Abstract
The introduction of an inducible suicide gene such as the herpes simplex virus thymidine kinase (HSV-TK) might allow exploitation of the antitumor activity of donor T cells after allogeneic hematopoietic cell transplantation (HCT) without graft versus host disease. However, HSV-TK is foreign, and immune responses to gene-modified T cells could lead to their premature elimination. We show that after the infusion of HSV-TK–modified donor T cells to HCT recipients, CD8+ and CD4+ T-cell responses to HSV-TK are rapidly induced and coincide with the disappearance of transferred cells. Cytokine flow cytometry using an overlapping panel of HSV-TK peptides allowed rapid detection and quantitation of HSV-TK–specific T cells in the blood and identified multiple immunogenic epitopes. Repeated infusion of modified T cells boosted the induced HSV-TK–specific T cells, which persisted as memory cells. These studies demonstrate the need for nonimmunogenic suicide genes and identify a strategy for detection of CD4+ and CD8+ T-cell responses to transgene products that should be generally applicable to monitoring patients on gene therapy trials. The potency of gene-modified T cells to elicit robust and durable immune responses imply this approach might be used for vaccination to elicit T-cell responses to viral or tumor antigens.
Introduction
The genetic modification of cells has the potential to improve upon current therapies for inherited and acquired diseases. For example, the introduction of a conditional suicide gene into T cells has been proposed as a strategy for enhancing the safety of adoptive T-cell therapy for cancer where the antigens targeted by transferred T cells are expressed on both normal and malignant cells1-6 or where T cells are modified to confer novel functions that might improve antitumor efficacy but could cause toxicity.7 However, a problem that is increasingly being recognized in clinical gene therapy is the development of immune responses to transgene-encoded proteins, and many of the suicide genes developed for clinical use are derived from pathogens and are potentially immunogenic.8,9
The herpes simplex virus thymidine kinase (HSV-TK) gene has been used most extensively in the clinic for regulating cell survival, but data on its immunogenicity are conflicting, perhaps reflecting differences in the immunocompetence of treated patients and/or the assays used to analyze immune responses.8-15 The first study to administer HSV-TK–modified T cells to patients used a retrovirus (HyTK) that encodes hygromycin phosphotransferase (Hy) and HSV-TK to transduce autologous HIV gag-specific CD8+ T-cell clones that were infused into HIV-infected individuals to augment virus-specific immunity. There was rapid clearance of HyTK-positive T cells after repeated infusions, and the elimination of T cells coincided with the induction of CD8+ cytotoxic T lymphocyte (CTL) responses directed against Hy and HSV-TK.11 In contrast, studies in allogeneic hematopoietic cell transplantation (HCT) recipients in which transferred donor T cells were modified with HSV-TK and adoptively transferred to induce a graft versus leukemia (GVL) effect have suggested that immune recognition of gene-modified T cells may be less of an impediment. Bonini et al12 used HSV-TK–modified donor lymphocyte infusion (DLI) to treat a small number of patients with Epstein-Barr virus (EBV) lymphoproliferative disease (LPD) or relapsed leukemia after allogeneic T-cell–depleted HCT. The transferred HSV-TK–positive T cells were detected for more than 12 months in vivo, exerted an antitumor effect, and could be eliminated by administration of ganciclovir (GCV).12 Tiberghien et al administered HSV-TK–positive T cells with a T-cell–depleted graft to 12 HCT recipients and confirmed the persistence of HSV-TK–modified cells.14 However, in a subsequent study in which HSV-TK–modified donor T cells were administered to patients with relapsed malignancies after unmodified allogeneic HCT, the gene-modified cells did not persist after transfer in most of the patients.16 Studies to evaluate immune responses to HSV-TK were not performed; thus, it was unclear if the failure of gene-modified T cells to persist was due to immune rejection or to the in vitro culture conditions used to generate the cells.16,17
Here we report the results of a detailed immunologic analysis of 3 consecutive HCT recipients who received infusions of HSV-TK–modified donor T cells to treat relapsed leukemia after unmodified allogeneic HCT. The administration of a single dose of HyTK-positive donor T cells was sufficient to elicit potent CD8+ T-cell responses that were directed against several distinct epitopes of HSV-TK and rapidly eliminated the gene-modified cells. In vitro cytotoxicity assays and detection of cytokine-producing T cells after stimulation with panels of overlapping peptides were used to monitor immunogenicity and permitted the rapid and sensitive detection of both CD8+ and CD4+ T cells responding to the transgene product. These results illustrate the importance of monitoring patients on gene therapy trials for transgene-specific immune responses and suggest that suicide-gene therapy to control graft versus host disease (GVHD) resulting from DLI after unmodified allogeneic HCT will require the expression of a nonimmunogenic protein that can be used to promote T-cell death.
Patients, materials, and methods
Clinical protocol and patient characteristics
The clinical trial to administer allogeneic donor T cells modified with HSV-TK to HCT recipients with posttransplantation relapse of chronic myeloid leukemia (CML) was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board, the Food and Drug Administration, and the Recombinant DNA Advisory Committee. Patients with cytogenetic relapse or morphologic relapse in chronic phase (CP) or accelerated phase (AP) were eligible for this trial if they had no evidence of GVHD and exhibited donor T-cell chimerism by fluorescence in situ hybridization with X and Y chromosome–specific probes or analysis of informative variable number tandem repeat markers. The protocol enrolled a total of 3 patients and was terminated early because the transgene product was immunogenic, as reported in this study. The clinical characteristics of these patients are shown in Table 1. The initial cell doses were 0.5 × 108 CD3+HyTK-positive T cells per kilogram for the patient with an HLA-matched related donor and 0.1 × 108 CD3+HyTK-positive T cells per kilogram for the 2 patients with an unrelated or HLA-mismatched related donor. Infusions of higher cell doses were permitted if patients failed to respond to the prior infusion by 60 days or had progressive leukemia and had less than grade 2 GVHD or other toxicity with the prior infusion. Assessment of acute and chronic GVHD was made according to clinical and histologic criteria reported previously.18,19 Patients were followed long-term for toxicity, GVHD, and disease response.
Retroviral vector
Clinical grade retroviral vector encoding the HyTK fusion gene under the transcriptional control of the Moloney murine leukemia virus long terminal repeat was produced by the National Gene Vector Laboratory (Indiana University, Indianapolis) in the PA317 packaging cell line (Targeted Genetics, Seattle, WA).20 For in vitro experiments to analyze the specificity of immune responses, the PA317/Hy and PLNT 3.3 retrovirus vectors encoding Hy and HSV-TK, respectively, were used as described previously.11
Generation and characterization of HyTK-modified polyclonal CD4+ and CD8+ donor T cells
Peripheral blood was obtained from the HCT donors after informed consent, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation (GE Healthcare BioSciences, Piscataway, NJ). CD4+ and CD8+ T cells were purified and transduced separately with retroviral supernatant and then combined to achieve a defined ratio of CD4+ to CD8+ T cells of 60:40 comparable to freshly isolated PBMCs. The rationale for this was to overcome potential differences in the number of CD4+ and CD8+ cells infused as a consequence of differences in transduction efficiency or growth of the T-cell subsets.21 Briefly, PBMCs were incubated with immunomagnetic beads (Dynal, Oslo, Norway) coated with monoclonal antibody (mAb) MCA 66.1 (CD4) or MCA 51.1 (CD8), selected according to the manufacturer's instruction, and resuspended in RPMI 1640 medium containing 25 mM HEPES, 4 mM l-glutamine, 25 μM 2-mercaptoethanol, and 10% human AB serum (T-cell medium). For transduction, cells were stimulated for 48 hours with anti-CD3 mAb (30 ng/mL OKT3; Ortho-Biotech, Raritan, NJ) in the presence of γ-irradiated autologous PBMCs as feeder cells to provide costimulation and recombinant IL-2 (10 U/mL; Chiron, Emeryville, CA). On days 2 and 3, cells were exposed to HyTK retroviral supernatant containing 5 μg/mL Polybrene (Sigma, St Louis, MO) and IL-2 (10 U/mL), spun at 1000g for 1 hour at 32°C, and incubated for 8 hours. T cells were harvested and cultured for 11 days in T-cell medium containing IL-2 (10 U/mL) and drug-selected with hygromycin B (300 μg/mL; Boehringer Mannheim, Indianapolis, IN). Viable cells were cryopreserved in aliquots for subsequent expansion and reinfusion. To expand T cells for adoptive transfer, HyTK-transduced CD4+ and CD8+ T cells were stimulated separately with anti-CD3 mAb (30 ng/mL) in the presence of γ-irradiated autologous PBMCs (35 Gy [3500 rad]) and autologous EBV-lymphoblastoid cell lines (LCLs; 70 Gy [7000 rad]) and IL-2 (10 to 25 U/mL).22 After 14 days of culture, transduced CD4+ and CD8+ T cells were combined and administered over 30 minutes intravenously.
Prior to transfer, the transduced T cells were analyzed by cell surface staining with fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–coupled mAbs to CD3, CD4, CD8, and isotype-matched irrelevant control mAbs (Becton Dickinson [BD], Mountain View, CA). All analyses were performed on a FACSCalibur flow cytometer, and data were analyzed using CellQuest Software (BD). Aliquots of the T cells were analyzed by Southern blot of genomic DNA to confirm the presence of the HyTK proviral DNA in an unrearranged form as described.11,20 Transduced T cells were also assayed and shown to be negative for replication-competent retrovirus.23 Additional quality control testing included viability assessment by trypan blue exclusion; endotoxin, bacterial, and fungal sterility assays; and Mycoplasma testing.11
GCV sensitivity of HyTK-modified cells
Sensitivity of unmodified or HyTK-positive T cells to GCV was determined by stimulating aliquots of T cells (5 × 105/mL) with anti-CD3 mAb for 2 days and then exposing cells to various concentrations of GCV (0.1 to 10 μg/mL). Cell death was determined daily by trypan blue exclusion. The criterion for acceptance was more than 90% cell death within 120 hours of exposure to GCV at more than 3 μg/mL compared with control cells cultured without GCV.
Cytotoxicity assays and flow cytometry
Cytotoxic T-cell responses specific for Hy and HSV-TK were examined as described.11,24 Briefly, PBMCs obtained before and after infusion were stimulated twice 1 week apart with γ-irradiated (35 Gy [3500 rad]) donor HyTK-positive T cells and assayed in a chromium release assay for recognition of donor 51Cr-labeled EBV-LCLs either unmodified or modified to express the Hy, HSV-TK, or HyTK genes.11 In some experiments, CD8+ T cells were eliminated from the cultures by depletion (unique patient number [UPN] 4103) or by enrichment for CD4+ T cells (UPN 1574 and 7826) using immunomagnetic beads (Dynal or Miltenyi Biotec, Auburn, CA).
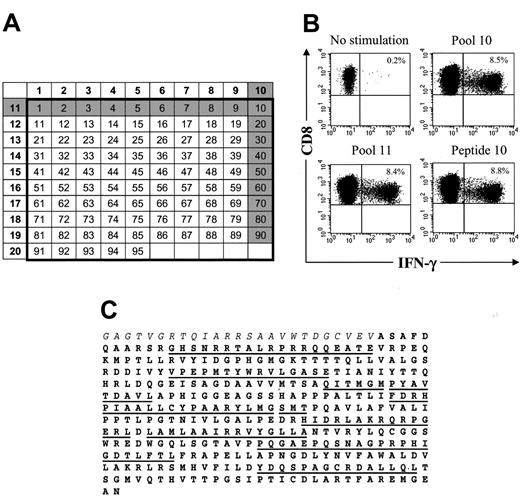
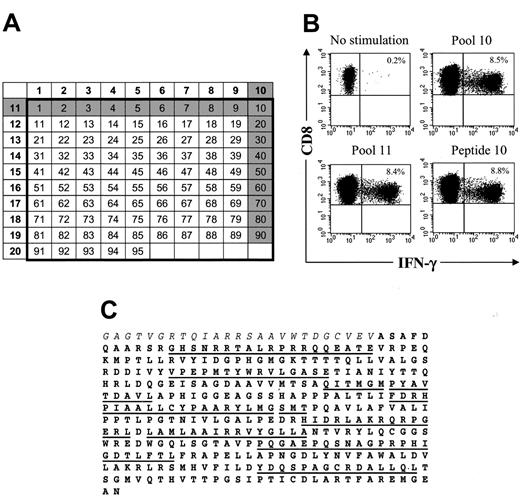
The fine specificity of HSV-TK–specific T-cell responses was determined using intracellular cytokine staining after stimulation with pools of 15-mer peptides with 11 amino acid (aa) overlap spanning a 367 aa sequence of the HSV-TK protein (HSV-TK10-376; GenBank accession nos. V000467, CAA23741) and adjacent HyTK-fusion site (Hy300-324). Ninety-five peptides were synthesized using standard FMOC chemistry (NMI, Reutlingen, Germany), and an analytic grid composed of 20 pools each containing 10 peptides was constructed. Aliquots of HyTK-specific T-cell lines or PBMCs obtained before and after HyTK-positive DLI were stimulated for 6 hours at 37°C with the peptides or medium alone in the presence of anti-CD28 and anti-CD49d mAbs and 10 μg/mL brefeldin A (Sigma). Staphylococcal enterotoxin B (1 μg/mL) served as positive control. In some experiments, PBMCs were stained with anti-CD62L and anti-CD8 mAbs, sorted on a Vantage BD instrument in a CD8+CD62L+ and CD8+CD62L– subset, and used in the assay. Cells were stained for cell surface expression of CD8β and CD4, permeabilized using FACS Permeabilizing Solution, and then stained with FITC-labeled interferon-gamma (IFN-γ) mAb (BD). Data files were analyzed with CellQuest software (BD). Immunogenic peptides were identified by the responses of T cells to intersecting peptides in the grid, and the assays were repeated with each individual peptide to confirm specificity.
Fluorescent probe PCR assay (TaqMan)
Polymerase chain reaction (PCR) amplifications and analyses were performed using a quantitative real-time PCR assay (Perkin Elmer Applied Biosystems, Foster City, CA).24 DNA was isolated using the Qiagen QiAmp kit (Valencia, CA) and amplified using PCR primers and a TaqMan probe (Synthegen, Houston, TX) designed to detect a unique HyTK sequence. The PCR primers were 5′TACACAAATCGCCCGCAGA3′ and 5′AGCCTGGTCGAACGCAGAC3′ and were used with a fluorescent-tagged probe 5′-FAM-CGACTTCTACACAGCCATCGGTCCAGA-TAMRA-3′ encompassing the junction of the Hy and HSV-TK genes. Standards consisted of serial dilutions of DNA derived from HyTK-positive T cells in DNA obtained from preinfusion recipient PBMCs. Each reaction contained 2 × 105 cells. Preinfusion PBMCs served as negative control.
Results
Safety of adoptively transferred HSV-TK–positive polyclonal T cells
Three patients with relapsed CML after unmodified allogeneic HCT received a total of 4 infusions of polyclonal HyTK-positive donor lymphocytes. All patients in this study had cytogenetic or hematologic relapse of CML, which was first detected 9 to 69 months after HCT (Table 1). All patients were initially treated with α-interferon, resulting in a transient response.25,26 The patients were referred for DLI for persistent or progressive disease. All 3 patients had normal or only slightly decreased absolute lymphocyte counts and were not receiving immunosuppressive drugs at the time HyTK-modified donor lymphocytes were administered.
Peripheral blood donor T cells used for DLI were modified to express HyTK and selected with hygromycin B to ensure a pure population of gene-modified cells. The function of the HSV-TK gene was assessed prior to infusion by culturing an aliquot of transduced T cells and unmodified T cells in the presence of GCV (0.1 to 10 μg/mL). The viability of untransduced T cells was not affected at 3 μg/mL, whereas more than 95% of HyTK-positive T lymphocytes were killed after 120 hours of exposure to 3 μg/mL or more GCV (data not shown).
An initial dose of 0.1 × 108/kg (2 patients) or 0.5 × 108/kg (1 patient) HyTK-positive donor T cells containing a defined ratio of CD4+ to CD8+ cells was administered, and patients were monitored for toxicity, GVHD, and antileukemia response. The first infusion of HyTK-positive donor T cells was well tolerated in all 3 recipients. Three months after the first infusion, a second, higher dose of HyTK-positive T cells (0.5 × 108/kg) was given to UPN 1574. This patient experienced fever and chills that developed 5 to 6 hours after the T-cell infusion, but these symptoms resolved spontaneously within 24 hours. None of the patients developed symptoms of GVHD, and no antileukemic effects were observed in response to the transferred cells.
Persistence of adoptively transferred HyTK-modified donor T cells
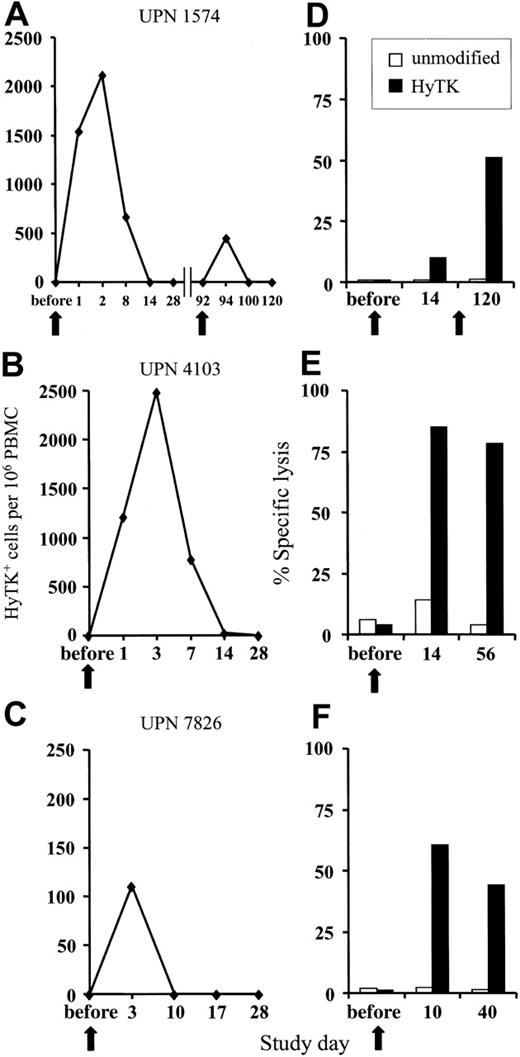
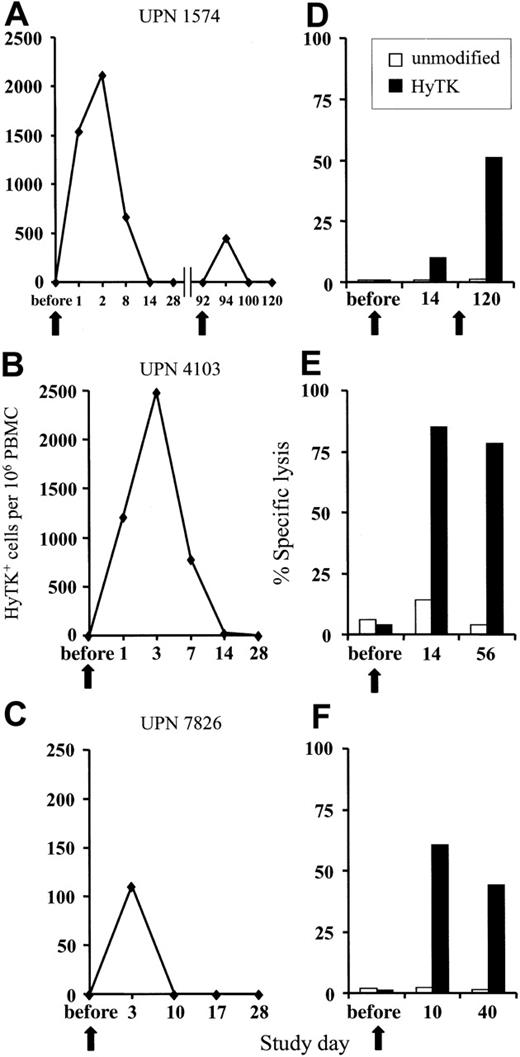
GVHD and GVL effects after unmodified DLI are delayed; therefore, the donor HyTK-positive T cells must persist in vivo to allow the subset of T cells that are specific for minor histocompatibility or leukemia-associated antigens to undergo activation and expansion.27-30 The persistence of the HyTK-positive T cells in peripheral blood was examined by quantitative real-time (TaqMan) PCR specific for transgene sequences.24 In UPN 1574, 1539 HyTK copies per 106 cells were detected 1 day after the first infusion, and these cells peaked at day 2 after transfer at 2117 HyTK copies per 106 cells (Figure 1A). HyTK-positive T cells remained detectable 8 days after the infusion but were no longer detectable in the blood 14 days after transfer. A second, higher dose of HyTK-positive T cells (0.5 × 108/kg) was given 3 months later. However, HyTK-positive cells were detected for only 2 days after the second infusion and at substantially lower levels as compared with the first cell dose (Figure 1A). A similar pattern of in vivo persistence of the transferred cells was observed after a single infusion in both UPN 4103 and 7826. HyTK-positive cells were detected in the peripheral blood of UPN 4103 at a frequency of 1214 HyTK copies per 106 cells on day 1 and peaked at day 3 at 2485 HyTK copies per 106 cells. However, the levels of HyTK-positive cells declined rapidly, dropping by day 14 to undetectable levels (Figure 1B). In UPN 7826, the transferred HyTK-modified T cells were no longer detectable 10 days after transfer (Figure 1C). These results suggested that the short in vivo persistence of the HyTK-positive T cells was at least in part responsible for the failure of these T cells to induce GVHD or a GVL effect.
Adoptively transferred HyTK-modified T cells. (A-C) Persistence of adoptively transferred HyTK-modified T cells in vivo. PBMCs from each of the 3 patients were obtained at various time points before and after infusion of HyTK-positive T cells and evaluated for the presence of HyTK-transduced cells by quantitative PCR as described in “Patients, materials, and methods.” (D-F) Rapid induction of a HyTK-specific CTL response after adoptive transfer of HyTK-modified T cells. PBMCs from each of the 3 patients were obtained prior to and at various times after infusion and stimulated in vitro with γ-irradiated HyTK-positive donor T cells. Aliquots of these cultures were then evaluated in a chromium release assay for recognition of HyTK-positive target cells (▪) or unmodified controls (□). The killing of HyTK-positive LCLs is shown at an effector-target ratio of 10:1. The arrows indicate the day of the HyTK-positive T-cell infusion.
Adoptively transferred HyTK-modified T cells. (A-C) Persistence of adoptively transferred HyTK-modified T cells in vivo. PBMCs from each of the 3 patients were obtained at various time points before and after infusion of HyTK-positive T cells and evaluated for the presence of HyTK-transduced cells by quantitative PCR as described in “Patients, materials, and methods.” (D-F) Rapid induction of a HyTK-specific CTL response after adoptive transfer of HyTK-modified T cells. PBMCs from each of the 3 patients were obtained prior to and at various times after infusion and stimulated in vitro with γ-irradiated HyTK-positive donor T cells. Aliquots of these cultures were then evaluated in a chromium release assay for recognition of HyTK-positive target cells (▪) or unmodified controls (□). The killing of HyTK-positive LCLs is shown at an effector-target ratio of 10:1. The arrows indicate the day of the HyTK-positive T-cell infusion.
HyTK-specific CD8+ CTL responses develop rapidly after HyTK-positive DLI
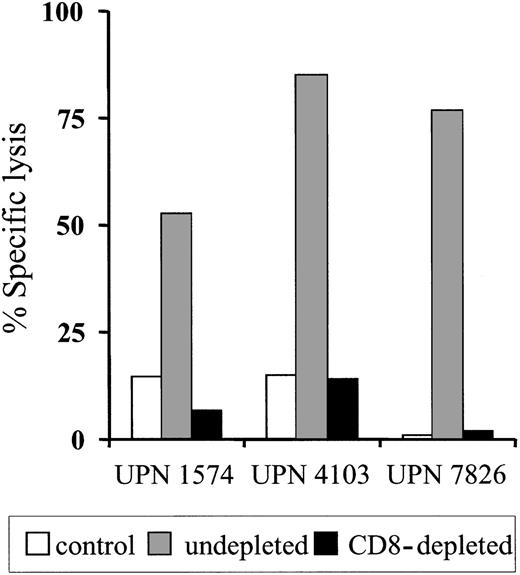
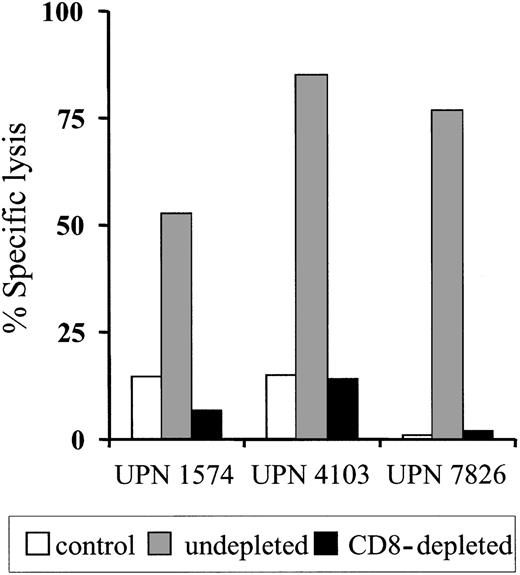
To determine if the clearance of the adoptively transferred T cells might be due to the induction of an immune response against transgene-encoded proteins, PBMCs obtained from each patient before and after T-cell transfer were stimulated in vitro with donor HyTK-positive T cells as antigen-presenting cells and then assayed for lytic activity against HyTK-positive and unmodified donor EBV-LCLs as target cells. There was no detectable HyTK-specific cytolytic activity in the preinfusion PBMCs in any of the 3 patients on multiple assays, indicating the absence of a significant preexisting CTL response to Hy or HSV-TK (Figure 1D-F). However, HyTK-specific T-cell responses were present 10 to 14 days after the first T-cell infusion in all 3 patients and were augmented in UPN 1574 after the second dose of HSV-TK–positive T cells (Figure 1D). Selective depletion of CD8+ but not CD4+ T cells from the cultures abrogated lytic activity, indicating that CD8+ T cells mediated target-cell lysis (Figure 2). Collectively, these results indicate that a single infusion of HyTK-positive donor T cells was sufficient to prime potent HyTK-specific CD8+ CTL responses, and the induction of this immune response correlated with the clearance of the transferred cells.
Evaluation of specificity and HLA restriction of the transgene-specific CD8+ CTL response
The immune response to the HyTK protein could be targeted either to sequences within the bacterial Hy or viral HSV-TK protein products or to novel epitopes derived from the junctions between the protein domains. If only Hy were recognized, alternative nonimmunogenic marker genes such as truncated versions of the human low-affinity nerve growth factor receptor (ΔLNGFR), CD20, or CD34 gene could be employed to replace Hy within the fusion gene.12,31-34 If only a few epitopes within HSV-TK were recognized, it might be possible to mutate these to nonimmunogenic sequences. To map the specificity of the immune response, postinfusion PBMCs were stimulated in vitro with HyTK-positive donor T cells and examined for recognition of target cells expressing exclusively Hy, HSV-TK, or both Hy and HSV-TK. The T-cell lines from UPN 1574 and UPN 4103 recognized both Hy-positive and HSV-TK–positive target cells, consistent with the induction of CTL responses to epitopes derived from each domain of the fusion protein (Figure 3A-B). In UPN 7826, the CTL response detected by this assay appeared to only be specific for Hy-expressing target cells (Figure 3C and data not shown). To determine if a HSV-TK–specific CTL response might have been induced but be obscured by selective outgrowth of Hy-specific CTLs in the culture, a separate aliquot of PBMCs from UPN 7826 was stimulated with T cells modified to express exclusively the HSV-TK gene.11 Under these conditions, a HSV-TK–specific CTL response was readily identified (Figure 3D).
CD8+ T cells recognize peptides derived from processing of intracellular proteins and presented by class I major histocompatibility complex (MHC) molecules. To identify the class I MHC restricting alleles for the HyTK-specific T cells induced by gene transfer, the T-cell lines derived from each patient were evaluated for the ability to lyse Hy-positive or HSV-TK–positive target cells from unrelated donors that shared only a single class I MHC allele. The HyTK-specific T-cell line isolated from UPN 4103 recognized target cells that expressed 2 (HLA-A24, HLA-B8) of 4 class I alleles of the patient, the line from UPN 1574 was predominantly restricted by a single class I MHC allele, and the T-cell lines from UPN 7826 were restricted by HLA-B57 and HLA-A3 (Figure 3).
HyTK-specific immunity is mediated by HyTK-specific CD8+cytotoxic T cells. Postinfusion PBMCs were obtained from UPN 1574, UPN 4103, and UPN 7826 after the HyTK-positive T-cell infusion and stimulated in vitro with γ-irradiated HyTK-expressing T cells twice 1 week apart. Aliquots of these cultures were depleted of CD8+ T cells as described in “Patients, materials, and methods” and then evaluated in a chromium release assay for recognition of HyTK-positive target cells (▪) or untransduced LCLs (□). Aliquots of the undepleted cultures (▦) were assayed in a similar fashion for HyTK-positive target cell lysis. Data are shown at an effector-target ratio of 10:1.
HyTK-specific immunity is mediated by HyTK-specific CD8+cytotoxic T cells. Postinfusion PBMCs were obtained from UPN 1574, UPN 4103, and UPN 7826 after the HyTK-positive T-cell infusion and stimulated in vitro with γ-irradiated HyTK-expressing T cells twice 1 week apart. Aliquots of these cultures were depleted of CD8+ T cells as described in “Patients, materials, and methods” and then evaluated in a chromium release assay for recognition of HyTK-positive target cells (▪) or untransduced LCLs (□). Aliquots of the undepleted cultures (▦) were assayed in a similar fashion for HyTK-positive target cell lysis. Data are shown at an effector-target ratio of 10:1.
HyTK-specific CD8+CTL recognize epitopes derived from both the Hy and TK domain of theHyTKfusion gene and are restricted by multiple class I MHC alleles. Samples of PBMCs were obtained from UPN 1574, UPN 4103, and UPN 7826 after the HyTK-positive T-cell infusion and stimulated twice 1 week apart with γ-irradiated HyTK-expressing donor T cells (A-C) or with HSV-TK–expressing donor T cells (UPN 7826 [D]). To map the HLA-restricting alleles, EBV-transformed LCL lines that matched at all 4 class I MHC alleles (Auto), allogeneic LCL lines that matched only at a single class I MHC allele, and mismatched LCLs (MM) were modified to express either the Hy (▦) or the HSV-TK (▪) domain of the HyTK fusion protein. Aliquots of the CD8+ CTL lines were then examined for recognition of nontransduced LCL- (□), Hy- (▦), or HSV-TK– (▪) modified target cells. Data are shown at an effector-target ratio of 5:1.
HyTK-specific CD8+CTL recognize epitopes derived from both the Hy and TK domain of theHyTKfusion gene and are restricted by multiple class I MHC alleles. Samples of PBMCs were obtained from UPN 1574, UPN 4103, and UPN 7826 after the HyTK-positive T-cell infusion and stimulated twice 1 week apart with γ-irradiated HyTK-expressing donor T cells (A-C) or with HSV-TK–expressing donor T cells (UPN 7826 [D]). To map the HLA-restricting alleles, EBV-transformed LCL lines that matched at all 4 class I MHC alleles (Auto), allogeneic LCL lines that matched only at a single class I MHC allele, and mismatched LCLs (MM) were modified to express either the Hy (▦) or the HSV-TK (▪) domain of the HyTK fusion protein. Aliquots of the CD8+ CTL lines were then examined for recognition of nontransduced LCL- (□), Hy- (▦), or HSV-TK– (▪) modified target cells. Data are shown at an effector-target ratio of 5:1.
Multiple epitopes of HSV-TK are recognized by CD8+ and CD4+ T cells
We next analyzed the fine specificity of the T-cell response to HSV-TK and the fusion site between Hy and TK to determine if there was a single epitope or a few epitopes responsible for immunogenicity that might be modified to nonimmunogenic sequences while conserving HSV-TK function. The peptide epitopes were mapped using intracellular cytokine staining following stimulation with individual pools of 15-mer peptides overlapping by 11 aa and representing the entire HSV-TK sequence including the fusion region with Hy (Figure 4A,C). The initial experiments were performed using transgene-specific T-cell lines from the 3 patients because the frequency of reactive T cells would be enriched after in vitro stimulation and facilitate the detection of T cells specific for individual peptides by cytokine flow cytometry (CFC). In all patients, significant HSV-TK–specific CD8+ T-cell responses were observed in several pools (Figure 4B-C; Table 2), and the assays were then repeated using individual candidate peptides predicted by the initial screen. The results identified several peptides that induced responses in more than 1% of CD8+ T cells in the polyclonal cultures (Table 2). Multiparameter analysis also revealed that in all 3 patients several of the HSV-TK peptide pools induced IFN-γ production in a significant fraction of CD4+ T cells (UPN 1574, 0.73% to 2.03%; UPN 4103, 0.52% to 1.8%; UPN 7826, 2.6%; data not shown). Collectively, these results indicate that a broad immune response consisting of both CD8+ and CD4+ T cells and directed against multiple HSV-TK epitopes dispersed throughout the protein sequence was elicited by the adoptive transfer of HyTK-positive T cells.
Transgene product-specific T cells are detectable in PBMCs, boosted by repeated infusion of gene-modified cells, and persist as memory cells
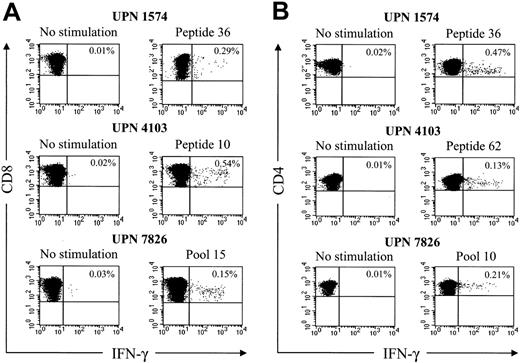
The use of overlapping peptide panels and CFC could provide a sensitive methodology to detect immune responses to transgene products in gene therapy trials. Thus, we next ascertained whether this approach could be used to measure HSV-TK–specific T cells directly in PBMC samples obtained from the patients after DLI. Cryopreserved PBMC samples were thawed, stimulated with the 15-mer HSV-TK peptides that elicited CD8+ and CD4+ responses from the T-cell lines, and stained for IFN-γ. In all 3 patients, significant responses (0.15% to 0.54% of CD8+ T cells, 0.13% to 0.47% of CD4+ T cells) were detected in PMBC samples obtained 14 to 28 days after the first T-cell infusion (Figure 5; Table 2). No CD8+ or CD4+ T-cell responses above background levels were detected in pretreatment PBMC samples, consistent with the absence of a preexisting T-cell response to HSV-TK (data not shown). These results illustrate the use of stimulation with peptide pools and CFC for the sensitive detection of T-cell responses to transgene products.
HSV-TK–specific CD8+CTLs can be visualized by CFC and recognize multiple epitopes within the HSV-TK protein. (A) Design of peptide pools. The numbers of the pools (left column and top row) are shown in bold. Individual 15-mer peptides overlapping by 11 aa (n = 95) in these 20 pools correspond to the numbers in the respective columns and rows. Peptides 1 to 3 comprise the C-terminal aa sequence of the Hy protein (Hy300-324), peptides 4 to 7 encompass the HyTK fusion site, and peptides 8 to 95 span the aa sequence of the HSV-TK protein (HSV-TK10-376). The gray shading illustrates how a candidate peptide was chosen. (B) Identification of an immunogenic peptide from the peptide pools that induced IFN-γ production by CD8+ T cells. Samples of postinfusion PBMCs were obtained from UPN 4103 stimulated in vitro with γ-irradiated HyTK-positive donor T cells twice 1 week apart. Aliquots of the cultures were incubated for 6 hours with medium alone or with each 1 of the 20 peptide pools or the individual 15-mer peptides. Cells were then stained with FITC-coupled anti–IFN-γ and PE-coupled anti-CD8β mAbs and examined by flow cytometry. Cells were gated to identify CD8+ T cells and stained for intracellular IFN-γ. Values indicate the percentage of cells in the culture that produced IFN-γ in response to peptide stimulation. (C) Multiple 15-mer peptides throughout the sequence of the HSV-TK protein (boldface) are immunogenic. The underlined sequences indicate the 15-mer candidate peptides within the HSV-TK protein that were identified to induce IFN-γ production by CD8+ T cells in aliquots of the T-cell lines or postinfusion PBMCs from the 3 patients. Immunogenic peptides within the adjacent Hy protein (italics) or fusion site were not detected in these patients.
HSV-TK–specific CD8+CTLs can be visualized by CFC and recognize multiple epitopes within the HSV-TK protein. (A) Design of peptide pools. The numbers of the pools (left column and top row) are shown in bold. Individual 15-mer peptides overlapping by 11 aa (n = 95) in these 20 pools correspond to the numbers in the respective columns and rows. Peptides 1 to 3 comprise the C-terminal aa sequence of the Hy protein (Hy300-324), peptides 4 to 7 encompass the HyTK fusion site, and peptides 8 to 95 span the aa sequence of the HSV-TK protein (HSV-TK10-376). The gray shading illustrates how a candidate peptide was chosen. (B) Identification of an immunogenic peptide from the peptide pools that induced IFN-γ production by CD8+ T cells. Samples of postinfusion PBMCs were obtained from UPN 4103 stimulated in vitro with γ-irradiated HyTK-positive donor T cells twice 1 week apart. Aliquots of the cultures were incubated for 6 hours with medium alone or with each 1 of the 20 peptide pools or the individual 15-mer peptides. Cells were then stained with FITC-coupled anti–IFN-γ and PE-coupled anti-CD8β mAbs and examined by flow cytometry. Cells were gated to identify CD8+ T cells and stained for intracellular IFN-γ. Values indicate the percentage of cells in the culture that produced IFN-γ in response to peptide stimulation. (C) Multiple 15-mer peptides throughout the sequence of the HSV-TK protein (boldface) are immunogenic. The underlined sequences indicate the 15-mer candidate peptides within the HSV-TK protein that were identified to induce IFN-γ production by CD8+ T cells in aliquots of the T-cell lines or postinfusion PBMCs from the 3 patients. Immunogenic peptides within the adjacent Hy protein (italics) or fusion site were not detected in these patients.
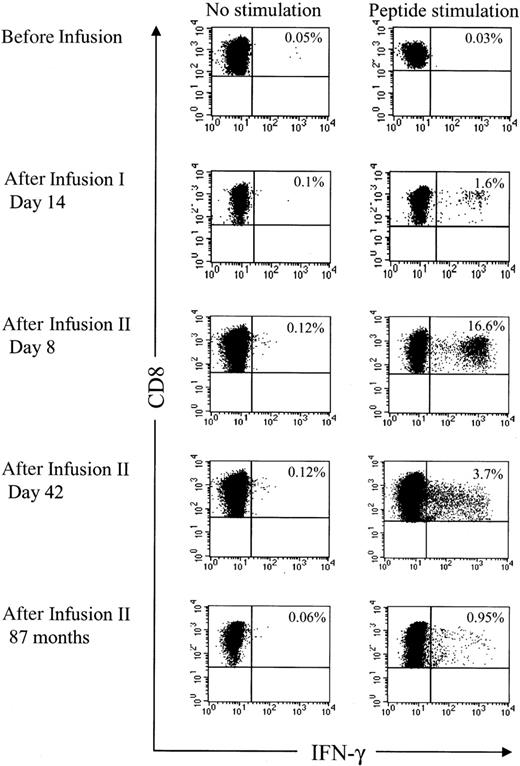
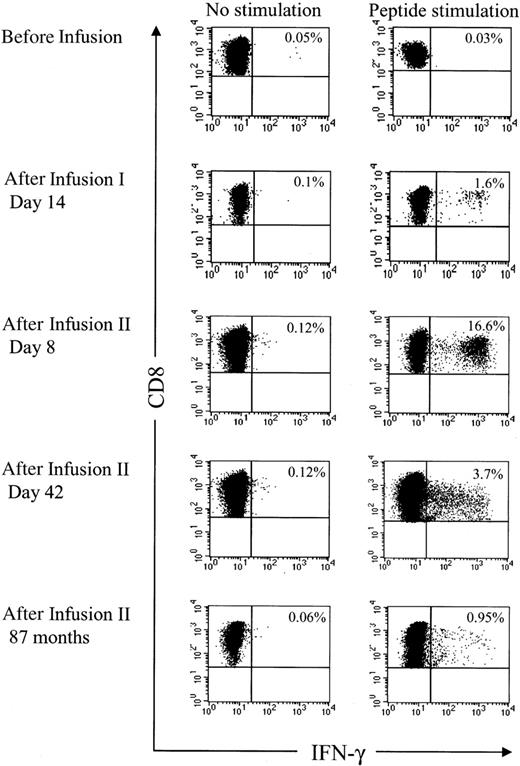
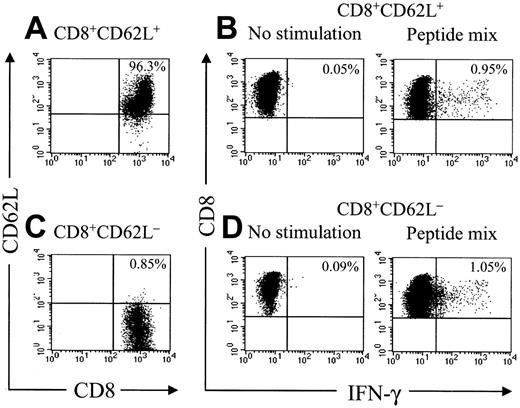
Analysis of HyTK-specific T-cell responses in UPN 1574 using cytolytic assays suggested the response was augmented after the second dose of HSV-TK–positive T cells (Figure 1D). To examine the frequency of the HSV-TK–specific CD8+ T cells with greater precision, samples of PBMCs obtained from UPN 1574 before and after the first and second T-cell dose were stimulated with a pool of the antigenic HSV-TK peptides and examined by CFC. HSV-TK–specific T-cell responses were not detected in PBMCs before the first HyTK-positive T-cell infusion but were easily detected in PBMCs obtained at day 14 after the first infusion. After the second HyTK-positive T-cell infusion, the responses were boosted dramatically, and up to more than 16% of CD8+ T cells in PBMCs obtained 8 days after the second infusion produced IFN-γ in response to the HSV-TK peptide pools (Figure 6). HSV-TK–specific CD8+ T cells had declined to 3.7% by 6 weeks after the second infusion of HyTK-positive T cells but remained detectable in PBMCs obtained more than 7 years after HyTK-positive DLI. A substantial fraction of the HSV-TK–specific CD8+ T cells that persisted long-term were in the CD62L+ subset (Figure 7), consistent with the formation of a durable memory T-cell response.
Outcome of the patients
Despite the failure of the HyTK-modified T cells to induce a GVL effect, 2 patients subsequently responded to alternative therapies such as nontransduced DLI, α-interferon, or imatinib mesylate (Table 1). A complete remission (CR) was achieved in UPN 1574 after 2 infusions of unmodified DLI, but this treatment was complicated by GVHD, and the disease response was not sustained. A molecular CR was achieved after 7 months of treatment with imatinib mesylate. UPN 4103 remained in stable cytogenetic relapse for 18 months. Subsequent disease progression was successfully treated with imatinib mesylate, resulting in a hematologic CR. UPN 7826 received treatment with α-interferon and imatinib mesylate and remained in stable cytogenetic relapse without hematologic features. All 3 patients are alive 66 to 90 months after receiving gene-modified cells with a Karnofsky score of 80% to 100%. None of the patients has displayed adverse long-term effects that could be attributed to the gene-modified T cells.
HSV-TK–specific CD8+and CD4+T cells can be directly visualized by CFC in samples of peripheral blood after HyTK-positive DLI. Samples of PBMCs obtained from the 3 patients after the first T-cell infusion were incubated for 6 hours with medium alone, the candidate 15-mer peptides, or with peptide pools that elicited responses from the HSV-TK–reactive T-cell lines. Cells were then stained with FITC-coupled anti–IFN-γ, PE-coupled anti-CD8β, and peridinin chlorophyll protein (PerCP)-Cy5.5–coupled anti-CD4 mAbs, respectively, and examined by flow cytometry. (A) Gated on CD8+ T cells. (B) Gated on CD4+ T cells. Values indicate the percentage of cells producing IFN-γ. Data are shown for representative HSV-TK peptides.
HSV-TK–specific CD8+and CD4+T cells can be directly visualized by CFC in samples of peripheral blood after HyTK-positive DLI. Samples of PBMCs obtained from the 3 patients after the first T-cell infusion were incubated for 6 hours with medium alone, the candidate 15-mer peptides, or with peptide pools that elicited responses from the HSV-TK–reactive T-cell lines. Cells were then stained with FITC-coupled anti–IFN-γ, PE-coupled anti-CD8β, and peridinin chlorophyll protein (PerCP)-Cy5.5–coupled anti-CD4 mAbs, respectively, and examined by flow cytometry. (A) Gated on CD8+ T cells. (B) Gated on CD4+ T cells. Values indicate the percentage of cells producing IFN-γ. Data are shown for representative HSV-TK peptides.
The HSV-TK–specific CD8+T cells are boosted after reinfusion of HyTK-positive donor T cells. Samples of PBMCs were obtained from UPN 1574 before and at various time points after the first and second infusion of HyTK-modified donor T cells. Aliquots of these cells were incubated for 6 hours with medium alone or with all 6 candidate 15-mer peptides that were identified to induce IFN-γ production in samples of PBMCs obtained from this patient. After 6 hours of stimulation, cells were permeabilized, stained with anti–IFN-γ and anti-CD8 mAbs, and examined by flow cytometry. Values indicate the percentage of CD8+ T cells producing IFN-γ.
The HSV-TK–specific CD8+T cells are boosted after reinfusion of HyTK-positive donor T cells. Samples of PBMCs were obtained from UPN 1574 before and at various time points after the first and second infusion of HyTK-modified donor T cells. Aliquots of these cells were incubated for 6 hours with medium alone or with all 6 candidate 15-mer peptides that were identified to induce IFN-γ production in samples of PBMCs obtained from this patient. After 6 hours of stimulation, cells were permeabilized, stained with anti–IFN-γ and anti-CD8 mAbs, and examined by flow cytometry. Values indicate the percentage of CD8+ T cells producing IFN-γ.
Adoptive transfer of HSV-TK–modified T cells results in the induction of a durable memory T-cell response. Samples of PBMCs were obtained from UPN 1574 more than 7 years after HSV-TK–modified DLI, stained with anti-CD8 and anti-CD62L mAbs, and separated by fluorescence-activated cell sorting (FACS) in a CD8+CD62L+ and CD8+CD62L– fraction. These fractions were then incubated with medium alone or stimulated for 6 hours with a peptide mix consisting of the 6 candidate 15-mer peptides as described in “Patients, materials, and methods.” After 6 hours of stimulation, cells were then stained with FITC-coupled anti–IFN-γ and PE-Cy7–coupled anti-CD8 mAbs and examined by flow cytometry. Cells were gated to identify CD8+ T cells and assessed for cytokine production.
Adoptive transfer of HSV-TK–modified T cells results in the induction of a durable memory T-cell response. Samples of PBMCs were obtained from UPN 1574 more than 7 years after HSV-TK–modified DLI, stained with anti-CD8 and anti-CD62L mAbs, and separated by fluorescence-activated cell sorting (FACS) in a CD8+CD62L+ and CD8+CD62L– fraction. These fractions were then incubated with medium alone or stimulated for 6 hours with a peptide mix consisting of the 6 candidate 15-mer peptides as described in “Patients, materials, and methods.” After 6 hours of stimulation, cells were then stained with FITC-coupled anti–IFN-γ and PE-Cy7–coupled anti-CD8 mAbs and examined by flow cytometry. Cells were gated to identify CD8+ T cells and assessed for cytokine production.
Discussion
Donor T cells mediate both GVHD and a GVL effect after allogeneic HCT, and the separation of GVL from GVHD has proven to be a formidable problem.5,6 The expression of an inducible suicide gene in donor T cells was conceived as a potential way to provide for the abrogation of GVHD after leukemic cells were eradicated. The most extensively studied suicide genes are derived from pathogens and include the HSV-TK and bacterial cytosine deaminase genes, which encode enzymes that metabolize GCV and 5-fluorocytosine, respectively, and generate toxic compounds.8-10,35 A potential problem is that these proteins are foreign and could elicit immune responses that may compromise the survival of transferred gene-modified T cells. Indeed, the induction of a CD8+ cytotoxic T-cell response to HSV-TK was observed in the first clinical study in which HSV-TK was used to modify T cells for immunotherapy in nontransplantation patients.11 In this study, the precursor frequency of HSV-TK–specific CD8+ T cells was shown to be less than 1 in 900 000 prior to the transfer of HSV-TK–positive T cells, consistent with the absence of preexisting immunity to HSV-TK. Thus, it was anticipated that gene-modified T cells may exhibit improved persistence after allogeneic HCT because the posttransplantation immune deficiency might impair T-cell priming.
In the study reported here, long-term persistence of adoptively transferred HSV-TK–modified donor T cells was not achieved in HCT recipients. The failure of T cells to persist was due to the rapid development of CD8+ and CD4+ T-cell responses specific for epitopes of both HSV-TK and the Hy selectable marker protein that mediated lysis of the gene-modified T cells. No GVHD or GVL effects were observed after T-cell therapy, reflecting the short duration the T cells persisted, but the treatment did not interfere with response to subsequent infusions of unmodified DLI or pharmacologic therapy. Our results differ from a previous report in which HCT patients with EBV-LPD or leukemic relapse after T-cell–depleted transplantation were treated with donor T cells modified with a retroviral vector encoding both the ΔLNGFR and HSV-TK genes.12 Antitumor effects were observed in 5 of 7 evaluable patients, and the administration of GCV resolved GVHD in 2 of 3 patients in which it occurred.12 Similarly, in vivo persistence of the transferred cells and GCV-sensitive alloreactivity was observed after prophylactic administration of HSV-TK–positive DLI with a T-cell–depleted allograft.14 The likely explanation for the different outcome is that patients treated in these 2 studies had received a T-cell–depleted HCT, which results in a prolonged and severe immunodeficiency that may compromise the ability to mount an immune response against HSV-TK.12,14 The fact that a subset (2 of 8) of the patients reported by Bonini et al suffered from EBV-LPD, which occurs only in severely immunodeficient patients, is consistent with this explanation.12 In studies by Tiberghien et al, 3 of 12 patients receiving T-cell depleted HCT and up-front infusion of HSV-TK–positive donor T cells developed EBV-LPD, and this was fatal in 2 of the patients.14 Factors that improve immune function in transplant recipients such as time after transplantation and treatment with α-interferon may be important for the increased risk of immune elimination of gene-modified T cells observed in our study.
Other studies have suggested that T cells cultured in vitro to allow genetic insertion may be less functional in vivo.14,17,36-39 Improved culture conditions have been developed that preserve the T-cell repertoire and improve viability,17,38-41 but the results presented here demonstrate that immune responses to foreign transgene products pose an additional obstacle except in severely immunosuppressed hosts. Thus, sensitive methods to analyze the induction of T-cell immunity are needed to monitor patients and ensure that subsequent therapy with the same transgene is avoided in patients that have already developed immunity. We have used in vitro stimulation of PBMCs with gene-modified cells to detect CD8+ CTL responses to transgene products.11,24,42 This assay is sensitive and reproducible but requires 1 to 2 weeks to perform and expertise in T-cell culture and cytotoxicity assays. In this paper, we demonstrate the utility of flow cytometry to directly detect T cells in the peripheral blood that produce IFN-γ following stimulation with pools of overlapping peptides corresponding to the sequence of the foreign protein.43,44 This assay is rapid, sensitive, and can be applied to directly quantitate the frequency of both CD8+ and CD4+ T cells that recognize the transgene product in patient blood samples and provide information on the precise epitopes that are recognized.
Strategies have been proposed to overcome immune rejection of gene-modified cells. The finding in our study that the T-cell response to HSV-TK recognized multiple epitopes suggests that modification of immunogenic sequences in transgene products is unlikely to be effective, particularly in humans where the diversity of HLA alleles will allow the recognition of multiple immunogenic epitopes. We previously investigated the coexpression of immune evasion genes from CMV or HSV that selectively interfere with class I MHC antigen presentation and recognition by CD8+ T cells, but this approach did not allow complete escape from immune recognition despite a 1 log10 reduction in class I MHC expression on gene-modified cells.42 Because class I MHC molecules engage inhibitory receptors on NK cells, a potential problem with this strategy is that it may also enhance elimination by NK cells.45 Immunosuppressive conditioning has been evaluated in animal models to circumvent host immune responses to transgene-encoded proteins.24,46 Nonmyeloablative conditioning with low-dose TBI combined with immunosuppressive drugs significantly prolonged the in vivo persistence of transferred T cells that expressed an immunogenic transgene but did not prevent eventual immune elimination and is limited by the risk of infectious complications and other toxicities.24,46 Thus, further investigation is needed to define approaches that can prevent immunologic rejection of cells expressing a foreign transgene product and be translated to the clinic.
Progress has been made in developing alternative suicide genes that might be used to abrogate GVHD after allogeneic HCT based on engineering human proapoptotic chimeric proteins that can be activated by a nontoxic synthetic ligand.9,39,47-50 Recent reports in which Fas or caspase-9 were used as the death-effector molecule demonstrated that the administration of a bivalent nontoxic drug (AP1903) can safely regulate T-cell survival in vitro and in vivo.39,48-52 The caspase-9–based suicide gene was superior to the Fas-based system and exerted lower basal toxicity50-52 (C.B. and S.R.R., unpublished data, August 18, 2005). The enhanced efficacy of caspase-9 in part reflects its function independent of antiapoptotic proteins such as BCL-xL and c-Flip that are expressed in T cells after activation and interfere with Fas signaling.53-55 These novel suicide genes contain fewer potential immunogenic sequences and are actively being developed for use in human HCT.
An unanticipated finding in our study was the magnitude, diversity, and stability of the T-cell immune response elicited after the adoptive transfer of T cells expressing a foreign protein. A frequent goal of vaccination for malignancy or infectious disease is to induce strong effector and memory T cells, which has often proven difficult to achieve in humans after inoculation with recombinant viruses, protein or peptide preparations, or dendritic cells pulsed with antigen.56 The remarkable ability of HSV-TK–modified T cells to induce strong primary T-cell responses to HSV-TK and to boost the induced responses to very high levels has encouraged us to further investigate the mechanisms responsible for immunogenicity and the utility of gene-modified T cells as vehicles for vaccination to induce immunity to viral or tumor-associated antigens.
Prepublished online as Blood First Edition Paper, November 10, 2005; DOI 10.1182/blood-2005-08-3503.
Supported by National Institutes of Health grants CA18029 (S.R.R.), HL66947 (S.R.R.), and CA224536 (S.R.R.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We appreciate the assistance of Dusty Miller in screening HyTK-modified T cells for helper virus and Targeted Genetics (Seattle, WA) for quality control testing of T cells for one of the patients. We thank Rici de Fries-Hallstrand for technical support and Kenneth G. Cornetta and Lilith A. Reeves (National Gene Vector Laboratory, Indiana University) for vector production.

![Figure 3. HyTK-specific CD8+ CTL recognize epitopes derived from both the Hy and TK domain of the HyTK fusion gene and are restricted by multiple class I MHC alleles. Samples of PBMCs were obtained from UPN 1574, UPN 4103, and UPN 7826 after the HyTK-positive T-cell infusion and stimulated twice 1 week apart with γ-irradiated HyTK-expressing donor T cells (A-C) or with HSV-TK–expressing donor T cells (UPN 7826 [D]). To map the HLA-restricting alleles, EBV-transformed LCL lines that matched at all 4 class I MHC alleles (Auto), allogeneic LCL lines that matched only at a single class I MHC allele, and mismatched LCLs (MM) were modified to express either the Hy (▦) or the HSV-TK (▪) domain of the HyTK fusion protein. Aliquots of the CD8+ CTL lines were then examined for recognition of nontransduced LCL- (□), Hy- (▦), or HSV-TK– (▪) modified target cells. Data are shown at an effector-target ratio of 5:1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/6/10.1182_blood-2005-08-3503/4/m_zh80060692630003.jpeg?Expires=1769915935&Signature=3QpLoSaEFrPijGdf6R21B2LWuAdJKl6n3PPOdT~NdbBL21KI0cY6J--wjXzXI87GxqtwdfI~YqD9wwn6wT7BL4rscEJzWv4oBP1kp~tHyE~cOy7pSVimIh8xB7ta1oOVskQ194btB2QYMiiJJ4sxBLyPol4aWH3R4MNvN6G4Qf-OnfYtOuxw7Kzmqg5~wldGTUsiQ72ecVYxjarK9QUqeIiLj94qWwUDGGoG0PGjT9eh6dF60X4rocszeN4GwHbvlAwGZpvcJnEMTFFwrNn2Bdi04H7QxJu1kTXLNrKCcCLR~D9MmvQT7Kp7gZucZe5zWHPt8KElqTP5qCvzkqe2FA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. HyTK-specific CD8+ CTL recognize epitopes derived from both the Hy and TK domain of the HyTK fusion gene and are restricted by multiple class I MHC alleles. Samples of PBMCs were obtained from UPN 1574, UPN 4103, and UPN 7826 after the HyTK-positive T-cell infusion and stimulated twice 1 week apart with γ-irradiated HyTK-expressing donor T cells (A-C) or with HSV-TK–expressing donor T cells (UPN 7826 [D]). To map the HLA-restricting alleles, EBV-transformed LCL lines that matched at all 4 class I MHC alleles (Auto), allogeneic LCL lines that matched only at a single class I MHC allele, and mismatched LCLs (MM) were modified to express either the Hy (▦) or the HSV-TK (▪) domain of the HyTK fusion protein. Aliquots of the CD8+ CTL lines were then examined for recognition of nontransduced LCL- (□), Hy- (▦), or HSV-TK– (▪) modified target cells. Data are shown at an effector-target ratio of 5:1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/6/10.1182_blood-2005-08-3503/4/m_zh80060692630003.jpeg?Expires=1769915936&Signature=ykCCfBef5Q5wvNvtA-jv3WGUqcjh-gPjGBofN3-iz1fuaT6Jup-Vyk9GfJ~jqFYNX9BHGGua3QFLVAKwmLlgKorHNnKL7YfXusYUcBalMPU4zOftX4GM5wEETRPm6CF7-8obwgyr4AIdyDyb2Gh4eejx5LZR8qkslRZrstCt63bs~d0f7cH8huXnD9W5vn7VaH8WXLbEi0kDTSHg~TAwFs2D6DEQC7yNawwlC2H0-Zm6XdvLlHaerpURCeDa68y2QqR0cZVk8r6sSkP3rRqeXvEVby6E9i92BlSr8FXq~BbTLKu5aNa47j4Bl-~VyzY7HyWMekHMdHb64CuQgGvb4w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)