Despite a rapidly accumulating clinical experience with autologous stem cell transplantation (ASCT) as a treatment for severe refractory autoimmune disease, data on the mechanisms by which ASCT induces immune tolerance are still very scarce. In this study it is shown that ASCT restores immunologic self-tolerance in juvenile idiopathic arthritis (JIA) via 2 mechanisms. First, ASCT induces a restoration of the frequency of FoxP3 expressing CD4+CD25bright regulatory T cells (Tregs) from severely reduced numbers before ASCT to normal levels after ASCT. This recovery is due to a preferential homeostatic expansion of CD4+CD25+ Tregs during the lymphopenic phase of immunereconstitution, as measured by Ki67 and CD44 expression, and to a renewed thymopoiesis of naive mRNA FoxP3 expressing CD4+CD25+ Tregs after ASCT. Second, using artificial antigen-presenting cells to specifically isolate self-reactive T cells, we demonstrate that ASCT induces autoimmune cells to deviate from a proinflammatory phenotype (mRNA interferon-γ [IFN-γ] and T-bet high) to a tolerant phenotype (mRNA interleukin-10 [IL-10] and GATA-3 high). These data are the first to demonstrate the qualitative immunologic changes that are responsible for the induction of immune tolerance by ASCT for JIA: the restoration of the CD4+CD25+ immune regulatory network and reprogramming of autoreactive T cells.
Introduction
Improved safety of autologous stem cell transplantation (ASCT) over the last decade has been followed by increasing acceptance of ASCT as an experimental treatment for severe autoimmune diseases that are resistant to conventional treatment. While we and others have convincingly described the beneficial effect of ASCT for some forms of refractory autoimmune disease, for instance, a persistent suppression of inflammatory disease activity, exactly how autografting achieves remission has yet to be defined.2-6 The initial temporary effect is likely to be attributable to the eradication of autoreactive lymphocytes and memory cells due to the high-dose lymphoablative conditioning regimen. However, althoughASCT permits intense host immune suppression, elimination of every resting lymphocyte with high-dose chemotherapy and/or radiation is probably not feasible. In addition, there must be a significant contribution from altered immune reconstitution that occurs after autologous transplantation.7 Isolated observations, including alterations in CD4/CD8 ratios, decreased mitogenic responsiveness, and restoration of reduced perforin expression have been made following autologous transplantation.8-10 Furthermore, fetal animal work is supportive of the hypothesis that exposure of the developing immune system to neoantigens in a period when the immune system is developing its repertoire leads to tolerance.11-13 These observations suggest that the success of ASCT is not only based on the loss of autoreactive T-cell clones, but also on the complete reassignment of imbalanced cellular and soluble networks. Confirming this hypothesis, recent analysis of the T-cell repertoire in multiple sclerosis patients treated with ASCT by Muraro et al14 showed the reconstitution of an overall broader clonal diversity and a renewal of clonal specificities compared with pretherapy. In our study we substantiated which exact immunologic changes within this new repertoire are responsible for the induction of immune tolerance after ASCT and analyzed the 2 cell types that are thought to be of most importance in the development of most autoimmune diseases: the autoreactive T cells themselves and the regulatory T cells that are normally supposed to control them.
To assure the timely and efficient dampening of immune responses, the immune system harbors a network of regulatory T cells (Tregs). The most important group of Tregs is currently identified by the expression of CD25 and the transcription factor FoxP3.15 CD4+CD25+ Tregs play a key role in the maintenance of immunologic tolerance to both self and foreign antigens. This occurs by suppressing aggressive T-cell responses, and in multiple experimental animal models it has been shown that, in the absence of these so-called CD4+CD25+ Tregs, the risk of developing autoimmunity is significantly increased.16,17 Studies on CD4+CD25+ Tregs in human disease are still limited.18 We recently published data showing that a lower number of CD4+CD25+ Tregs in the synovial fluid of children with juvenile idiopathic arthritis (JIA) is correlated with the development of a less favorable clinical course of the disease.19
JIA is the most frequent rheumatic disease in childhood.20 Since 1997 ASCT has been applied in a small group of treatment-resistant JIA patients.2,3 The results of a recently published survey of 34 cases, who received transplants in 9 different pediatric bone marrow transplantation units, were impressive with a prolonged drug-free follow-up of 12 to 60 months.2
To address the question whether changes in autoreactive T cells themselves and/or the regulatory cells that are supposed to control them play a role in the induction of tolerance by ASCT, we studied 12 patients with JIA that we prospectively followed for up to 5 years after receiving ASCT. Arthritis-related, autoreactive CD4+ T cells with the same peptide specificity before and after ASCT were analyzed by using artificial antigen-presenting cells (aAPCs).21 Our results provide evidence that 2 mechanisms are attributable to the induction of tolerance by transplantation: the recovery of the CD4+CD25+ regulatory network and the reprogramming of arthritis-related autoimmune T cells.
Patients, materials, and methods
Patients and transplant characteristics
Twelve patients who received ASCT for refractory JIA (Table 1) and 8 JIA patients on conventional therapy were analyzed. The inclusion and exclusion criteria for ASCT as well as the clinical follow-up of the 12 patients who received transplants is described in detail in earlier publications.2,22 The graft, harvested at least 1 month prior to ASCT, was either purged by T-cell depletion with CD2 and CD3 antibodies or by positive stem-cell selection using CD34 selection devices. These techniques yielded a final suspension with a CD34+ cell count of 0.44 to 6.0 × 106 cells per kg (mean 2.2 × 106 cells per kg) and with a CD3+ cell count of 0.5 to 28.4 × 105 cells per kg (mean 5.6 × 105 cells per kg). The conditioning regimen included 4 days of antithymocyte rabbit immunoglobulin (ATG, SangStat, Fremont, CA), 5 mg/kg from day -9 to -6, cyclophosphamide, 50 mg/kg/d from day -5 to -2, and low-dose total body irradiation (4 gray, single fraction) on day -1. Informed consent was obtained either from parents/guardians or from the children directly when they were older than 12 years old (The Netherlands). Approval for these studies was obtained from the Utrecht University Medical Center institutional review board.
Blood samples and cell separation
Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll Isopaque density gradient centrifugation (Ficoll-Paque, Pharmacia, Uppsala, Sweden). CD4+ T cells were purified by using magnetic bead-activated cell sorting (MACS, Miltenyi Biotec, Bisley, Surrey, Great Britain). In brief, PBMCs were incubated for 20 minutes at 4°C with CD4-coated magnetic beads (10 μL/10 × 106 cells). After washing, the cells were passed through lymphocyte-depletion (LD) columns within the MACS device. The resulting CD4+ T-cell fraction was subsequently sorted into CD4+CD25-, CD4+CD25bright, CD4+CD25+CD45RO, and CD4+CD25+CD45RA T cells by FACS (FACSVantage, Becton Dickinson, San Jose, CA). The buffer used throughout the whole procedure was phosphate-buffered saline (PBS) supplemented with 2% fetal calf serum (FCS) and 2 mM EDTA. T-cell purity was more than 97%.
Functional assays
For functional assays 5 × 103 FACS-sorted CD4+ T cells depleted or not of the CD4+CD25bright fraction were cultured in a 96-well plate and coated with anti-CD3 (OKT-3, 1 μg/mL) with or without the addition of interleukin-2 (IL-2) (10 ng/mL, Eurocetus, Amsterdam, The Netherlands). PBMCs depleted of T cells using anti-CD3 beads and irradiated with 3500 rad were used as antigen-presenting cells (APCs). We added 3 × 104 APCs to each well. The cells were incubated at 37°C for 6 days, the last 18 hours in the presence of [3H]thymidine (1 μCi[0.037 MBq]/well). Proliferative responses were calculated as the mean [3H]thymidine incorporation (cpm) of triplicate wells.
Flow cytometry
The following phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)-, cychrome (CY)-, or APC-labeled mAbs were used: anti-human CD4 (RPA-T4), CD25 (2A3), CD45RA (L48), CD45RO (4CHL-1), CD44 (G44-26), CCR4 (1G1), GITR (110416), CTLA-4 (BN13), and Ki67 (MIB-1). For intracellular staining of CTLA-4 and Ki67, the cells were first surface stained, then fixed in Cytofix/Cytoperm solution (20 minutes, 4°C) and washed twice in Perm/Wash solution (Cytofix/perm kit, Becton Dickinson), followed by incubation with anti-CTLA-4 or Ki67 mAb. GITR-specific mAb was obtained from R&D (Bad Nauheim, Germany) and Ki-67 from Immunotech (Marseilles, France). All other mAbs were obtained from Becton Dickinson. Stained mononuclear cells were diluted in sheath fluid and run on a FACSCalibur (Becton Dickinson). CellQuest software (BD Biosciences, San Jose, CA) was used for analysis.
mRNA analysis by quantitative PCR
Total RNA was isolated using Tripure isolation reagent (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. First-strand cDNA was synthesized from total RNA using Moloney murine leukemia virus reverse transcriptase (M-MLV, Promega, Madison WI) with 1 μg/μL oligo (dT) and 10 mM dNTPs (both from Amersham Pharmacia Biotech AB, Uppsala, Sweden). The reaction mixture was incubated at 40°C for 90 minutes, followed by incubation at 70°C for 15 minutes. Messenger RNA expression levels of FoxP3, IL-10, IFN-γ, GATA3, and T-bet were determined by real-time quantitative polymerase chain reaction (PCR) on a LightCycler (Roche Diagnostics). β2-microglobulin (B2M) was analyzed as a housekeeping gene. The following combination of primers and probes were used: FoxP3 forward 5′ TCA AGC ACT GCC AGG CG 3′, FoxP3 reverse 5′ CAG GAG CCC TTG TCG GAT 3′, IL-10 forward 5′ TGA GAA CAG CTG CAC CCA CTT, IL-10 reverse 5′ GCT GAA GGC ATC TCG GAG AT, IFN-γ forward 5′ GCA GAG CCA AAT TGT CTC CT, IFN-γ reverse 5′ ATG CTC TTC GAC CTC GAA AC, T-bet forward 5′ CCC CAA GGA ATT GAC AGT TG, T-bet reverse 5′ GGG AAA CTA AAG CTC ACA AAC, Gata3 forward 5′ CTG CAA TGC CTG TGG GCT C, Gata3 reverse 5′ GAC TGC AGG GAC TCT CGC T, β2m forward 5′ CCA GCA GAG AAT GGA AAG TC 3′, β2m reverse 5′ GAT GCT GCT TAC ATG TCT CG 3′.
To quantify mRNA amounts, the following protocol was used. A standard curve was generated with a dilution series of a reference cDNA sample, which was run at the same time as the unknown samples. Data are expressed as normalized gene expression, which was obtained by dividing the relative quantity of the gene of interest for each sample by the relative quantity of B2M for the same sample.
T-cell capture and artificial APC
This technique is extensively described earlier.21 Compared to the previously described protocol, a few improvements were made. In short, PBMCs of 2 DR4 homozygotic, JIA patients who received transplants, obtained before and after transplantation, were cultured with or without a peptide of human heat shock protein 60, hsp60 280-294 (GEALSTLVLNRLKVG).23 After 4 days the cells were prestained with anti-CD4-CY and subsequently incubated with artificial antigen-presenting cells (aAPCs) for 2 hours at 37°C. Before sorting the cells on the FACSVantage (Becton Dickinson), cells and aAPCs were washed twice and resuspended in FACS buffer. The aAPCs were prepared as follows. Phosphatidylcholine and cholesterol (Sigma, St Louis, MO) were combined in a glass tube at a molar ratio of 7:2. The solvent was evaporated under an Argon stream for 30 minutes and dispersed at a final concentration of 10 mg/mL in 140 mM NaCl and 10 mM Tris·HCl, pH 8 (buffer A) containing 0.5% sodium deoxycholate. Monosialoganglioside-GM1 (Sigma G-7641) was added at a final concentration of 0.28 mM. The solution was sonicated until clear and was stored at -20°C. Liposomes were formed through dialysis at 4°C against PBS in a 10-kDa Slide-A-Lyzer (Pierce, Rockford, IL) for 48 hours. Biotinylated recombinant major histocompatibility complex (MHC) was incubated with the peptide (6 hours, room temperature). The resulting MHC-peptide complexes were incorporated in rafts, engineered on the aAPC surface. The rafts were constructed by mixing biotinylated HLA-DR4 molecules, biotinylated antibodies to CD28 and anti-LFA-1, and biotinylated cholera toxin subunit B-FITC conjugated (CTB-FITC; Sigma) in the appropriate (equal) molar ratio. Next, neutravidin (NA; Pierce) was added in a molar ratio of 4 biotinylated moieties per molecule of NA. CTB-FITC was used to visualize T cells bound by the aAPCs. After incubation (1.5 hours at room temperature), the Raft-NA mixture was added to the liposomes for 2 hours, again at room temperature, and washed 3 times in PBS. Finally, once the aAPCs were generated, they were incubated with the stained cells.
Statistical analysis
Basic descriptive statistics were used to describe the patient population. A Wilcoxon-signed rank test was used to compare numbers of and the expression of molecules on CD4+CD25+ T cells before and at different time points after ASCT. A Mann-Whitney U test was used to compare the frequency of CD4+CD25+ T cells between the different patient groups.
Results
Rapid reconstitution of CD4CD25bright T cells after ASCT
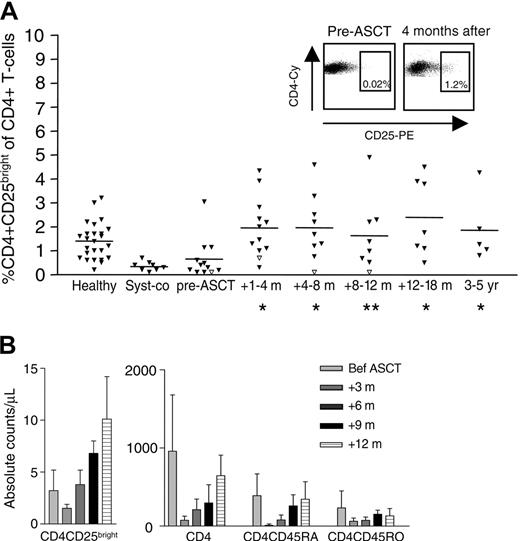
Frequencies of CD4+CD25bright T cells were determined in 8 JIA patients on conventional therapy and 12 JIA patients who received ASCT for JIA (Table 1). Both conventionally treated and ASCT-treated patients before transplantation displayed significantly lower frequencies of CD4+CD25bright T cells (Figure 1A) than previously found in healthy children (mean ± SEM: 1.6% ± 0.1%) and children with oligoarticular JIA (mean ± SEM: 1.2% ± 0.2%), a subtype of JIA with a much milder clinical course and without the systemic features.19 As early as 6 to 8 weeks after transplantation, the patient group who received transplants showed a significant increase in the relative number of CD4+CD25bright T cells. Interestingly, the patient who suffered a complete relapse 6 months after ASCT showed a clear decrease in CD4+CD25bright T-cell frequency at the time of relapse (Figure 1A, open triangles). None of the patients who gained a long-lasting and drug-free remission by ASCT showed this phenomenon.
In evaluating absolute numbers of CD4+ T cells after ASCT, the findings were consistent with published literature on patients treated with intensive chemotherapy (Figure 1B).5,24 All children analyzed displayed a profound CD4+ T-cell depletion in the first months after ASCT. The reconstitution of the CD4+CD25bright T cells followed the same pattern as the total CD4+ T-cell population. However, while it took 12 months (range, 6-18 months) for CD4+ T-cell counts to recover to baseline counts (Wilcoxon-signed rank test), absolute CD4+CD25bright T-cell counts exceeded pre-ASCT levels within 6 months (range, 4-8 months) of ASCT. Twelve months after ASCT, absolute CD4+CD25bright T-cell counts were 1.3 to 6.5 times higher compared to baseline levels before ASCT.
Recovery of CD4+CD25bright T-cell frequency after ASCT. The relative number of CD4+CD25bright T cells in 25 healthy controls, 8 systemic JIA patients on conventional therapy (syst-co), and 12 children who received ASCT for refractory JIA was measured by FACS staining. The patient represented by open triangles suffered a complete relapse 6 months after ASCT. Since it has been shown that the regulatory CD4+ T cells preferentially reside within the CD4+CD25bright population, only the CD4+CD25bright T cells and not the CD4+CD25 total T cells were analyzed. *P < .05; **P = .06. The dotplots show a representative example of the increase in CD4+CD25bright T cells after ASCT (A). Mean and SEM of the absolute CD4+ T-cell counts per microliter in 12 children before and after ASCT for refractory JIA. For comparison, mean CD4+ T-cell count in healthy children of the same age: 1.0 × 109/L (1000/μL); range, 0.3-2.0 × 109/L (300-2000/μL) (B).1
Recovery of CD4+CD25bright T-cell frequency after ASCT. The relative number of CD4+CD25bright T cells in 25 healthy controls, 8 systemic JIA patients on conventional therapy (syst-co), and 12 children who received ASCT for refractory JIA was measured by FACS staining. The patient represented by open triangles suffered a complete relapse 6 months after ASCT. Since it has been shown that the regulatory CD4+ T cells preferentially reside within the CD4+CD25bright population, only the CD4+CD25bright T cells and not the CD4+CD25 total T cells were analyzed. *P < .05; **P = .06. The dotplots show a representative example of the increase in CD4+CD25bright T cells after ASCT (A). Mean and SEM of the absolute CD4+ T-cell counts per microliter in 12 children before and after ASCT for refractory JIA. For comparison, mean CD4+ T-cell count in healthy children of the same age: 1.0 × 109/L (1000/μL); range, 0.3-2.0 × 109/L (300-2000/μL) (B).1
FoxP3 has been identified as a specific marker of CD4+CD25+ Tregs, distinguishing them from recently activated, nonregulatory CD4+CD25+ T cells.15,25 The early-reconstituted CD4+CD25bright T cells expressed high amounts of mRNA FoxP3 and thus can be considered as professional regulatory T cells. The levels of mRNA FoxP3 in these CD4+CD25+ T cells were significantly higher than expressed by their counterparts obtained from time points before transplantation and comparable to levels found in healthy controls (Table 2). Furthermore, the early-reconstituted CD4+CD25bright T cells (1 month after ASCT) showed an increased expression of CTLA4, GITR, and CCR4 compared to their counterparts before ASCT (Table 3). CTLA4, GITR, and CCR4 are constitutively expressed on CD4+CD25+ Tregs.26-28 It is conceivable that the increase found in CTLA4, GITR, and CCR4 expression represents the increased proportion of professional Tregs within the analyzed CD4+CD25+ T cells.
The division rates of CD4+CD25bright Tregs and CD4CD25- T cells are not equally increased early after ASCT
After ASCT the immune system is reconstituted via 2 distinct pathways.29 In the first 2 months after transplantation the T-cell compartment repopulates rapidly through lymphopenia-induced expansion of mature T cells that have survived the preconditioning and/or the residual T cells co-infused with the graft. The alternative way of reconstitution is thymic dependent and might be considered as a recapitulation of ontogeny. It was hypothesized that the rapid recovery of the CD4+CD25bright T-cell frequency after ASCT is the result of a preferential proliferation of CD4+CD25bright T cells in the early phase of the immune reconstitution. This was supported by the finding that CD4+CD25bright T cells showed a significantly higher expression of CD44 in the first 3 months after transplantation compared to CD4+CD25- T cells and compared to their counterparts before transplantation (Table 3). To further substantiate this hypothesis, cell proliferation in CD4+CD25bright and CD4+CD25- T cells were measured separately by the analysis of Ki67. Ki67 is a protein pivotal for cell division and is expressed exclusively by cells that are in cell cycle.30 Since infections may induce increased proportions of T cells to divide, only patients who did not show any sign of an infection in this early period after transplantation were analyzed on Ki67. In each patient analyzed the same pattern of Ki67 staining was seen. As could be expected,31 before ASCT CD4+CD25bright T cells showed a higher division rate compared to CD4+CD25- T cells (Ki67 + mean ± SEM, pre-ASCT: 3.8% ± 0.5% versus 0.8% ± 0.1%). Due to lymphopenia-induced homeostatic proliferation in the early period after ASCT, the division rate was highly increased in both populations (25.3% ± 9.4% versus 14.7% ± 4.9%). Confirming the hypothesis, the most pronounced increase in division rate was seen in the CD4+CD25bright T-cell population (+21.5% versus +13.9% in CD4+CD25- T cells). Given the assumption that CD4+CD25- and CD4+CD25bright T cells equally survived the conditioning regimen, it can be calculated from these division rates that after ASCT, fewer than 35 cycles of proliferation are needed to result in the observed 15-time increase in CD4+CD25bright T-cell frequency. Thirty-five cycles of proliferation are easily accomplished within 6 weeks.32 Thus, it is likely that the more pronounced increase in division rate in the CD4+CD25bright T cells explains the recovery of the CD4+CD25bright T-cell frequency as seen within 6 weeks of ASCT.
The thymus contributes substantially to the reconstitution of CD4+CD25+ regulatory T cells after transplantation
We next investigated the contribution of the thymus to the reconstitution of CD4+CD25+ Tregs. The best way to determine the source of the early-reconstituted CD4+CD25bright Tregs is to correlate FoxP3 mRNA levels in sorted CD4+CD25bright Tregs with the presence of T-cell rearrangement excision circles (TRECs). However, not enough material was available to sort CD4+CD25bright T cells and perform both quantitative assays. We therefore used an alternative way to identify recent thymic emigrants. It has been suggested that after transplantation, the expression of CD45RA can be used as a surrogate approximation of T-cell neogenesis and confound accurate estimates of thymic activity.33-35 Since CD45RA may switch to CD45RO in the periphery and vice versa, this approximation is the most accurate at the time point the thymus just becomes active, 4 to 6 months after transplantation.
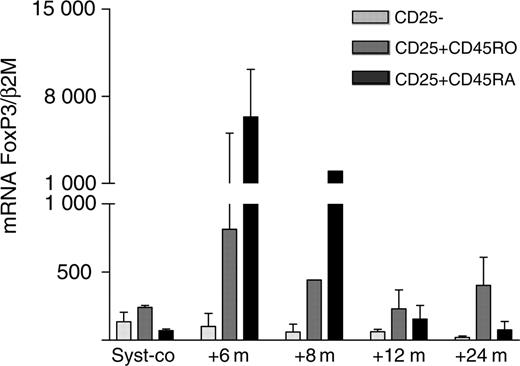
The frequencies of CD4+CD45RAand CD4+CD25+CD45RAcells and the expression of FoxP3 mRNA by these cells were analyzed at different time points after transplantation. The first CD4+CD45RA cells were detectable 3 to 9 months after transplantation, and 12 to 18 months after transplantation the ratio of naive and memory T cells resembled the ratio before transplantation.2 CD25 expression on CD4+CD45RA T cells was readily detectable and expressed in the same percentage as on CD4+CD45RO cells. However, while the CD25 density on the CD4+CD45RA cells was less bright than on CD4+CD45RO cells, as reflected by a lower mean fluorescence intensity, CD4+CD25+CD45RA cells sorted at time points 6 and 8 months after transplantation expressed extremely high levels of mRNA FoxP3 (Figure 2). Hereafter, mRNA FoxP3 levels rapidly decreased again until pretransplantation levels, most conceivably due to the conversion of CD25+CD45RA to CD25+CD45RO cells and vice versa, and to a decreased output of Tregs by the thymus once the immune reconstitution was complete.
CD4+CD25bright T cells are partly responsible for the hyporesponsive state of PBMCs during the early phases of immune reconstitution
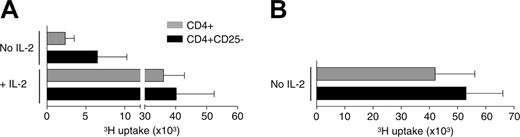
T cells of allogeneic as well as autologous patients who received transplants show a markedly decreased mitogenic responsiveness in vitro during the first 6 to 12 months after transplantation.36 In the patients evaluated in this report, in vitro mitogenic responses were diminished for 12 months (mean ± 1.3 months) after ASCT.2 Gavin et al37 previously have shown that homeostatic proliferation does not abolish but rather augments CD4+CD25+ Treg-cell function. This observation raised the question whether the described in vitro hyporesponsiveness of CD4+ T cells obtained during the early phases of reconstitution could be due to the increased frequency and/or function of homeostatic proliferated CD4+CD25+ Tregs. Impaired by the low cell counts early after ASCT, we tested this hypothesis by comparing proliferative responses of CD4+ T cells and CD4+ T cells depleted of CD4+CD25bright cells upon anti-CD3 stimulation. Depletion of CD4+CD25bright T cells indeed resulted in a marked increase in the response of the remaining CD4+CD25- T cells (Figure 3A). However, this response was still impaired when compared to the response of CD4+ and CD4+CD25- T cells obtained from healthy controls (Figure 3B). A complete reversion of the hyporesponsive state of the CD4+CD25- T cells could be induced only by the addition of high-dose IL-2 to the cultures (Figure 3A). Blocking mRNA IL-2 transcription in responder cells is one of the mechanisms CD4+CD25+ Tregs use to exert their regulatory function.38 Therefore, these data may indicate that under lymphopenic conditions CD4+CD25+ Tregs can induce an IL-2 transcription block in CD4+CD25- responder T cells that holds even in the absence of Tregs. Alternatively, the lymphopenic conditions may have induced intrinsic changes in the CD4+CD25- responder cells, independent of CD4+CD25+ Tregs. Notably, Setoguchi et al39 recently have shown that though IL-2 is indispensable for the peripheral maintenance of CD4+CD25+ Tregs in a normal nonlymphopenic environment, the homeostatic proliferation of CD4+CD25+ Tregs in a lymphopenic environment is IL-2 independent.
High expression of mRNA FoxP3 in thymus-derived CD4+CD25+ T cells. CD4+CD25-, CD4+CD25+CD45RO, and CD4+CD25+CD45RA T cells were isolated from the PB of 3 systemic JIA patients on conventional therapy and from 3 systemic JIA patients at different time points after ASCT by FACS sorting. mRNA FoxP3 was measured by quantitative PCR. Data are expressed as the mean normalized gene expression (± SEM) of the 3 patients.
High expression of mRNA FoxP3 in thymus-derived CD4+CD25+ T cells. CD4+CD25-, CD4+CD25+CD45RO, and CD4+CD25+CD45RA T cells were isolated from the PB of 3 systemic JIA patients on conventional therapy and from 3 systemic JIA patients at different time points after ASCT by FACS sorting. mRNA FoxP3 was measured by quantitative PCR. Data are expressed as the mean normalized gene expression (± SEM) of the 3 patients.
Changes in arthritis-related autoreactive T cells after ASCT
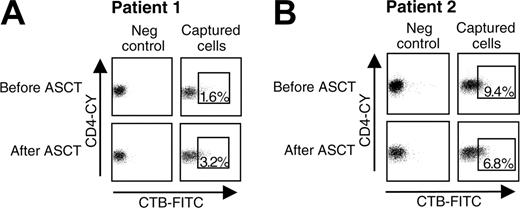
Seven to 18 months after transplantation the hyporesponsive state of CD4+ T cells had completely reversed. Although theoretically the change in the CD4+CD25bright Treg-cell population observed after ASCT alone may explain the induction of tolerance by ASCT, evidence exists that autoreactive T cells themselves may have undergone persistent intrinsic changes as well. The only way to investigate this hypothesis properly is by comparison of antigen-specific T cells before and after ASCT. A newly developed technique was used that uses artificial aAPCs and is designed to capture peptide-specific CD4+ T cells.21,40 Artificial APCs containing HLA-DR4 molecules were loaded with a peptide of human heat shock protein 60 (hsp60). Previous studies have extensively described the involvement of T-cell reactivity to autologous hsp60 in down-regulating inflammation in adjuvant arthritis,41,42 as well as JIA.43-46 Recently, we identified novel pan-DR binding T-cell epitopes from hsp60 that allow the study of the arthritis-related T-cell repertoire at the molecular level.23 Using aAPCs loaded with one of the new self-hsp60 epitopes enabled us to isolate self-hsp60-specific T cells from a DR4 heterozygotic patient who gained complete remission after ASCT (Figure 4A, Table 4). The cells were obtained from 2 time points, before and 2 years after ASCT. Furthermore, with the same epitope, self-hsp60-specific T cells were sorted from a patient who showed a complete relapse of disease after having been in complete remission for 7 years by ASCT. From this DR4 heterozygotic patient, cells were sorted from a time point the patient was still in remission (5 years after ASCT) and from a time point during the relapse (Figure 4B, Table 4). Analysis of mRNA expression levels revealed major differences between the hsp60-specific T cells obtained during active disease periods and during the tolerant period induced by ASCT. An increased expression of mRNA GATA-3 and IL-10 and a decreased expression of mRNA IFN-γ clearly indicated a more Th2 or regulatory phenotype of the human hsp60-specific T cells present after ASCT. Thus, besides the recovery of the CD4+CD25+ regulatory network, ASCT also induces tolerizing changes in the autoreactive T-cell clones.
The hyporesponsive state of CD4+ T cells obtained early after ASCT is partially reversed by the depletion of CD4+CD25bright cells and completely reversed by the addition of IL-2. FACS-sorted CD4+ T cells and CD4+ T cells depleted of CD4+CD25bright T cells (CD4+CD25- T cells) of 4 patients obtained 3 to 6 months after ASCT (A) and 3 healthy controls (B) were cultured in the presence of plate-bound anti-CD3, APCs, and with or without the addition of IL-2 (10 ng/mL). Proliferation was measured after 5 days by tritium incorporation.
The hyporesponsive state of CD4+ T cells obtained early after ASCT is partially reversed by the depletion of CD4+CD25bright cells and completely reversed by the addition of IL-2. FACS-sorted CD4+ T cells and CD4+ T cells depleted of CD4+CD25bright T cells (CD4+CD25- T cells) of 4 patients obtained 3 to 6 months after ASCT (A) and 3 healthy controls (B) were cultured in the presence of plate-bound anti-CD3, APCs, and with or without the addition of IL-2 (10 ng/mL). Proliferation was measured after 5 days by tritium incorporation.
Differences in mRNA expression in peptide-specific T cells before and after ASCT. (A) PBMCs of a DR4 heterozygotic patient, from time points before (full-blown disease) and 2 year after ASCT (complete remission) and (B) from a DR4 heterozygotic patient, from time points 5 years after ASCT (patient still in remission) and 7 years after ASCT (complete relapse of disease) were stimulated with a peptide derived of human hsp60. After 4 days, peptide-specific CD4+ T cells were captured and sorted, using the indicated gates. CTB-FITC and CD4-CY were used to visualize CD4+ T cells bound by the aAPCs. Liposomes containing complete rafts but no MHC-peptide complexes were used as negative controls. The sorted peptide-specific CD4+ T cells were lysed and analyzed on the mRNA expression of the transcription factors T-bet and GATA-3 and the cytokines IL-10 and IFN-γ by quantitative PCR.
Differences in mRNA expression in peptide-specific T cells before and after ASCT. (A) PBMCs of a DR4 heterozygotic patient, from time points before (full-blown disease) and 2 year after ASCT (complete remission) and (B) from a DR4 heterozygotic patient, from time points 5 years after ASCT (patient still in remission) and 7 years after ASCT (complete relapse of disease) were stimulated with a peptide derived of human hsp60. After 4 days, peptide-specific CD4+ T cells were captured and sorted, using the indicated gates. CTB-FITC and CD4-CY were used to visualize CD4+ T cells bound by the aAPCs. Liposomes containing complete rafts but no MHC-peptide complexes were used as negative controls. The sorted peptide-specific CD4+ T cells were lysed and analyzed on the mRNA expression of the transcription factors T-bet and GATA-3 and the cytokines IL-10 and IFN-γ by quantitative PCR.
ASCT has emerged in recent years as the first opportunity to offer patients with refractory forms of autoimmune disease a potentially curative treatment. While clinical experience with this relatively new treatment is rapidly accumulating, still very little is known about which changes in the immune system induced by ASCT are responsible for the favorable effect.
Three nonmutually exclusive hypotheses can be formulated: (1) immune ablation eliminates autoreactive T-cell clones; (2) autoreactive T-cell clones are rendered tolerant; and (3) regulatory networks controlling the autoreactive T cells are restored. In an attempt to address this issue a study was conducted of both the immune reconstitution and function of CD4+CD25+ Tregs, and self-antigen-specific T cells before and after ASCT in a group of 12 patients who received ASCT for JIA.
Normally, for a given age and genetic background, CD4+CD25bright Tregs represent a stable proportion of the CD4+ T cells in the steady state, suggesting that the homeostasis of Tregs is tightly regulated.47 The data from this study showed a severely reduced frequency of CD4+CD25bright Tregs in the peripheral blood of systemic JIA patients, which in all but one patient was completely restored, even after long-term follow-up, by the treatment with ASCT. This one patient was one of the 2 patients who showed a complete relapse of the disease: all others gained a partial or complete remission of the disease after ASCT. Since ASCT obviously is not successful in the restoration of genetic defects, it can now be concluded that the low frequency of CD4+CD25bright Tregs found in systemic JIA patients is the result of either environmental factors envisioned before the onset of disease, the disease itself, or the immunosuppressive drugs the patients received in an attempt to control the disease.
After ASCT the CD4+CD25+ Tregs reconstitute via the same 2 pathways as described for CD4+ T cells in general.29,35,48 Thus, in the first period after transplantation, CD4+CD25+ Tregs reconstitute via clonal expansion, while in the course of several months, a thymic-dependent regeneration of naive CD4+CD25+ Tregs is seen. As early as 2 to 3 month after ASCT, during this first period of clonal expansion, the severely reduced CD4+CD25+ Treg-cell frequency, as seen in each of our patients before ASCT, had completely recovered and reached percentages of CD4+ T cells as found in healthy controls (1.5%-3%).19 This early recovery of the CD4+CD25+ Treg frequency is at least partly the result of a highly preferential proliferation of CD4+CD25+ Tregs above CD4+CD25- T cells. It is conceivable that this preferential proliferation of CD4+CD25+ Tregs during lymphopenia is explained by differences in response to TCR versus lymphopenia-induced signals between conventional CD4+CD25- T cells and CD4+CD25+ Tregs.49,50 Besides the preferential proliferation of CD4+CD25+ Tregs afterASCT, the CD4+CD25+ Tregs may have selectively survived the conditioning regimen, a mechanism that was recently shown in a murine model for chronic graft-versus-host disease.51
After the first period of clonal proliferation the first naive, thymus-derived T cells appeared. These naive T cells showed an intermediate expression of CD25, but nevertheless extremely high levels of mRNA FoxP3, reaching levels even 10 times higher than found in CD4+CD25bright T cells of healthy children.19 Thus, both an increased preferential proliferation during lymphopenia and a significant output by the thymus contribute to the reconstitution of CD4+CD25+ Tregs after ASCT and, thus, to the recovery of these cells in these patients.
During the first 12 months after ASCT, the regenerated CD4+ T cells showed an impaired proliferation to polyclonal stimulators in vitro. This hyporesponsive state was partly abrogated by the depletion of CD4+CD25bright T cells. A complete reversion of the hyporesponsive state of CD4+CD25- T cells was observed only when IL-2 was added to the cultures. Given the fact that CD4+CD25+ Tregs function by suppressing the transcription of IL-2 mRNA in their target cells, this finding may suggest that during the lymphopenic period after ASCT CD4+CD25+ Tregs suppress newly reconstituting CD4+CD25- T cells.37,38 In other words; the rapidly reconstituting CD4+CD25+ Tregs, not unlikely in interaction with other regulatory cells not studied yet, seem to provide a tolerant environment in which the reconstitution of the rest of the immune system subsequently takes place.
After 12 months the hyporesponsive state was reversed, heralding the time the immune system had fully reconstituted. To investigate whether ASCT induced permanent changes in autoreactive T cells, such as changes that persist at time points at which no additional suppressive effect of homeostatic proliferation can be expected, a newly developed technique was used to capture peptide-specific T cells. Using this technique, it was found that the same autoantigen-specific T cells show a clear deviation to a more regulatory phenotype during the tolerant period induced by ASCT. Thus, besides restoring the CD4+CD25+ regulatory network, ASCT brought about persistent changes in autoreactive T cells as well.
On evaluation of the clinical follow-up of 34 JIA patients who received ASCT, 90% of all partial and complete relapses of disease occurred in the first 9 months after ASCT.2 This observation correlates markedly well with the recovery of naive CD4+CD25+ Tregs by the thymus that reaches a maximum in this same period. Thus, while the rapidly reconstituting CD4+CD25+ Tregs provide a tolerant environment in the first months after ASCT, a well-functioning thymus subsequently seems needed to optimize the CD4+CD25+ Treg repertoire and sustain the remission. Although it has been shown that children recover faster and have a better reconstitution of their T-cell repertoire than adults,52,53 recent analysis of the T-cell repertoire in adult multiple sclerosis patients treated with ASCT showed a significant thymus-dependent immune rejuvenation in these adult patients as well.14 More extensive studies in children as well as adults are now needed to confirm a correlation between thymic regeneration and clinical outcome.
In conclusion, ASCT for refractory JIA seems to induce immunologic self tolerance by reprogramming arthritis-related autoimmune T cells and restoring the CD4+CD25+ regulatory network. These observations could guide the design of new protocols for ASCT, aiming at higher remission rates. The data suggest that dose intensification, or new preparative regimens that cause a further depletion of T cells, is not the answer to improving outcomes. Directing the reconstitution and/or activity of regulatory T cells during the first months of immune reconstitution may provide a mechanism by which a higher incidence of patients can be cured.
Prepublished online as Blood First Edition Paper, November 1, 2005; DOI 10.1182/blood-2005-07-2800.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Wilco de Jager and Brenda Hendriks for their help on the molecular work, Erica Roks for excellent secretarial assistance, and Lindi Belfield for reading and editing the manuscript.