infiltration of the salivary glands and the thyroid, respectively. Chronic inflammation and the ensuing cellular damage are associated with massive exposure of autoantigens to the immune system. B lymphocytes infiltrating the salivary glands in SS and the thyroid in HT progressively organize into a lymphoid infiltrate that reproduces the distinctive histologic architecture of normal MALT, including the presence of numerous reactive follicles.114 The risk for developing a B-cell lymphoma is increased by a factor of 44 in patients with SS.8 Similarly, indolent lymphomas of the thyroid, most often of the MALT-type, develop on a background of autoimmunity.115,116 Thus, utoantigenic stimulation observed during SS and AT appears to recapitulate the chronic microbial antigenic stimulation observed during persisting infections, and as in chronic infections, the failure to eradicate the antigenic source in autoimmunity leads to sustained B-cell stimulation, thus favoring lymphoid transformation and lymphomas.
Pathophysiologic aspects of antigen-driven MZ lymphomagenesis
H pylori and gastric MALT lymphoma
Converging evidence supports a causative role for H pylori in gastric MALT lymphomas117,118 : T cells from patients with gastric MALT lymphomas are able to sustain the in vitro proliferation of autologous malignant B cells in the presence of H pylori extracts, in a CD40-CD40L–dependent manner, supporting the role of H pylori in triggering the lymphoproliferation119 (Figure 1A). Strikingly, neoplastic B cells from gastric MALT lymphoma are not specific for H pylori antigens but rather for autoantigens found in the gastric mucosa. These autoreactive B cells are thought to receive cognate help from H pylori–specific T cells displaying cross-reactivity with gastric autoantigens (Figure 1A and “Microbial persistence: implications in lymphoproliferation and autoimmunity”). Thus, the malignant B cells could derive from TD GC B cells that homed to the MZ and thus display MZ-type phenotype and function. Alternatively, they could also be true MZ B cells participating in a TD response.28,30
H pylori–infected gastric mucosal cells produce proinflammatory cytokines (such as lymphotoxin beta) and B-cell homing factors (such as BCA-1), leading to the emergence of MALT in the gastric mucosa.120 Infection of mice with Helicobacter species, including H pylori and the related species H felis and H heilmannii, also leads to the development of chronic gastritis and gastric MALT lymphomas with similarities to the human disease.117,118
Thus H pylori not only fulfills the Koch postulate for gastric ulcer and carcinomas, but can also be convincingly incriminated in gastric MALT lymphomagenesis. H pylori–associated gastric MALT lymphomagenesis thus stands as the best-defined paradigm for infection-associated indirect lymphoid transformation.
Microbial persistence: implications in lymphoproliferation and autoimmunity
Pathogens inducing chronic infection have selected countless mechanisms allowing them to persist in the host and colonize their specific niches.
Molecular mimicry, a situation in which microbial pathogens express antigenic motifs shared with the host, is also a mechanism that favors microbial persistence, given the tolerization of the immune system toward autoantigens. Several H pylori antigens resemble autoantigens, notably the fucosylated Lewis antigens expressed on the surface of the gastric mucosa and the epitopes of self-gastric parietal-cell H(+)K(+)-ATPase57,121 (Figure 1A). Other examples of autoreactivity elicited by antimicrobial immune responses have been described for the aforementioned microbial species associated with antigen-driven lymphomagenesis: C jejuni is associated with Guillain-Barré syndrome, an acute polyneuropathy induced by cross-reactive antibodies directed against C jejuni lipo-oligosaccharides (LOSs) and nervous system gangliosides122 ; B burgdorferi OspA protein is structurally homologous to human lymphocyte function antigen-1 (LFA1), which may play a role in the autoimmune manifestations of the disease.123 Chlamydia species also share immunoreactivity with eukaryotic heat shock proteins,124 and this has been proposed to play a role in autoimmunity associated with this bacterium. Finally, the basis for the strong association between the immune response to HCV and the detection of an RF may lie in the structural and antigenic homologies between the N-terminal region of the HCV E2 envelope protein and the human immunoglobulin variable domains, and as such can be recognized by antihuman antibodies.125,126
All the aforementioned microbial pathogens may also evade the immune system by antigenic variation, as has been documented in detail for H pylori,57 B burgdorferi,127 and HCV.128 This process also contributes to chronic stimulation of the immune system by continuously modifying microbial antigenic determinants.
Evidence for antigenic selection in MZ lymphomas
Indirect evidence for the role of an antigen in B-cell proliferation can be deduced from the analysis of the V gene use and SHM in immunoglobulin V genes, because they constitute molecular signatures for antigen selection.129-131 All MZ lymphomas associated with chronic infection and/or autoimmunity exhibit a biased immunoglobulin V gene use and SHM.132-134 The recent finding that MALT lymphomas stand out among other B-cell lymphomas as frequently expressing immunoglobulin V genes with strong homology to RF135 underscores the links between chronic antigenic stimulation, autoimmunity, and development of MZ-derived lymphoproliferations. Analysis of the immunoglobulin specificity from 2 HCV-associated lymphoma tumor cells demonstrated that they bound the HCV E2 glycoprotein similarly to human anti-E2 antibodies.125 Furthermore, B-cell clones in HCV-associated EMC and lymphomas often use the VH1-69 gene segment, which is also used by anti-E2 antibodies elicited by HCV.133,134,136 Many autoantigens have been identified in both SS and AT, and a common feature to both conditions is the frequent presence of RF; some cases of salivary gland lymphomas arising in SS also use immunoglobulin segments with RF activity, further supporting the role of chronic antigen stimulation in the pathogenesis of this condition.137
Ongoing mutations in proliferating lymphocytes
Reactive oxygen species (ROSs) produced during inflammation are genotoxic and favor the occurrence of oncogenic DNA damage in proliferating lymphocytes.138 The intrinsic genetic instability of B cells during isotype class-switching and SHM139,140 also increases the risk of transformation during protracted proliferation associated with inflammation.
H pylori chronic infection is associated with the production of ROSs, chronic inflammation, and DNA damage.57,141,142 Most isolates of C jejuni produce a toxin called CdtB (cytolethal distending toxin B) that causes direct DNA damage.143 CdtB induces double-strand DNA breaks and growth arrest in T lymphocytes and may thus participate in immune evasion mechanisms during infection with CdtB-producing bacteria,143 as well as the emergence of the DNA breaks in B cells leading to the synthesis of a truncated immunoglobulin as seen in IPSID patients.71 The conjunction of CdtB and SHM occurring in the MALT during C jejuni chronic infection could lead to large deletions of the variable region of the αHC, precluding the association with a light chain and leading to the synthesis of a truncated αHC.139,143
Mechanisms of clonal progression of antigen-dependent B cells
Inactivation of cell-cycle regulating genes such as the cyclin-dependent kinase inhibitors p15 and p16 is observed in early stages of gastric MALT lymphomas (Figure 1B).144 Fas/CD95, involved in apoptosis and homeostasis of normal and autoreactive B cells,145 is often mutated in nongastric MALT lymphomas and in other MZ lymphomas.146,147 These alterations confer a clonal advantage to antigen-specific B cells and ultimately lead to transformation. Early transformed B cells would still rely on signals from the antigen receptor for their proliferation and survival, as attested by their antigen dependence, which is illustrated by the efficacy of antigenic eradication (Figure 1B).
Molecular pathogenesis and cytogenetic features of MZ lymphomas
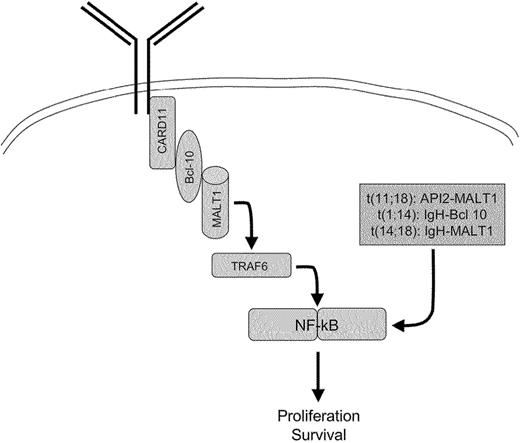
Recurrent cytogenetic abnormalities are found in most MZ/MALT lymphomas. The t(11;18), which fuses the API2 and MALT1 genes and generates a functional API2-MALT1 fusion product, has been found in several cases of MALT lymphomas arising in various mucosal sites.18 t(11;18) is usually the sole chromosomal aberration and occurs early. Other translocations, including t(1;14) and t(14;18), which fuse the BCL10 and MALT1 genes, respectively, to the IGH locus (Figures 1C, 5), have also been described, and can be associated with other cytogenetic aberrations such as chromosome 3 trisomy.18 More recently, the occurrence of a t(3;14) translocation has been described in 10% of MALT lymphomas, but not in nodal or splenic MZ lymphomas. This translocation that fuses FoxP1 to the IgH locus is mutually exclusive with the other MALT lymphoma–specific translocations, t(11;18), t(1;14), and t(14;18).148
In SMZLs, the most common cytogenetic abnormality is the deletion of the long arm of chromosome 7 (7q21-32), which likely involves cdk6, and trisomy 3,54 whereas the t(11;18), t(1;14), and t(14;18) translocations are not found.
The oncogenic activity of the 3 chromosomal translocations t(11;18), t(1;14), and t(14;18) is linked to the physiologic role of BCL10 and MALT1 in antigen receptor–mediated NF-κB activation and inhibition of apoptosis.22,149,150 Constitutive activation of the NF-κB pathway by these translocations bypasses the requirement for the B-cell receptor signaling and accounts for the antigen independence of cells harboring these translocations67,151,152 (Figures 1 and 5). The oncogenic role of the IgH-FoxP1 fusion transcript is unknown.22
Gastric MALT lymphoma translocations lead to a bypass of the BCR-mediated NF-κB activation.
Gastric MALT lymphoma translocations lead to a bypass of the BCR-mediated NF-κB activation.
Alterations of B-cell functions by HCV
The HCV E2 glycoprotein interacts with CD81 on the surface of B lymphocytes and is a target of the humoral response against the virus.125,153 CD81 engagement on B cells enhances signaling through the BCR.154 Engagement of CD81 and virus-specific BCR by E2 could perturb B-cell function and lead to lymphoma. Mutations in the p53, Bcl6, and β-catenin genes may occur in B-cell lines infected in vitro with HCV as well as in peripheral-blood mononuclear cells from patients with chronic HCV infection. Induction of nitric oxide synthase by the viral core protein (C) and nonstructural protein 3 (NS3) has been implicated in the occurrence of these mutations.155,156
Although HCV can infect B cells in vitro, possibly through CD81,153 only one case of B-cell lymphoma associated with direct infection of B cells by HCV has been described so far.157 A lymphoid cell line derived from an HCV-infected patient presenting with a mantle-cell lymphoma produced virus in vitro.158 Thus, direct infection of lymphocyte by HCV does not appear to be a prerequisite for most HCV-associated lymphomas,159 in agreement with what one would expect for an antigen-driven lymphoproliferation according to the “indirect” model of lymphomagenesis.160 Moreover, recombinant HCV E2 binding to CD81 on B cells has been shown to induce hypermutations at the immunoglobulin locus.161 E2 is exposed on the virion surface and can interact externally with the CD81 coreceptor on B cells. This mechanism of mutagenesis would be independent of direct infection of B cells by HCV.
Conclusions and perspectives
Antigen-driven lymphoproliferations constitute a pathophysiologic concept that took root with the description of the MALT-lymphoma entity in the early 1980s and bloomed with the identification of H pylori as the causal agent of gastric MALT lymphoma in the early 1990s. This concept assumes that the marginal zone B lymphocyte is the cell from which these types of lymphomas derive, through a protracted proliferation induced by a persisting antigen, mostly of microbial origin. This entity now includes numerous additional examples of lymphoproliferations, which fall into the wider category of “MZ lymphomas.” The recent deciphering of the antigenic specificity of B-cell responses from the MZ has yielded important clues in relation to MZ lymphomagenesis associated with persistent antigenic stimulation.16 The spectacular responses observed after microbial eradication in a number of MZ/MALT lymphomas associated with chronic infections are of great pathophysiologic but also clinical importance, because many patients can be treated without antineoplastic chemotherapy, at least during the early stage of the disease. Unbiased approaches aimed at the identification of novel or unsuspected pathogens associated with MZ/MALT lymphomas will undoubtedly lead to the lengthening of the list of microbial pathogens associated with lymphoproliferations. Additional work is now critically needed to provide irrefutable evidence demonstrating not only the association but also the causative role of the microbial pathogens identified with this approach.
Prepublished online as Blood First Edition Paper, January 5, 2006; DOI 10.1182/blood-2005-09-3679.
Note added in proof. C psittaci association with adnexal lymphoma has not been confirmed by other groups,162 emphasizing the possible geographic heterogeneity of the association.
We thank Nicole Brousse, Antoine Martin, and Françoise Valensi for kindly providing pictures for Figure 4 and Bruno Varet for his support. We apologize to colleagues whose work could not be cited because of space limitations.

![Figure 1. H pylori and gastric MALT lymphomagenesis paradigm for infection-associated indirect lymphoid transformation. (A) Persisting antigens (Ag's) elicit a polyclonal B-cell response. Costimulation is provided by cytokines and members of the tumor necrosis factor superfamily (CD40-CD40L in T-cell–dependent responses and B-cell activating factor [BAFF]/B-lymphocyte stimulator [BLyS] or a proliferation-inducing ligand [APRIL] produced by dendritic cells in T-cell–independent responses). In the case of H pylori, T cells specific for H pylori epitopes provide help to B cells that recognize cross-reactive autoantigens present in the gastric mucosa such as fucosylated sialyl–Lewis X through CD40-CD40L costimulation. (B) Occurrence of genetic events such as p15 and p16 hypermethylation provide a selective advantage leading to the outgrowth of an antigen-responsive clone. Antigen dependence reflects the requirement for BCR signals such as NF-κB activation (see also Figure 5). (C) Progression toward antigen-independent (therefore antimicrobial-insensitive) MALT lymphoma is associated with the occurrence of additional genetic events. Chromosomal translocations involving MALT1 and Bcl-10 lead to a constitutive activation of NF-κB, bypassing the requirement for BCR-dependent signals (Figure 5). The t(11;18) translocation occurs early during B-cell proliferation and accelerates the transformation process in an antigen-independent fashion. The t(1;14) and t(14;18) translocations also lead to BCR-dependent NF-κB activation, but occur later after an antigen-dependent phase and can be associated with additional cytogenetic abnormalities. The t(3;14) translocation produces an IgH-FoxP1 transcript in 10% of MALT lymphomas that do not harbor other translocations involving the MALT1/Bcl-10 pathway. The precise role of t(3;14) is not known. P53 mutation is associated with transformation to high-grade lymphoma.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/8/10.1182_blood-2005-09-3679/4/m_zh80080694000001.jpeg?Expires=1765955722&Signature=2L3lCpzjlp~XxnQhd3~7NZ70Q~azna8RQtUE9VVwIGRUMhyYUr6TacFtxTrtFDU7pivQ50hCWuGvN5cSxndP9SLGdkY3hI2HhhVC98mI-sVD1an6ERGE38JZTwzXqCQsKhhlZy7P302dCDcejUcSzxJZlFvpIfA8u5GeR~UnkSeleO~qzC1X865WC-EyLbfKTkaqJHzY--PjICNrY9RaimXfyD3ygh0t4l-79Wh0lMN98ABTrpnjukn1q0nX~6Qfh7G6OULfSOSA0E6J1ox8mmo3Q0pVXh1agsjvkYPWOBkyaCyZNINB1lxneT00ZuJWuIqqlhbI54c8R5o5kEx~Mw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)