Abstract
Several studies have demonstrated that marrow stromal cells (MSCs) can suppress allogeneic T-cell responses. However, the effect of MSCs on syngeneic immune responses has been largely overlooked. We describe here that primary MSCs derived from C57BL/6 mice behave as conditional antigen-presenting cells (APCs) and can induce antigen-specific protective immunity. Interferon gamma (IFNγ)-treated C57BL/6 MSCs, but not unstimulated MSCs, cocultured with ovalbumin-specific major histocompatibility (MHC) class II-restricted hybridomas in the presence of soluble ovalbumin-induced significant production of interleukin-2 (IL-2) in an antigen dose-dependent manner (P < .005). IFNγ-treated MSCs could further activate in vitro ovalbumin-specific primary transgenic CD4+ T cells. C57BL/6 MSCs, however, were unable to induce antigen cross-presentation via the MHC class I pathway. When syngeneic mice were immunized intraperitoneally with ovalbumin-pulsed IFNγ-treated MSCs, they developed antigen-specific cytotoxic CD8+ T cells and became fully protected (10 of 10 mice) against ovalbumin-expressing E.G7 tumors. Human MSCs were also studied for antigen-presenting functions. IFNγ-treated DR1-positive human MSCs, but not unstimulated human MSCs, induced significant production of IL-2 when cocultured with DR1-restricted influenza-specific humanized T-cell hybridomas in the presence of purified influenza matrix protein 1. Taken together, our data strongly suggest that MSCs behave as conditional APCs in syngeneic immune responses. (Blood. 2006;107:2570-2577)
Introduction
Preclinical and clinical studies have demonstrated that bone marrow stromal cells (MSCs) can be used for tissue repair,1-4 delivery of therapeutic gene products,5-11 and to enhance engraftment of autologous peripheral blood stem cells.12 MSCs can differentiate along multiple cell lineages, including adipocytes, chondrocytes, osteocytes, myocytes, astrocytes, neurons, endothelial cells, and lung epithelial cells.1,6,13-16 MSCs are generally isolated based on their adherence to tissue-culture plates, resulting in a semihomogenous population characterized by the absence of CD45 and CD31 expression, and by the expression of CD105, CD73, and CD44.4 MSCs express low levels of major histocompatibility complex (MHC) class I molecules while, as a general rule, they do not constitutively express MHC class II molecules.17-19 One study, however, reported constitutive MHC class II expression on MSCs.20 Both MHC class I and class II molecules get up-regulated following interferon γ (IFNγ) treatment.17,21,22 Costimulatory molecules such as CD80, CD86, CD40, and CD40L are not known to be expressed nor induced on human MSCs, while mouse MSCs can be found to express CD80.22
MSCs are also known to secrete a wide spectrum of growth factors and cytokines implicated in different aspects of hematopoiesis2 and lymphopoiesis.23 One important feature of MSCs is their recently identified in vitro immunosuppressive properties against allogeneic immune responses.17-19,22,24-28 The immunosuppressive potential of MSCs has been exemplified by Le Blanc and colleagues,29 who reported in a case study that administration of haploidentical human MSCs following allogeneic stem cell transplantation could reverse the severe grade IV acute graft-versus-host disease (GVHD) of a patient. Recently, Zappia et al further demonstrated that administration of MSCs effectively ameliorates experimental autoimmune encephalomyelitis in mice.30 At present, the exact mechanism responsible for MSC-mediated immunosuppression remains imprecise. Soluble factors such as hepatocyte growth factor (HGF), transforming growth factor (TGF)-β1,19 indoleamine 2,3-dioxygenase (IDO),31 interleukin-10 (IL-10),27 and unidentified factors,18,24,32 as well as contact-dependent mechanisms,22,27 have been implicated.
If the immunosuppressive effects of MSCs on allogeneic or third-party immune responses have been well described, the effect of MSCs on syngeneic immune responses has been largely unexplored. To further characterize the effect of MSCs on autologous immunity, we investigated the immunomodulatory properties of MSCs during a syngeneic antigen-specific immune response. Unexpectedly, we observed that syngeneic MSCs behave as conditional antigen-presenting cells (APCs). We demonstrated that IFNγ can induce mouse MSCs to process and present antigenic peptides derived from a soluble xenoprotein (ovalbumin) and activate in vitro antigen-specific T cells. When injected in vivo into syngeneic mice, ovalbumin-pulsed IFNγ-treated MSCs induced potent ovalbumin-specific cellular immune responses and protected mice against ovalbumin-expressing tumors. We further demonstrated that human MSCs can also acquire antigen-presenting functions upon IFNγ stimulation. Taken together, our results strongly suggest that in syngeneic conditions, IFNγ-stimulated MSCs behave as conditional APCs able to activate antigen-specific immune responses.
Materials and methods
Animals and cell lines
Mice were 4- to 8-week-old female C57BL/6 or BALB/c purchased from Charles River (La Prairie, QC, Canada). C57BL/6 mouse embryonic fibroblasts, EL4 cells, and E.G7 cells were purchased from the American Type Culture Collection (ATTC; Manassas, VA). DC2.4 cells, MF2.2D9 cells, and RF33.70 cells have been described previously,33 and were a generous gift from Dr Ken L. Rock (University of Massachusetts, Worcester). C57BL/6 OT-II mice were kindly provided by Dr C. Piccirillo (McGill University, Montreal, QC, Canada). The anti-SIINFEKL/H2-Kb monoclonal antibody (mAb)-producing hybridoma 25D1.16 was a gift from Dr Ronald N. Germain (National Institutes of Health, Bethesda, MD).34 Synthetic SIINFEKL peptide was purchased from Sheldon Biotechnology Centre (McGill University). Purified influenza matrix protein 1 as well as humanized DR1-restricted influenza-specific T-T hybridomas have been described previously,35 and were a generous gift from Dr David Canaday (Case Western Reserve University, Cleveland, OH).
MSC harvest
Mouse MSCs were isolated from female C57BL/6 mice as previously described.11 Briefly, whole marrow from the femurs and tibias was flushed in Dulbecco modified Eagle medium (DMEM; Wisent, St-Bruno, QC, Canada), 10% fetal bovine serum (FBS; Wisent), and 50 U/mL Pen/Strep (Wisent Technologies), plated for 5 days, and washed; fresh media were added to the adherent cells every 3 to 4 days. When 80% confluent, adherent cells were trypsinized (0.05% Trypsin at 37°C for 5 minutes; Wisent Technologies), harvested, and expanded until a homogenous population was obtained (ie, approximately 20 population doublings) before being used for antigen presentation assays. Human MSCs were isolated as previously described.28 Briefly, whole marrow was collected from human patients, diluted in DMEM, added to a Ficoll gradient (Amersham Bioscience, Oakville, ON, Canada) and centrifuged at 900g for 30 minutes. Mononuclear cells were plated at 2 × 105 cells/cm2 on 10-cm2 tissue-culture dishes in DMEM, 10% FBS, and 50 U/mL Pen/Strep. The nonadherent cells were removed after 48 hours and media were replaced every 3 to 4 days. When 80% confluent, adherent cells were trypsinized (0.05% Trypsin at 37°C for 5 minutes), harvested, and expanded for a minimum of 10 population doublings before being used for flow cytometry analysis and antigen presentation assays. Human MSCs did not express CD45 or CD31, were positive for CD105 and CD73 surface expression, and could differentiate into osteogenic and adipogenic cells.
Differentiation of mouse MSCs
For osteogenic differentiation, MSCs were cultured in complete media supplemented with β-glycerol phosphate (10 mM), dexamethasone (10-8 M), and ascorbic acid 2-phosphate (5 μg/mL) (all from Sigma-Aldrich, Oakville, ON, Canada) for 4 weeks, renewing the media every 2 to 3 days. Alizarin Red S (2% [pH 4.1] in ammonium hydroxide) was then used to stain calcium in the mineralized extracellular matrix. To induce adipogenic differentiation, MSCs were cultured in complete media supplemented with indomethacin (46 μM), 3-isobutyl-methylxanthine (0.5 mM), dexamethasone (1 μM), and insulin (10 μg/mL) (all from Sigma-Aldrich) for 7 days, renewing the media twice. Oil Red O (Sigma-Aldrich) was used for lipid droplet staining.
Flow cytometry analysis
Flow cytometry analysis was performed in phosphate-buffered saline (PBS) with 2% FBS with the following mAbs: R-phycoerythrin (PE)-conjugated anti-mouse CD45 (clone 30-F11), H-2Kb (clone AF6-88.5), I-Ab (clone AF6-120.1), CD40 (clone 3/23), CD54 (clone 3E2), CD28 (clone 37.51; eBioscience, San Diego, CA), B7-DC (clone TY25; eBioscience), B7-H1 (clone MIH5; eBioscience), and 4-1BBL (clone TKS-1; eBioscience), as well as biotin-conjugated anti-mouse CD105 (clone MJ7/18; eBioscience), CD80 (clone 16-10A1), CD86 (clone PO3), and ICOS-L (clone HK5.3). Isotypic control analyses were performed in parallel. Except where indicated, Abs were from BD Pharmingen (San Diego, CA). Biotinylated Abs were revealed by allophycocyanin (APC)-streptavidin (BD Pharmingen). Flow cytometry was performed using a FACS Calibur cytometer (BD) and analyzed using Cellquest software (BD).
Two-way mixed lymphocyte cultures
In triplicate, 105 C57BL/6 splenocytes and 105 BALB/c splenocytes per well were cocultured in a round-bottom 96-well plate in 200 μL complete medium (RPMI with 10% FBS, 50 U/mL Pen-Strep) with or without 105 C57BL/6 MSCs, pretreated or not with recombinant mouse IFNγ (50 ng/mL; BioSource International, Camarillo, CA) for 20 hours followed by extensive washing in PBS. After 3 days, the cocultures were centrifugated and 100 μL of supernatant was collected for measurement of mouse IFNγ using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN).
Ovalbumin-specific T-T hybridoma assays
DC2.4 or mouse embryo fibroblasts (MEFs) (5 × 104 cells) were cocultured for 20 hours with 105 MF2.2D9 cells in flat-bottom 96-well plates with or without soluble ovalbumin (Sigma-Aldrich) at the indicated concentration in 200 μL complete media (RPMI with 10% FBS 50 U/mL Pen/Strep). Where indicated, 5 × 104 naive or IFNγ-treated MSCs (50 ng/mL for 20 hours) were added to the cocultures or in replacement of DC2.4 cells. Where indicated, recombinant mouse IFNγ was added to the cocultures (final 50 ng/mL). Where indicated, conditioned supernatant from naive or IFNγ-treated MSCs (50 ng/mL for 20 hours) were added to DC2.4 and MF2.2D9 cocultures. Where indicated, naive or IFNγ-pretreated MSCs were fixed in 1% paraformaldehyde, washed once with DMEM, once with 0.125M d-l lysine buffer for 30 minutes (Sigma-Aldrich), 4 times with DMEM, and then added to DC2.4 and MF2.2D9 cocultures. In some experiments, DC2.4 cells were first pulsed with soluble ovalbumin for 20 hours and then cocultured with the indicated cells for another 20 hours. Where indicated, MSCs were treated with chloroquine (100 μM; Sigma-Aldrich) 30 minutes prior to and during antigen exposure. After 20 hours, supernatant was collected from the cocultures and tested for the presence of IL-2 by commercial ELISA (eBioscience).
OT-II antigen presentation assays
C57BL/6 MSCs or DC2.4 were first pretreated with recombinant mouse IFNγ (50 ng/mL) and soluble ovalbumin (2.5 mg/mL) for 20 hours. The next day, ovalbumin-specific CD4+ T cells were isolated from the spleens and lymph nodes of transgenic OT-II mice using the SpinSep kit following the manufacturer's instructions (Stem Cell Technologies, Vancouver, BC, Canada). IFNγ-treated ovalbumin-pulsed DC2.4 or MSCs (5 × 104 cells) were then cocultured for 48 hours with purified CD4+ OT-II cells in flat-bottom 96-well plates in 200 μL complete media (RPMI with 10% FBS, 50 U/mL Pen/Strep). Where indicated, purified anti-mouse CD80 (clone 16-10A1) or isotype control Abs (50 μg/mL; BD Pharmingen) were added to the MSCs or DC2.4 30 minutes prior to and during coculture with OT-II cells.
In vivo immunization of mice
C57BL/6 MSCs or MEF cells were treated in vitro with recombinant IFNγ (50 ng/mL) and soluble ovalbumin (2.5 mg/mL) for 20 hours, washed with PBS, and injected (0.1 × 106 cells) intraperitoneally into syngeneic C57BL/6 mice. Two weeks later, the same mice were injected a second time (0.2 × 106 cells) and 1 week after, serum samples and splenocytes of immunized mice were collected. For antibody titering, serum samples were diluted in PBS, incubated for 2 hours at 37°C onto ovalbumin-coated (10 μg/mL) 96-well plates and revealed using anti-mouse immunoglobulin-horseradish peroxidase (Ig-HRP) antibody (1: 1000 in PBS with 10% FBS; BD Pharmingen) and TMB substrate (eBioscience). For cytotoxic T-cell (CTL) assays, 50 × 106 pooled splenocytes from immunized mice were restimulated in vitro with 106 Mitomycin-C (Sigma-Aldrich)-treated E.G7 cells in complete media (RPMI with 10% FBS 50 U/mL Pen/Strep, 50 μM β-mercaptoethanol) for 5 days. Then, CD8+ T cells were purified using the SpinSep kit and used as effectors in annexin-V-based CTL assays against 5 × 104 PKH26-labeled (Sigma-Aldrich) EL4 or E.G7 targets and analyzed by flow cytometry as previously described.11,36
Human MSC antigen presentation assay
Human MSCs were human leukocyte antigen (HLA)-typed (Montreal Royal Victoria Hospital), and DR1-positive MSCs were used in antigen presentation assays. Where indicated, human MSCs were pretreated for 24 hours with recombinant human IFNγ (100 ng/mL; InterMune Pharmaceuticals, Brisbane, CA) and subsequently cocultured for 24 hours with influenza matrix protein 1-specific DR1-restricted T-cell hybridomas and/or 100 μg/mL purified influenza matrix protein 1 in complete media (RPMI with 10% FBS 50 U/mL Pen/Strep). After coculture, supernatant was collected and tested for mouse IL-2 release by ELISA (eBioscience).
Results
Phenotypic characterization of primary MSCs
Primary MSCs were isolated from C57BL/6 mice as previously described.10,11 Cultured in differentiation media, MSCs were able to give rise to osteogenic and adipogenic cells (data not shown). Phenotypically, MSCs were negative for CD45, CD31, CD54, CD86, and CD40 expression and were positive for CD105, MHC class I (H-2Kb), and CD80 expression as determined by flow cytometry. When exposed to recombinant mouse IFNγ (50 ng/mL) for 20 hours, MSCs up-regulated MHC class I, MHC class II, and CD54, but not CD80, while they remained negative for CD86, CD40, and CD45 expression (Figure S1 on the Blood website; see the Supplementary Figures link at the top of the online article).
Immunosuppressive effects of MSCs
We evaluated the immunosuppressive effect of C57BL/6-dervied mouse MSCs on allogeneic mixed lymphocyte cultures. In accordance with previous studies,22 the addition of MSCs to allogeneic cocultures of C57BL/6 and BALB/c splenocytes significantly inhibited the activation levels of the cocultures (P < .05 by t test; Figure 1A). Also consistent with previous studies,17 prestimulation of MSCs with recombinant IFNγ did not hinder their allogeneic immunosuppressive effect (P > .05 by t test; Figure 1A). To test whether MSCs could suppress syngeneic immune responses, we used a previously described ovalbumin-specific T-T hybridoma assay.33 In this assay, immortalized dendritic cells (DC2.4 cells) are cocultured for 20 hours with syngeneic MHC class II-restricted ovalbumin-specific T-T hybridomas (MF2.2D9 cells) in the presence of increasing doses of soluble ovalbumin. Twenty hours later, antigen-specific T-cell activation was assessed by measuring the level of IL-2 released in the supernatant. When soluble ovalbumin was added to DC2.4 and MF2.2D9 cocultures, significant levels of IL-2 were produced as determined by ELISA (Figure 1B). The addition of syngeneic MSCs to these cocultures significantly inhibited IL-2 release (P < .05 by t test, performed twice; Figure 1B). In contrast, the addition of IFNγ-treated syngeneic MSCs significantly enhanced IL-2 release. By comparison, the addition of IFNγ-treated allogeneic MSCs inhibited IL-2 release (P < .05 by t test; Figure 1B). Because IFNγ did not, on its own, induce syngeneic MSCs to release IL-2 (data not shown), this suggested that IFNγ modulated the syngeneic MSCs to become permissive to T-cell activation. We thus performed experiments to assess the nature of this permissiveness. Specifically, we wanted to determine whether IFNγ-treated MSCs enhanced or failed to suppress DC2.4-mediated antigen presentation. First, we assessed the effect of conditioned supernatant from naive or IFNγ-treated MSCs on DC2.4-mediated antigen presentation. As shown in Figure 1C, conditioned supernatant from naive or IFNγ-treated MSCs had no significant effect on DC2.4-mediated antigen presentation (P > .05 by t test). This suggested that IFNγ-treated MSCs did not enhance DC2.4 antigen presentation through a secreted factor. Secondly, we tested whether IFNγ-treated MSCs enhanced DC2.4 antigen presentation in a contact-dependent manner. As shown in Figure 1D, both naive MSCs and IFNγ-treated MSCs fixed with paraformaldehyde similarly suppressed DC2.4 antigen presentation (P < .05 by t test). Taken together, our data suggested that IFNγ treatment induced MSCs to play an active role in the activation of the hybridomas. We therefore hypothesized that IFNγ induced MSCs to acquire antigen-presenting functions on their own.
Effect of MSCs on allogeneic and syngeneic immune responses. (A) Two-way mixed lymphocyte reactions were performed with 105 C57BL/6 splenocytes and 105 BALB/c splenocytes in the presence or absence of 105 C57BL/6 naive or IFNγ-treated MSCs.After 3 days, supernatant was collected and tested for IFNγ release by ELISA. (B) DC2.4 cells (5 × 104 cells) were cocultured for 20 hours with ovalbumin-specific MHC class II-restricted T-T hybridomas (MF2.2D9; 105 cells) in the presence or not of 2.5 mg/mL soluble ovalbumin. Where indicated, 5 × 104 naive or IFNγ-pretreated (20 hours) MSCs from C57BL/6 or BALB/c mice were added to the cocultures. After 20 hours, supernatant was collected and tested for IL-2 release by ELISA. (C) Same as panel B, and where indicated, conditioned supernatant from naive or IFNγ-treated MSCs were added to the cocultures in replacement of MSCs. (D) Same as panel B, and where indicated, 5 × 104 naive or IFNγ-pretreated paraformaldehyde-fixed MSCs were added to the cocultures in the presence of ovalbumin (▪). Alternatively, DC2.4 cells were first pulsed with soluble ovalbumin for 20 hours and then cocultured with the indicated cells for another 20 hours (▤). Means of triplicates ± standard deviations of 1 of 2 representative experiments are shown.
Effect of MSCs on allogeneic and syngeneic immune responses. (A) Two-way mixed lymphocyte reactions were performed with 105 C57BL/6 splenocytes and 105 BALB/c splenocytes in the presence or absence of 105 C57BL/6 naive or IFNγ-treated MSCs.After 3 days, supernatant was collected and tested for IFNγ release by ELISA. (B) DC2.4 cells (5 × 104 cells) were cocultured for 20 hours with ovalbumin-specific MHC class II-restricted T-T hybridomas (MF2.2D9; 105 cells) in the presence or not of 2.5 mg/mL soluble ovalbumin. Where indicated, 5 × 104 naive or IFNγ-pretreated (20 hours) MSCs from C57BL/6 or BALB/c mice were added to the cocultures. After 20 hours, supernatant was collected and tested for IL-2 release by ELISA. (C) Same as panel B, and where indicated, conditioned supernatant from naive or IFNγ-treated MSCs were added to the cocultures in replacement of MSCs. (D) Same as panel B, and where indicated, 5 × 104 naive or IFNγ-pretreated paraformaldehyde-fixed MSCs were added to the cocultures in the presence of ovalbumin (▪). Alternatively, DC2.4 cells were first pulsed with soluble ovalbumin for 20 hours and then cocultured with the indicated cells for another 20 hours (▤). Means of triplicates ± standard deviations of 1 of 2 representative experiments are shown.
Activation of MHC class II-restricted hybridomas by IFNγ-treated MSCs
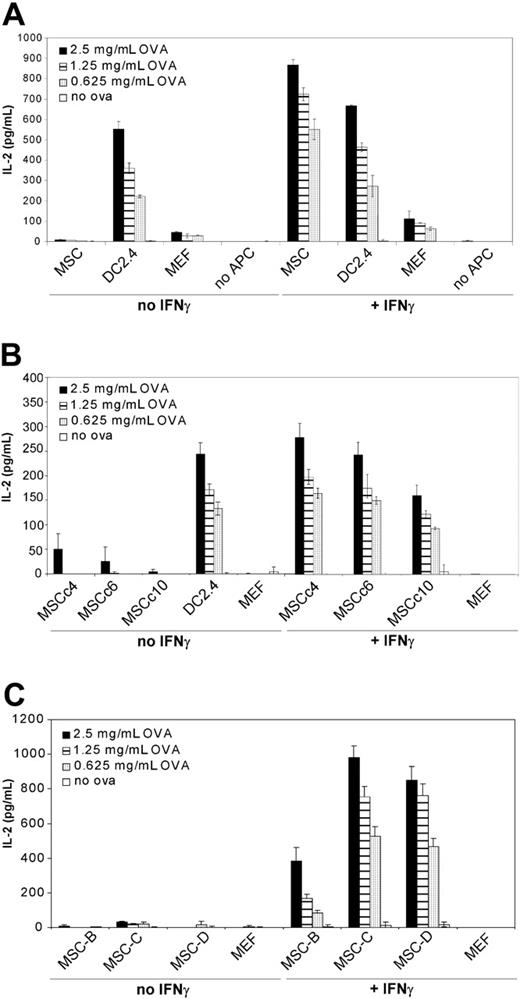
To investigate whether IFNγ-treated MSCs could behave as syngeneic antigen-presenting cells, soluble ovalbumin was added at increasing doses to cocultures of IFNγ-treated MSCs and MHC class II-restricted ovalbumin-specific T-T hybridoma cells. When IFNγ-treated MSCs were exposed to soluble ovalbumin at doses of 2.5, 1.25, and 0.625 mg/mL and cocultured for 20 hours with class II-restricted hybridomas, significant levels of IL-2 were detected in the supernatants as measured by ELISA (867, 722, and 551 pg/mL of IL-2, respectively; Figure 2A). On the other hand, unstimulated MSCs failed to induce IL-2 release in identical conditions. IL-2 levels were below sensitivity of the assay (< 2 pg/mL) when IFNγ-treated MSCs were cocultured with hybridomas without ovalbumin, or when the hybridomas were cultured with ovalbumin without MSCs. This experiment was performed 5 times, each in triplicate, with similar results.
To rule out the possibility that the observed MSC-mediated antigen presentation was the result of an idiosyncratic effect, distinct clonal (Figure 2B) and polyclonal (Figure 2C) populations of C57BL/6-derived MSCs were tested with comparable results. Phenotypically, the distinct MSC populations were very similar (Figure S1), with the exception of MSC clone 10, which constitutively expressed MHC class II and failed to up-regulate MHC class II upon IFNγ treatment.
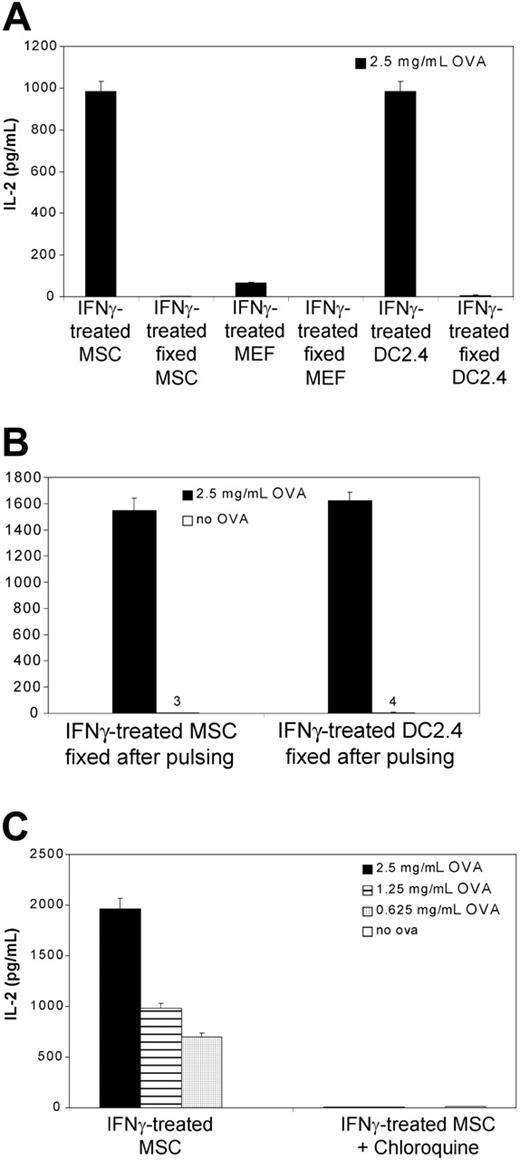
To exclude the possibility that free peptides in the ovalbumin preparation could have mediated antigen presentation in the absence of antigen processing as others have reported,37 the above-mentioned experiments were repeated using paraformaldehyde-prefixed MSCs subsequently exposed to ovalbumin. As shown in Figure 3A, prefixed IFNγ-treated MSCs did not induce IL-2 release when cocultured with hybridomas and ovalbumin. On the other hand, IFNγ-treated MSCs fixed with paraformaldehyde after ovalbumin pulsing retained their antigen-presenting properties (Figure 3B). This suggested that processing of ovalbumin is required for MSCs-mediated antigen presentation. To assess whether processing of ovalbumin was the result of endosomal protein proteolysis,33 we treated MSCs with chloroquine. Chloroquine is known to prevent protein hydrolysis by raising the pH in the endosomal and lysosomal compartments.38 As shown in Figure 3C, treatment with chloroquine inhibited the presentation of ovalbumin peptides on MHC class II molecules.
MSC-mediated activation of ovalbumin-specific T-T hybridomas. (A) C57BL/6 MSCs, DC2.4 or MEF (5 × 104 cells) were cocultured for 20 hours with ovalbumin-specific MHC class II-restricted T-T hybridomas (MF2.2D9; 105 cells) in the presence of increasing doses of soluble ovalbumin. Where indicated, recombinant mouse IFNγ (50 ng/mL final) was added to the cocultures. After 20 hours, supernatant was collected and tested for IL-2 release by ELISA. Means of triplicates ± standard deviations of 1 of 5 representative experiments are shown. (B) Same as panel A, except that clonal MSCs obtained by limiting dilution from the initial preparation were used. (C) Same as panel A, except that distinct polyclonal C57BL/6-derived MSC preparations were used. Means of triplicates ± standard deviations of 1 of 2 representative experiments are shown.
MSC-mediated activation of ovalbumin-specific T-T hybridomas. (A) C57BL/6 MSCs, DC2.4 or MEF (5 × 104 cells) were cocultured for 20 hours with ovalbumin-specific MHC class II-restricted T-T hybridomas (MF2.2D9; 105 cells) in the presence of increasing doses of soluble ovalbumin. Where indicated, recombinant mouse IFNγ (50 ng/mL final) was added to the cocultures. After 20 hours, supernatant was collected and tested for IL-2 release by ELISA. Means of triplicates ± standard deviations of 1 of 5 representative experiments are shown. (B) Same as panel A, except that clonal MSCs obtained by limiting dilution from the initial preparation were used. (C) Same as panel A, except that distinct polyclonal C57BL/6-derived MSC preparations were used. Means of triplicates ± standard deviations of 1 of 2 representative experiments are shown.
Antigen processing for MSC-mediated antigen presentation. (A) C57BL/6 MSCs, DC2.4, or MEFs (5 × 104 cells) were cocultured for 20 hours with ovalbumin-specific MHC class II-restricted T-T hybridomas (MF2.2D9; 105 cells) in the presence of 2.5 mg/mL soluble ovalbumin. Where indicated, MSCs were treated with IFNγ (50 ng/mL final). Where indicated, MSCs were first fixed with paraformaldehyde prior to coculture. After 20 hours, supernatant was collected and tested for IL-2 release by ELISA. (B) C57BL/6 MSCs or DC2.4 (5 × 104 cells) were first incubated with soluble ovalbumin (2.5 mg/mL) and IFNγ (50 ng/mL final) for 20 hours, then fixed with paraformaldehyde and cocultured for 20 hours with MF2.2D9 hybridomas (105 cells). (C) MSCs (5 × 104 cells) were cocultured for 20 hours with MF2.2D9 cells (105 cells) in the presence of increasing doses of soluble ovalbumin. Where indicated, MSCs were treated with chloroquine (100 μM) 30 minutes prior to and during antigen exposure. Means of triplicates ± standard deviations of 1 of 2 representative experiments are shown.
Antigen processing for MSC-mediated antigen presentation. (A) C57BL/6 MSCs, DC2.4, or MEFs (5 × 104 cells) were cocultured for 20 hours with ovalbumin-specific MHC class II-restricted T-T hybridomas (MF2.2D9; 105 cells) in the presence of 2.5 mg/mL soluble ovalbumin. Where indicated, MSCs were treated with IFNγ (50 ng/mL final). Where indicated, MSCs were first fixed with paraformaldehyde prior to coculture. After 20 hours, supernatant was collected and tested for IL-2 release by ELISA. (B) C57BL/6 MSCs or DC2.4 (5 × 104 cells) were first incubated with soluble ovalbumin (2.5 mg/mL) and IFNγ (50 ng/mL final) for 20 hours, then fixed with paraformaldehyde and cocultured for 20 hours with MF2.2D9 hybridomas (105 cells). (C) MSCs (5 × 104 cells) were cocultured for 20 hours with MF2.2D9 cells (105 cells) in the presence of increasing doses of soluble ovalbumin. Where indicated, MSCs were treated with chloroquine (100 μM) 30 minutes prior to and during antigen exposure. Means of triplicates ± standard deviations of 1 of 2 representative experiments are shown.
CD80-dependent activation of OT-II cells by IFNγ-treated MSCs
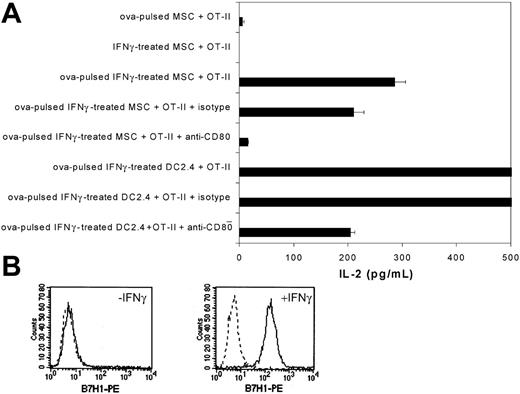
We next assessed whether IFNγ-treated mouse MSCs could activate primary transgenic T cells. Ovalbumin-specific CD4+ T cells were isolated from the spleens and lymph nodes of transgenic OT-II mice and purified by negative selection (> 80% purity; data not shown). When purified CD4+ OT-II cells were cocultured for 48 hours with ovalbumin-pulsed IFNγ-treated MSCs, we observed significant levels of IL-2 production (Figure 4A). We then investigated whether CD80 expression on mouse MSCs was required for OT-II activation. As shown in Figure 4A, the addition of a blocking antibody to CD80 inhibited by 90% the activation of CD4+ OT-II cells (P < .05 by t test).
B7-H1 expression is induced on mouse MSCs following IFNγ treatment
We investigated by flow cytometry the expression levels of other costimulatory molecules on naive and IFNγ-stimulated MSCs. Unstimulated as well as IFNγ-treated mouse MSCs were found to be negative for CD86, CD40, CD28, ICOSL, 41BBL, and B7-DC surface expression (data not shown). However, after IFNγ stimulation, mouse MSCs robustly up-regulated surface expression of B7-H1 molecules (Figure 4B).
MSC-mediated activation of primary OT-II CD4+ T cells. (A) C57BL/6 MSCs or DC2.4 were pretreated with recombinant mouse IFNγ (50 ng/mL) and soluble ovalbumin (2.5 mg/mL) for 20 hours and then cocultured (5 × 104 cells) for 48 hours with ovalbumin-specific purified CD4+ T splenocytes (105 cells; > 80% purity) from OT-II transgeneic mice. Where indicated, MSCs and DC2.4 were first incubated with a blocking antibody to mouse CD80 or an isotypic control 30 minutes prior to and during coculture. After coculture, supernatant was collected and tested for IL-2 release by ELISA. Means of triplicates ± standard deviations of 1 of 2 representative experiments are shown. (B) C57BL/6 MSCs were analyzed by flow cytometry for B7-H1 surface expression before and after recombinant IFNγ treatment (50 ng/mL for 20 hours). Plots show isotype control IgG staining profile (broken line) versus specific Ab staining profile (solid line).
MSC-mediated activation of primary OT-II CD4+ T cells. (A) C57BL/6 MSCs or DC2.4 were pretreated with recombinant mouse IFNγ (50 ng/mL) and soluble ovalbumin (2.5 mg/mL) for 20 hours and then cocultured (5 × 104 cells) for 48 hours with ovalbumin-specific purified CD4+ T splenocytes (105 cells; > 80% purity) from OT-II transgeneic mice. Where indicated, MSCs and DC2.4 were first incubated with a blocking antibody to mouse CD80 or an isotypic control 30 minutes prior to and during coculture. After coculture, supernatant was collected and tested for IL-2 release by ELISA. Means of triplicates ± standard deviations of 1 of 2 representative experiments are shown. (B) C57BL/6 MSCs were analyzed by flow cytometry for B7-H1 surface expression before and after recombinant IFNγ treatment (50 ng/mL for 20 hours). Plots show isotype control IgG staining profile (broken line) versus specific Ab staining profile (solid line).
MSCs cannot induce antigen cross-presentation
We further tested whether mouse MSCs could induce activation of MHC class I-restricted hybridomas in response to soluble ovalbumin. This experiment essentially measured the ability of MSCs to induce cross-presentation of exogenous antigens. While DC2.4 cells induced significant antigen cross-presentation as previously shown,33 unstimulated and IFNγ-stimulated MSCs could not induce IL-2 release (Figure S2). To determine whether MSCs could still process exogenous ovalbumin into MHC class I-associated peptides without inducing IL-2 production, we performed flow cytometry analysis of ovalbumin-pulsed MSCs using a monoclonal antibody specific for the SIINFEKL/H-2Kb complex (clone 25D1.16).34 While the antibody positively labeled IFNγ-treated MSCs pulsed with 10 μM of the synthetic SIINFEKL peptide, unstimulated as well as IFNγ-stimulated MSCs pulsed with soluble ovalbumin were not detected by the antibody (data not shown). Our results therefore suggested that mouse MSCs cannot perform exogenous antigen cross-presentation via the MHC class I pathway.
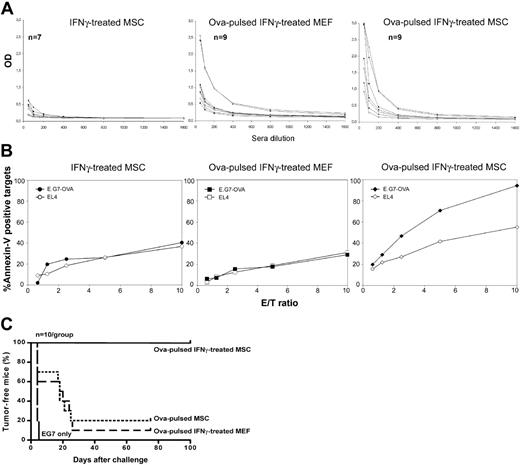
MSC-induced antigen-specific immune responses in vivo. C57BL/6 MSCs or MEF cells were treated in vitro with recombinant IFNγ and soluble ovalbumin for 20 hours, washed with PBS, and injected (0.1 × 106 cells) intraperitoneally into syngeneic C57BL/6 mice. Two weeks later, the mice were injected a second time with the corresponding cells (0.2 × 106) and 1 week after, ovalbumin-specific immune responses were assessed. (A) Serum samples of immunized mice were collected at day 20 after the first immunization, added at different dilutions to ovalbumin-coated 96-well plates and titered for antiovalbumin anti-bodies. (B) Splenocytes were isolated from immunized mice at day 21 after the first immunization and restimulated in vitro with mitomycin C-treated ovalbumin-expressing E.G7 cells. Five days later, CD8+ T cells were purified from the reactivated splenocytes (> 90% purity) and used as effectors in annexin-V-based CTL assays against EL4 or E.G7 target cells (1 of 2 representative experiments is shown). (C) Immunized mice were challenged at day 21 after the first immunization with a subcutaneous injection of 2 × 106 ovalbumin-expressing E.G7 tumor cells.
MSC-induced antigen-specific immune responses in vivo. C57BL/6 MSCs or MEF cells were treated in vitro with recombinant IFNγ and soluble ovalbumin for 20 hours, washed with PBS, and injected (0.1 × 106 cells) intraperitoneally into syngeneic C57BL/6 mice. Two weeks later, the mice were injected a second time with the corresponding cells (0.2 × 106) and 1 week after, ovalbumin-specific immune responses were assessed. (A) Serum samples of immunized mice were collected at day 20 after the first immunization, added at different dilutions to ovalbumin-coated 96-well plates and titered for antiovalbumin anti-bodies. (B) Splenocytes were isolated from immunized mice at day 21 after the first immunization and restimulated in vitro with mitomycin C-treated ovalbumin-expressing E.G7 cells. Five days later, CD8+ T cells were purified from the reactivated splenocytes (> 90% purity) and used as effectors in annexin-V-based CTL assays against EL4 or E.G7 target cells (1 of 2 representative experiments is shown). (C) Immunized mice were challenged at day 21 after the first immunization with a subcutaneous injection of 2 × 106 ovalbumin-expressing E.G7 tumor cells.
IFNγ-treated MSCs pulsed with soluble ovalbumin induced antigen-specific in vivo immune responses
Next, we investigated the ability of IFNγ-treated MSCs to induce antigen-specific in vivo immune responses. Polyclonal MSCs or control MEFs (both from C57BL/6 origin) were stimulated with recombinant IFNγ and soluble ovalbumin, IFNγ only, or ovalbumin only for 20 hours, washed with PBS, and injected intraperitoneally into syngeneic C57BL/6 mice. Two weeks later, the mice were injected a second time with the corresponding cells, and 1 week after, ovalbumin-specific immune responses were assessed. First, we assessed whether mice injected with ovalbumin-pulsed IFNγ-treated MSCs could generate antiovalbumin antibodies. Although few mice developed antiovalbumin antibodies, we observed no significant differences between MSC-injected versus MEF-injected mice (Figure 5A). Secondly, we investigated whether mice injected with ovalbumin-pulsed IFNγ-treated MSCs could generate ovalbumin-specific cytotoxic T lymphocytes (CTLs). For this, splenocytes from immunized mice were restimulated in vitro with mitomycin C-treated ovalbumin-expressing E.G7 cells, and 5 days later, CD8+ T cells were isolated by negative selection (> 90% purity; data not shown) and used as effectors in annexin-V-based CTL assays. Mice immunized with ovalbumin-pulsed IFNγ-treated MSCs developed a significant CD8+ ovalbumin-specific cytotoxic response (Figure 5B). This experiment was repeated once with similar results. To test whether MSC-mediated immunization induced systemic protective immunity, immunized mice were challenged with a subcutaneous injection of a tumorigenic dose (2 × 106 cells) of ovalbumin-expressing E.G7 tumor cells. Strikingly, 10 of 10 mice immunized with ovalbumin-pulsed IFNγ-treated MSCs were fully protected against E.G7 tumors (Figure 5C). In contrast, 1 of 10 mice immunized with ovalbumin-pulsed IFNγ-treated MEF cells was protected (P < .001 by log-rank test). Taken together, our data indicated that mouse MSCs can induce protective in vivo antigen-specific cellular immune responses.
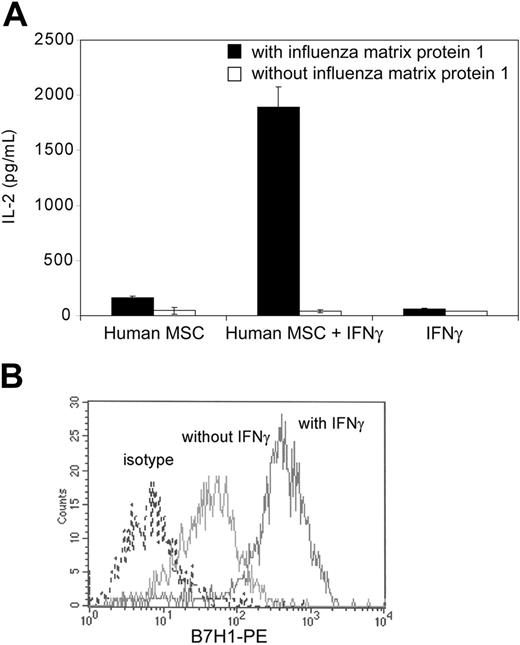
Activation of MHC class II-restricted hybridomas by IFNγ-treated human MSCs
We assessed the ability of human MSCs to acquire antigen-presenting functions following IFNγ stimulation. Human MSCs were isolated from healthy donors, culture expanded, and characterized by flow cytometry. Polyclonal MSCs populations from donors were HLA-typed, and the cells derived from an HLA class II DR1+ individual were used in the following experiments. When DR1+ IFNγ-treated human MSCs were cocultured for 24 hours with influenza matrix protein 1-specific DR1-restricted T-cell hybridomas in the presence of purified influenza matrix protein 1, significant levels of IL-2 were detected in the supernatant (Figure 6A). This indicated that IFNγ-stimulated human MSCs can efficiently process exogenous antigens and present antigen-derived peptides to MHC class II-restricted T cells. Human MSCs also significantly up-regulated surface expression of the costimulatory molecule B7-H1 upon IFNγ stimulation (Figure 6B). Taken together, our data suggested that human MSCs may behave as conditional antigen-presenting cells in syngeneic immune responses.
Human MSC-mediated activation of influenza matrix protein 1-specific T-T hybridomas. (A) Bone marrow-derived DR1-positive human MSCs were treated or not for 24 hours with recombinant human IFNγ (100 ng/mL) and subsequently cocultured for 24 hours with influenza matrix protein 1-specific DR1-restricted T-cell hybridomas with or without 100 μg/mL purified influenza matrix protein 1. Supernatant was collected and tested for IL-2 release by ELISA. Means of duplicates ± standard deviations of 1 of 2 representative experiments are shown. (B) Human MSCs were analyzed by flow cytometry for B7-H1 surface expression before and after recombinant IFNγ treatment (100 ng/mL for 24 hours). Plots show isotype control IgG staining profile (broken line) versus specific Ab staining profile (dark line).
Human MSC-mediated activation of influenza matrix protein 1-specific T-T hybridomas. (A) Bone marrow-derived DR1-positive human MSCs were treated or not for 24 hours with recombinant human IFNγ (100 ng/mL) and subsequently cocultured for 24 hours with influenza matrix protein 1-specific DR1-restricted T-cell hybridomas with or without 100 μg/mL purified influenza matrix protein 1. Supernatant was collected and tested for IL-2 release by ELISA. Means of duplicates ± standard deviations of 1 of 2 representative experiments are shown. (B) Human MSCs were analyzed by flow cytometry for B7-H1 surface expression before and after recombinant IFNγ treatment (100 ng/mL for 24 hours). Plots show isotype control IgG staining profile (broken line) versus specific Ab staining profile (dark line).
Discussion
The use of MSCs for regenerative and transgeneic cell therapy is generating promising preclinical and clinical results.5,39-45 The rising interest in MSC-based therapies comes from their plasticity and because they can be easily harvested and expanded to clinically relevant numbers.After allogeneic transplantation, MSCs have been described as inducing suppression of allogeneic immune responses.37 In autologous conditions, however, the immune modulatory effects of MSCs are less well defined. In this article, we investigated the immune modulatory properties of MSCs during syngeneic antigen-specific immune responses. Specifically, we studied the effect of MSCs on the syngeneic activation, in vitro and in vivo, of ovalbumin-specific immune responses using previously described ovalbumin-specific mouse T-cell activation assays. MSCs were isolated from C57BL/6 mice and characterized in vitro prior to assessing their immune modulatory effects. Functional characterization of the cells confirmed an MSC phenotype as demonstrated by mesenchymal plasticity and immunosuppressive effects when cocultured with allogeneic mixed lymphocytes in vitro. Flow cytometry analysis of the isolated MSCs was also in agreement with their reported phenotype.4,22
We first assessed the effect of syngeneic MSCs on dendritic cell (DC)-mediated T-cell hybridoma activation. We observed that in the absence of inflammatory stimuli, mouse MSCs significantly suppressed DC-mediated syngeneic T-cell activation. This suppressive effect was not significantly affected by paraformaldehyde fixation of the MSCs, nor could it be reproduced by conditioned media from MSCs, suggesting a pathway relying mainly on cell-contact mechanism. We cannot exclude, however, that soluble factor(s) induced by the interaction of MSCs with T cells might also be implicated, as others have suggested.24 We next investigated whether IFNγ could modulate the immune properties of MSCs. IFNγ is known to up-regulate MHC class I and induce MHC class II expression on MSCs.4 Strikingly, IFNγ-treated syngeneic MSCs, but not IFNγ-treated allogeneic MSCs, were permissive to DC-mediated T-cell activation as determined by IL-2 release. When prefixed with paraformaldehyde, IFNγ-treated syngeneic MSCs lost this permissiveness to antigen presentation. We thus hypothesized that IFNγ directly induced MSCs to acquire antigen-presenting functions. Using 2 distinct models (ie, ovalbumin-specific T-T hybridomas and primary transgenic OT-II activation assays), we demonstrated that IFNγ-treated C57BL/6-derived MSCs can: (1) efficiently process, via endocytosis, exogenous ovalbumin and present ovalbumin-derived peptides on MHC class II molecules; (2) efficiently activate, mainly in a CD80-dependent manner, MHC class II-restricted CD4+ T cells inducing IL-2 release; (3) do not induce exogenous antigen cross-presentation via the MHC class I pathway; (4) efficiently induce in vivo antigen-specific CD8+ T cells; and (5) efficiently induce cellular protective immunity against ovalbumin-expressing tumors when injected as a syngeneic cellular vaccine. We thus provide experimental evidence that IFNγ-treated MSCs can process exogeneous antigens and efficiently activate in vitro and efficiently induce in vivo antigen-specific immune responses. Our observation that MSCs can induce a strong CTL response in vivo despite being unable to perform cross-presentation in vitro suggests a role for host APCs in the generation of CTL response. Indeed, host APCs are known to internalize and present exogenous antigens acquired from other cell types, a phenomenon known as cross-priming.46 The implication of host APCs in our experiments is supported by the observation that ovalbumin-pulsed non-APC cells such as MEFs can induce a specific, albeit limited, immune response protecting 10% of mice against a tumor challenge (Figure 5). However, effective cross-priming of CTLs and subsequent secondary expansion of CTLs upon antigen re-encounter are dependent upon proper activation of CD4 helper T cells.47 We thus propose that CD4 T-cell activation by MSCs enhances host-derived CTL cross-priming, resulting in the generation of strong antigen-specific protective immunity.
MSCs isolated from different preparations, as well as distinct clonal MSCs populations were equally effective at activating ovalbumin-specific T-T hybridomas. Of particular interest, we observed that one of the clonal MSCs population (clone 10) expressed constitutively low levels of MHC class II and CD80 molecules, but failed to up-regulate these molecules upon IFNγ stimulation. Regardless, MSC clone 10 acquired antigen-presenting properties upon IFNγ stimulation, suggesting that contact-dependent molecule(s)—distinct from MHC class I, class II, and CD80—were up-regulated by IFNγ and implicated in MSC-mediated antigen presentation. This hypothesis was strengthened by the fact that blocking CD80 costimulation partially—rather than totally—inhibited OT-II activation. When we investigated by flow cytometry the surface expression levels of other known costimulatory molecules on naive and IFNγ-stimulated MSCs, we found MSCs to be consistently negative for CD86, CD40, ICOSL, 41BBL, and B7-DC surface expression. However, after IFNγ stimulation, every population of MSCs tested, including human MSCs, robustly up-regulated surface expression of B7-H1 molecules. The exact immune functions of B7-H1 are not fully understood and we can only hypothesize, at the moment, on its role during MSCs-mediated antigen presentation. B7-H1 (PD-L1) belongs to the B7 family members and is a ligand for programmed cell death-1 (PD-1) receptor expressed on activated T, B, and myeloid cells.48 B7-H1 is expressed on resting and up-regulated on activated T, B, myeloid, and DCs, and can be expressed on endothelial cells and other nonlymphoid organs.49-51 While B7-H1-/- mice suggest an essential role for B7-H1 in negatively regulating T-cell activation,52,53 other studies have demonstrated that B7-H1 expression can provide positive costimulation for T-cell priming in vitro and in vivo.54,55
An important aspect of our studies is the observation that human MSCs can also acquire antigen-presenting functions, strongly suggesting that both mouse and human MSCs behave as conditional antigen-presenting cells. We made use of a previously described transgenic mouse T-cell hybridoma that is restricted to human HLA-DR1 and specific for influenza matrix protein 1-derived peptides to study APC-like properties of human MSCs. Our results suggested that human MSCs can: (1) efficiently process soluble influenza matrix protein 1; (2) efficiently present influenza matrix protein 1-derived peptides on MHC class II molecules; and (3) efficiently activate antigen-specific T-cell hybridomas as determined by IL-2 release. It remains to be determined, however, whether human MSCs can provide proper T-cell costimulation in vivo.
In summary, our data suggest that MSCs possess a previously unrecognized dichotomy in their role as immune modulators, distinctively affecting allogeneic and syngeneic immune responses. We propose that MSCs constitute a novel subset of nonhematologic APCs. Few other cell types have been described to possess similar functions in the presence of proinflammatory stimuli, including vascular endothelial cells,56-58 keratinocytes,59,60 and enterocytes.61 An important aspect of the biology of MSCs that will further need investigation is whether MSC-mediated antigen presentation actually occurs in the bone marrow and, if so, whether it plays a significant role during endogenous immune responses. As the bone marrow is being revealed as a unique lymphoid organ able to activate naive T cells and to induce systemic immunity, in some cases more efficiently than peripheral lymph nodes,62,63 MSCs may represent a previously unrecognized player of physiologic immune responses.
Finally, the unique immune modulation afforded by MSCs in the autologous and allogeneic transplantation setting could have important repercussions in the development of MSC-based therapies. For instance, genetically engineered MSCs used for autologous transplantation in regenerative medicine may trigger potent immune rejection of these APC-like cells following inflammatory reactions. The APC-like properties of MSCs should also be taken into account in the development of immunosuppressive strategies based on MSC transplantation for treatment of GVHD. On the other hand, autologous transplantation of antigen-pulsed or genetically engineered IFNγ-treated MSCs could be profitably used to stimulate therapeutic antitumor or anti-infectious immune responses. While DCs are routinely studied in clinical trials for this purpose, MSCs may possess distinct APC-like properties inducing qualitatively divergent immune responses. Our in vivo observation that MSC-mediated immune responses were greatly biased toward a cellular type 1 response with minimal humoral response supports this hypothesis. In conclusion, MSCs have spurred much interest due to their mesenchymal plasticity. We show here that their immunologic plasticity merits a fresh introspective into their role in the physiologic immune response in health and disease and the harnessing of their unique properties for treatment of maladies amenable to immune modulation.
Prepublished online as Blood First Edition Paper, November 17, 2005; DOI 10.1182/blood-2005-07-2793.
Supported by the Canadian Institutes of Health Research (CIHR) operating grant MOP-15017. J.G. is a CIHR Clinician-Scientist. J.S. is a recipient of a Fond de Recherche en Santé du Québec doctoral training scholarship.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.