KBM5 cells, derived from a patient with blast crisis Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML), and imatinib-resistant KBM5 (KBM5-STI571) cells were found to express high levels of survivin. Inhibition of Bcr-Abl by imatinib significantly decreased survivin expression and cell viability in KBM5, but much less so in KBM5-STI571 cells. Inhibition of MEK, downstream of the Bcr-Abl signaling cascade decreased survivin expression and cell viability in both KBM5 and KBM5-STI571 cells. In addition, down-regulation of survivin by a survivin antisense oligonucleotide (Sur-AS-ODN) inhibited cell growth and induced maximal G2M block at 48 hours, whereas cell death was observed only at 72 hours in both KBM5 and KBM5-STI571 cells as shown by annexin V staining. Further, the combination of Sur-AS-ODN and imatinib induced more cell death in KBM5 cells than did either treatment alone. Down-regulating survivin also decreased colony-forming units (CFUs) in blast crisis CML patient samples. Our data therefore suggest that survivin is regulated by the Bcr-Abl/MAPK cascade in Ph+ CML. The facts that down-regulating survivin expression induced cell-growth arrest and subsequent cell death regardless of the cell response to imatinib and enhanced the sensitivity to imatinib suggest the potential therapeutic utility of this strategy in patients with CML, both imatinib sensitive and resistant.
Introduction
Survivin, a member of the inhibitor of apoptosis (IAP) family of proteins, plays important roles in both cell proliferation and cell death.1,2 Normally, it is highly expressed during fetal development but is undetectable in terminally differentiated adult tissues. However, survivin is prominently expressed in transformed cell lines, most common human cancers, and various hematopoietic malignancies.3 Indeed, its expression has been associated with poor prognosis and increased frequency of relapses in numerous cancers4-6 as well as in diffuse large B-cell lymphomas,7 and acute myelogenous leukemia (AML).8 In particular, survivin is overexpressed in AML blasts compared with normal CD34+ cells.9 Recently, survivin was also found to be expressed in accelerated/blast phase chronic myelogenous leukemia (CML) but was low10 or undetectable11,12 in chronic phase CML.
CML is a myeloproliferative disorder that results from the clonal expansion of transformed hematopoietic stem cells.13 Over 95% of patients with CML are Philadelphia chromosome positive (Ph+) due to the reciprocal translocation t(9;22) resulting in the formation of the BCR-ABL gene. This gene codes for a constitutively active tyrosine kinase that contributes to clonal expansion, confers growth advantage on CML blasts, and plays a critical role in leukemogenesis.14-16 Imatinib mesylate (STI571, Gleevec [imatinib]) is a specific tyrosine kinase inhibitor that inhibits Bcr-Abl, Tel-Abl, V-Abl, and cKit kinase activity17-21 as well as the growth and viability of cells transformed by any of the ABL genes.22 Indeed, imatinib is the most successful drug for targeted cancer therapies and has shown great efficacy in treating chronic phase patients with interferon-resistant CML (relapse rate at 18 months is about 10%).23 However, phase 2 studies of single-agent imatinib in interferon-refractory CML showed that relapse occurred in 40% of patients in an accelerated phase and 78% of patients in myeloid blast crisis.24,25 These findings demonstrate the limited ability of this drug to eradicate the Bcr-Abl+ clones. In addition, resistance to imatinib develops due to the amplification26-28 or point mutation26,29-33 of the BCR-ABL gene, increased expression of the Bcr-Abl protein, or other mechanisms such as overexpression of the multidrug resistance P-glycoprotein or Bcr-Abl–independent growth and survival due to changes in other signaling regulators.27,28,34-36 Furthermore, even in chronic phase of CML, molecular remissions are rare because Bcr-Abl mRNA is readily detected in 60% to 90% of patients treated with imatinib. Therefore, alternative therapies are needed for the treatment of CML, especially for patients in blast crisis and resistant to imatinib.
Bcr-Abl is a constitutively active tyrosine kinase that phosphorylates several substrates, including Ras, and activates multiple signal transduction pathways such as the mitogen-activated protein kinase kinase/extracellular signal regulated kinase (MAPK/ERK) pathway responsible for cell proliferation and survival.37-40 In previous studies in AML, we found that survivin expression is regulated through the MAPK signaling pathway9 and that down-regulation of survivin by the antisense oligonucleotide (Sur-AS-ODN) induces cell-cycle block at G2M, a cell-division defect, and finally cell death.41 The regulation and function of survivin in CML has not been studied, however. Here, we report that survivin expression is regulated in CML through Bcr-Abl/MAPK signaling and that the regulation of survivin by Bcr-Abl occurs at both transcriptional and posttranslational levels. Down-regulation of survivin expression by antisense oligonucleotide-induced cell-cycle block and subsequent cell death in both imatinib-sensitive KBM5 cells and imatinib-resistant KBM5-STI571 cells and decreased colony forming units (CFUs) in bone marrow cells from patients with CML. Our results suggest that survivin, a downstream effector of Bcr-Abl, could serve as a therapeutic target for Ph+ patients with CML regardless of their responses to imatinib.
Materials and methods
Cells and cell culture
KBM5 cells42 were derived from a Ph+ CML patient in blast crisis, and KBM5-STI571 cells are an imatinib-resistant KBM5 subline.43 KBM5-STI571 cells became imatinib resistant through acquisition of a single point mutation leading to a threonine-to-isoleucine substitution at position 315 of ABL with only a marginal increase in the number of copies of the BCR-ABL gene and its level of expression.43 KBM5 cells were cultured in Iscove modified Dulbecco medium (Gibco-BRL, Gaithersburg, MD) containing 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. KBM5-STI571 cells were maintained in the same medium in the presence of 1 μM imatinib. HL-60, OCI-AML3, U937, and HL-60p185 cells (kindly provided by Dr R. Arlinghaus, University of Texas, M. D. Anderson Cancer Center) and a HL-60 cell line stably transfected with BCR-ABL fusion gene expressing p18544 were cultured in RPMI 1640 medium containing 10% FCS, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. CML patient samples and normal bone marrow were obtained after informed consent was obtained in accordance with institutional guidelines set forth by the University of Texas MD Anderson Cancer Center (Houston, TX) and the Declaration of Helsinki. Mononuclear cells were purified by Ficoll-Hypaque (Sigma Chemical, St Louis, MO) density-gradient centrifugation and cultured in the same medium as KBM5 cells. Study protocols were approved by the institutional review board of the University of Texas MD Anderson Cancer Center.
Treatment of cells
Cells from cell lines, at a density of 0.2 × 106, or from CML patients, at a density of 1 × 106 were treated with various concentrations of the tyrosine kinase inhibitor imatinib (Novartis, Basel, Switzerland), the MEK inhibitor CI1040 (kindly provided by Dr J. Sebolt-Leopold, Pfizer, Ann Arbor, MI), or the proteasome inhibitor MG132 (Calbiochem, San Diego, CA). For KBM5 cells treated with both imatinib and MG132, MG132 was added 1 hour before imatinib.
The survivin antisense oligonucleotide (Sur-AS-ODN, ISIS-23722, 5′ TGTGCTATTCTGTGAATT 3′) and its control oligonucleotide (Sur-NS-ODN, ISIS-28598, 5′ TAAGCTGTTCTATGTGTT 3′) were obtained as generous gifts of Dr N. Dean from ISIS Pharmaceuticals (Carlsbad, CA). For the targeting of survivin expression, 3 × 106 exponentially growing KBM5 and KBM5-STI571 cells, or 3 × 106 Ficoll purified CML bone marrow and normal bone marrow cells were suspended in 100 μL nucleofection solution T and transfected with Sur-AS-ODN or Sur-NS-ODN using Nucleofector program K-17 (Amaxa Biosystems, Cologne, Germany) following the manufacturer's instructions.41,45
Cell-death analysis
Cell viability was determined by trypan blue exclusion. Apoptotic-cell death was analyzed by measuring externalized phosphatidyl serine with the Annexin V-FLUOS Staining Kit (Roche Diagnostics, Indianapolis, IN). Caspase activation was detected by flow cytometry using a fluorescein-conjugated cell-permeable peptide (FAM-VAD-FMK) that irreversibly binds to activated caspases (CaspaTag; Intergen, Purchase, NY). Changes in mitochondrial membrane potential were measured by staining cells with CMXRos and MitoTracker Green (both from Molecular Probes, Eugene, OR) followed by flow cytometry analysis according to a previously published method.46
Cell-cycle analysis
After being washed with phosphate-buffered saline (PBS), cells were fixed with 2 mL ice-cold ethanol (70%, vol/vol in water) overnight at -20°C. Cells were then collected by centrifugation and exposed to 400 μL propidium iodide (PI) staining solution (25 μg/mL PI, 180 U/mL RNase, 0.1% Triton X-100, and 30 mg/mL polyethylene glycol in 4 mM citrate buffer, pH 7.8, all from Sigma Chemical) for 30 minutes at 37°C, after which they were analyzed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). The cell-cycle distribution was then analyzed using ModFit LT software (Verity Software House, Topsham, ME).
Western blot analysis
The survivin and Bcr-Abl protein levels were determined by Western blot analysis as described previously9 using the antibodies from R&D Systems (Minneapolis, MN) and from Dr R. Arlinghaus, respectively. β-Actin was used as a loading control.
Reverse transcription and TaqMan PCR
Survivin RNA levels were measured by reverse transcription (RT) and TaqMan polymerase chain reaction (PCR). In brief, RNAs were isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) and reverse transcribed with random heximers (Roche Applied Science, Indianapolis, IN) by AMV reverse transcriptase (Boehringer Mannheim, Indianapolis, IN) at 42°C for 1 hour. The PCR amplification mixture (25 μL) contained cDNA, survivin forward primer (5′CTCAAGGACCACCGCATCTC3′), survivin reverse primer (5′CAGCCTTCCAGCTCCTTGAA3′), survivin probe (5′TTGGAGGGCTGCGCCTG3′), and TaqMan universal PCR master mix (PE Applied Biosystems, Foster City, CA). Thermal cycle conditions included holding the reaction at 50°C for 2 minutes and at 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and then 60°C for 1 minute. Results were collected and analyzed by an ABI Prism 7700 Sequence Detection System (PE Applied Biosystems). Relative RNA levels were calculated using ΔΔct with RNA levels of β2-microglobin used as internal controls.47
Colony assay
Statistics
All experiments were performed at least 3 times and the results are expressed as the mean plus or minus standard error (SE). The Student t test was used to compare the data from Sur-AS-ODN–treated and Sur-NS-ODN–treated cells. P values below .05 were considered to be statistically significant.
Results
Bcr-Abl tyrosine kinase regulates survivin expression in Ph+ CML cells
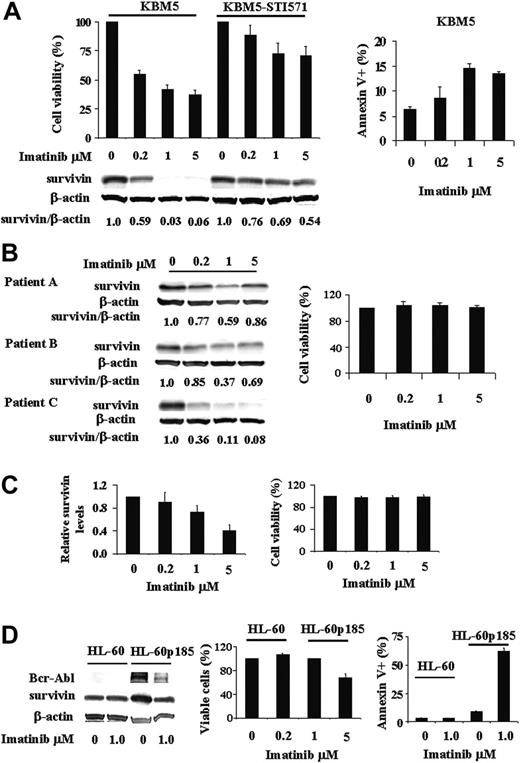
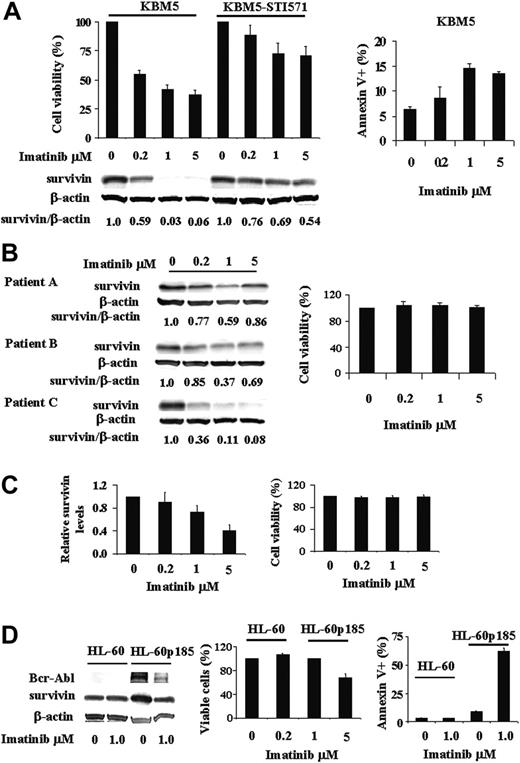
Survivin, absent in normal differentiated cells but present in many cancer cells, is regulated in AML by the MAPK/ERK pathway, which is constitutively activated in the majority of AML cases. Bcr-Abl, a constitutively activated tyrosine kinase expressed in over 95% of patients with CML, is an upstream regulator of many signal transduction pathways including MAPK/ERK. In this study, we asked the question whether survivin expression is regulated by Bcr-Abl. To answer this, we treated KBM5 and KBM5-STI571 cells with various concentrations of imatinib and cell viability and survivin protein levels were determined. As shown in Figure 1A, survivin is highly expressed in both KBM5 and KBM5-STI571 cells. The inhibition of Bcr-Abl by imatinib in KBM5 cells decreased viable cells and increased annexin V+ cells at 48 hours (Figure 1A). Survivin protein levels were significantly reduced in a dose-dependent manner at 24 hours, with maximum reduction at 48 hours. Indeed, less than 0.1% of survivin protein was detected at 48 hours in KBM5 cells treated with 1 μM or more imatinib (Figure 1A). In contrast, as expected, KBM5-STI571 cells were resistant to imatinib. In fact, at 48 hours, even at a dose of 5 μM imatinib, survivin levels in KBM5-STI571 cells were still more than 50% of those of untreated cells (Figure 1A), in agreement with the partial inhibition of p-tyrosine by imatinib at this concentration.43 We then treated cells from 3 accelerated/blast phase CML patient samples with imatinib. Their survivin levels and viabilities (n = 3) in response to imatinib ex vivo treatment are shown in Figure 1B. Survivin expression of patient A (10% blast; white blood cell [WBC] count, 26.1 × 109/L) and patient B (15% blast; WBC count, 11.6 × 109/L) was partially inhibited by imatinib. Both patients showed initial clinical responses to imatinib, then progressed and were resistant to imatinib ex vivo (Figure 1B) suggesting that similar to KBM5-STI571 cells, partial inhibition of the Bcr-Abl/survivin pathway is insufficient to overcome the effect of Bcr-Abl and that residual Bcr-Abl activity is sufficient to confer imatinib resistance clinically and experimentally. Interestingly, like KBM5 cells, survivin levels in the sample from patient C (62% blast; WBC count 71.2 × 109/L) was nearly diminished by imatinib (Figure 1B). Nevertheless, this patient progressed with imatinib treatment clinically and was resistant to imatinib ex vivo (Figure 1B). Molecular analysis revealed no mutation in the BCL-ABL gene of this patient, suggesting that additional survival pathways were acquired, which did not rely on Bcr-Abl/survivin signaling anymore.
Regulation of survivin expression by Bcr-Abl tyrosine kinase in Ph+ cells. (A) Survivin expression and viability of KBM5 and KBM5-STI571 cells treated with imatinib. KBM5 and KBM5-STI571 cells were treated with various concentrations of imatinib. Cell viability was determined by trypan blue exclusion and annexin V staining and survivin protein levels by Western blot analysis, both performed 48 hours after treatment. (B-C) Survivin levels by Western blot analysis and cell viability by trypan blue exclusion in 3 accelerated/blastic phase (B) and 4 chronic phase (C) CML samples treated 48 hours ex vivo with various concentrations of imatinib. (D) Survivin expression and viability of HL-60 and HL-60p185 cells treated with 1 μM imatinib for 48 hours. Cell viability was determined by trypan blue exclusion and annexin V staining and survivin protein levels by Western blot analysis.
Regulation of survivin expression by Bcr-Abl tyrosine kinase in Ph+ cells. (A) Survivin expression and viability of KBM5 and KBM5-STI571 cells treated with imatinib. KBM5 and KBM5-STI571 cells were treated with various concentrations of imatinib. Cell viability was determined by trypan blue exclusion and annexin V staining and survivin protein levels by Western blot analysis, both performed 48 hours after treatment. (B-C) Survivin levels by Western blot analysis and cell viability by trypan blue exclusion in 3 accelerated/blastic phase (B) and 4 chronic phase (C) CML samples treated 48 hours ex vivo with various concentrations of imatinib. (D) Survivin expression and viability of HL-60 and HL-60p185 cells treated with 1 μM imatinib for 48 hours. Cell viability was determined by trypan blue exclusion and annexin V staining and survivin protein levels by Western blot analysis.
To better understand the relationship between Bcr-Abl kinase inhibition with imatinib and survivin expression in primary cells, we also treated peripheral-blood samples from 8 chronic phase CML patients with imatinib ex vivo and determined survivin levels and cell viability after 48 hours. All of these patients had been treated clinically with imatinib. Among them, 4 samples showed undetectable baseline survivin levels. Interestingly, all but one of these 4 patients were imatinib sensitive and achieved cytogenetic remissions. The survivin levels of the other 4 samples in response to imatinib (Figure 1C) were similar to that of KBM5-STI571 cells (Figure 1A) and their cell viability was not affected by imatinib (Figure 1C). These 4 patients were clinically resistant or had relapses while on imatinib when the samples were taken.
Because imatinib also inhibits c-kit, Abl kinase, and other Abl fusion kinases, we treated Bcr-Abl- HL-60, OCI-AML3, and U937 cells with various concentrations of imatinib. Survivin expression was unaffected by imatinib up to doses of 5 μM at 24 and 48 hours (data not shown), indicating that the imatinib-induced down-regulation of survivin expression in KBM5 cells is the result of Bcr-Abl inhibition.
In addition, we compared the survivin levels in HL-60 and HL-60p185 cells, an HL-60 cell line stably transfected with the BCR-ABL fusion gene encoding the p185 protein, and investigated survivin levels in these cells after treatment with 1 μM imatinib. As demonstrated in Figure 1D, survivin protein levels were higher in HL-60p185 than in HL-60 cells. Imatinib had no effect on survivin protein level, cell growth, and survival of HL-60 cells. It inhibited cell growth and survivin protein level in HL-60p185 cells. The cell-growth inhibition was mainly due to apoptotic-cell death induced by imatinib. As shown in Figure 1D, 62.5% ± 2.5% HL-60p185 cells were annexin V+ at 48 hours after being treated with 1 μM imatinib. Imatinib inhibited survivin expression in HL-60p185 cells but not in HL-60 cells, further supporting the notion that survivin is regulated by Bcr-Abl tyrosine kinase in Ph+ cells.
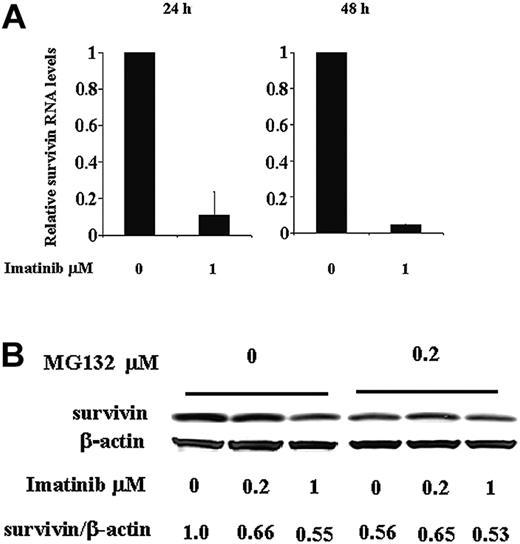
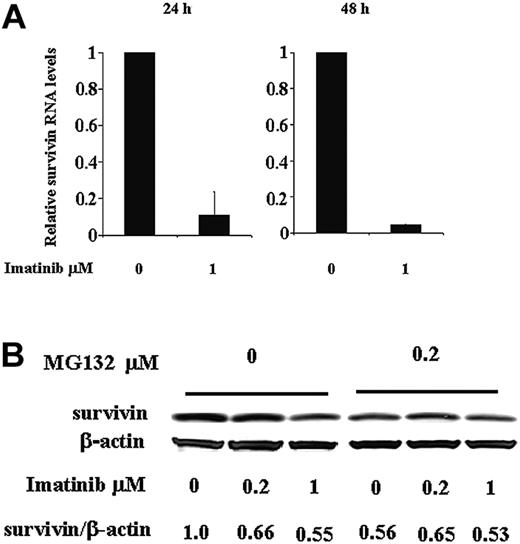
Regulation of survivin expression by imatinib at both RNA and protein levels in Ph+ KBM5 cells. (A) Survivin RNA levels in KBM5 cells treated with 1 μM imatinib determined by TaqMan RT-PCR at 24 and 48 hours. (B) Survivin protein levels at 24 hours in KBM5 cells treated with 1 μM imatinib in the presence or absence of proteasome inhibitor MG132.
Regulation of survivin expression by imatinib at both RNA and protein levels in Ph+ KBM5 cells. (A) Survivin RNA levels in KBM5 cells treated with 1 μM imatinib determined by TaqMan RT-PCR at 24 and 48 hours. (B) Survivin protein levels at 24 hours in KBM5 cells treated with 1 μM imatinib in the presence or absence of proteasome inhibitor MG132.
Bcr-Abl tyrosine kinase regulates survivin expression at both RNA and protein levels
To understand how Bcr-Abl inhibition reduces survivin expression, we first analyzed survivin RNA levels in KBM5 cells after imatinib treatment using TaqMan quantitative PCR. At 24 hours, survivin RNA levels were significantly reduced in cells treated with 1 μM imatinib compared to control cells treated with dimethyl sulfoxide (DMSO) and an even greater decrease was observed at 48 hours (Figure 2A). We then treated KBM5 cells with imatinib in the presence of the proteasome inhibitor MG132. As illustrated in Figure 2B, at 24 hours, imatinib reduced survivin protein in KBM5 cells, but this reduction was prevented in the cells also treated with MG132, which inhibited proteasome degradation. We therefore conclude that Bcr-Abl tyrosine kinase regulates survivin expression at both the RNA and protein levels. Inhibition of Bcr-Abl by imatinib down-regulates survivin protein level by decreasing its RNA transcription and protein stability through inducing its proteasome degradation.
Survivin expression is regulated through Bcr-Abl/MAPK cascade
The constitutive activation of the tyrosine kinase activity of the BCR-ABL fusion gene is essential for its transforming capacity50 and, in turn, activates multiple signaling pathways, such as the MAPK/ERK pathway, through which Bcr-Abl exerts profound effects on the cell cycle, cell adhesion, and apoptosis. Our previous study in AML cells showed that survivin expression is regulated through both the MAPK/ERK and phosphatidylinositol 3-kinase (PI3K) pathways, but probably predominantly through MAPK/ERK signaling.9
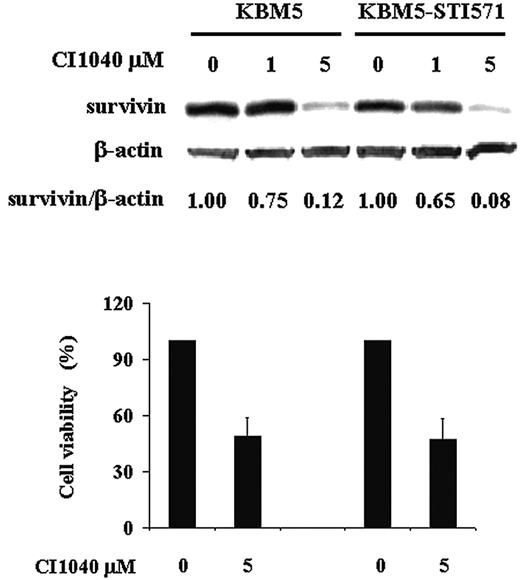
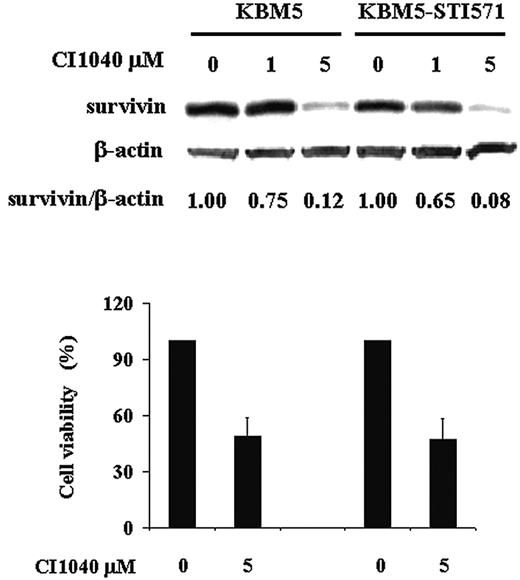
To further elucidate the role of MAPK/ERK signaling in the regulation of survivin expression in Ph+ CML, we analyzed survivin levels in both KBM5 and KBM5-STI571 cells in the presence of the MEK-specific inhibitor, CI1040. As shown in Figure 3, when MEK was inhibited, survivin levels were reduced by approximately 90% at 48 hours in both KBM5 and KBM5-STI571 cells. Cell viability was also decreased, to a similar degree in both cells (Figure 3). The MEK inhibitor U0126 produced similar results (data not shown). Our data therefore indicate that despite the changes in the Bcr-Abl tyrosine kinase in KBM5-STI571 cells that render them resistant to imatinib, the downstream MAPK signal transduction pathways in KBM5 and KBM5-STI571 cells are intact. Thus, targeting effector molecules downstream of the Bcr-Abl tyrosine kinase may be feasible to overcome imatinib resistance.
Down-regulation of survivin expression induces G2M cell-cycle block and cell death in both KBM5 and KBM5-STI571 cells
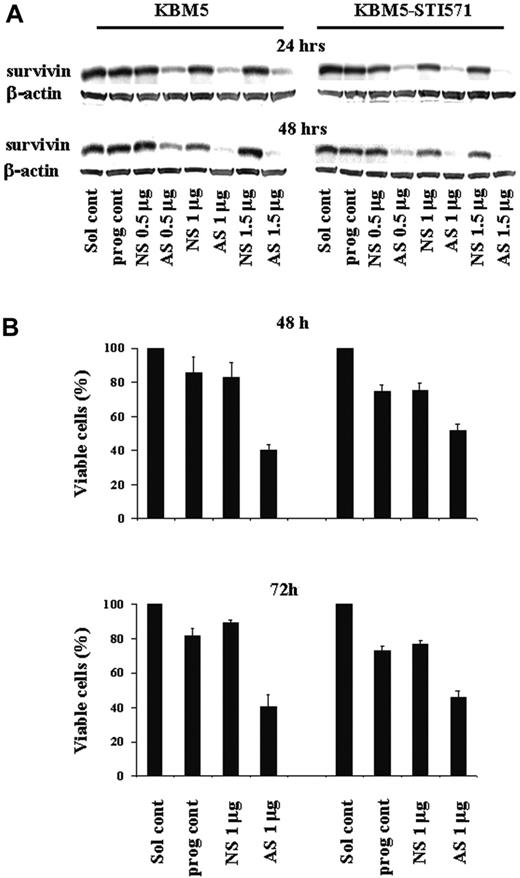
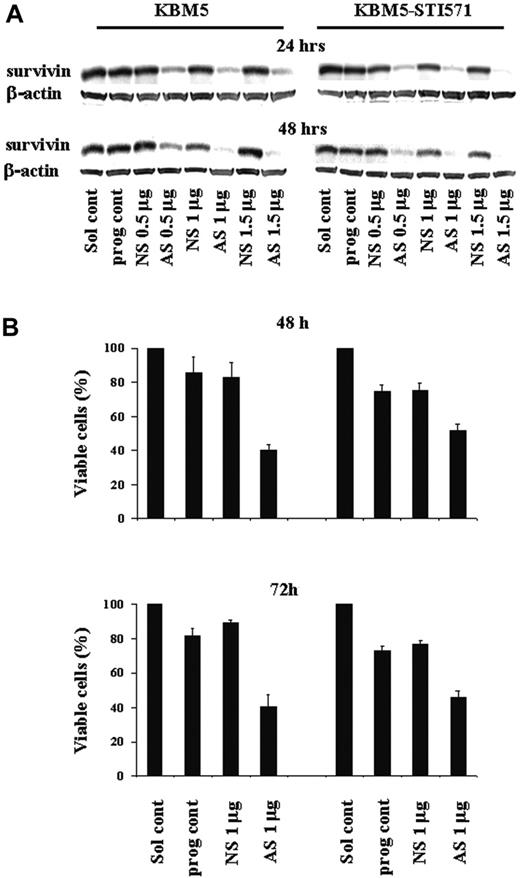
Our studies suggested that the Bcr-Abl tyrosine kinase asserts its biologic effect at least in part through survivin. To determine the biologic importance of survivin for cell proliferation and survival of Ph+ CML cells, we targeted survivin expression in KBM5 and KBM5-STI571 cells with increasing amounts of Sur-AS-ODN (0.5-1.5 μg) by nucleofection. Sur-AS-ODN resulted in the dose- and time-dependent down-regulation of survivin protein levels in both KMB5 and KBM5-STI571 cells at 24 and 48 hours (Figure 4A). A Sur-AS-ODN dose of 1 μg was chosen for the subsequent experiments. Concomitant with the down-regulation of survivin expression, a decrease in the number of viable cells (Figure 4B) was observed. Of particular importance, both imatinib-sensitive KBM5 and imatinib-resistant KBM5-STI571 cells were sensitive to the down-regulation of survivin (Figure 4B).
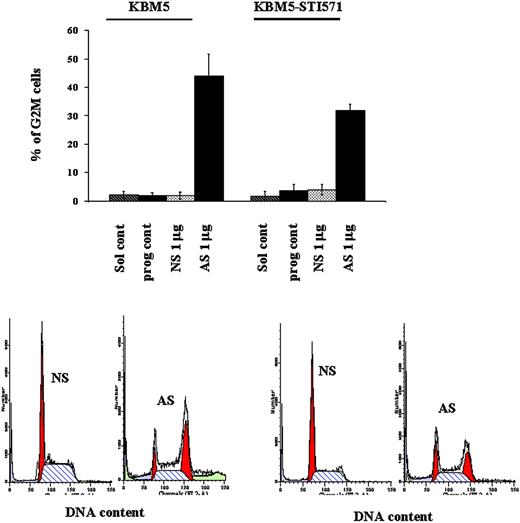
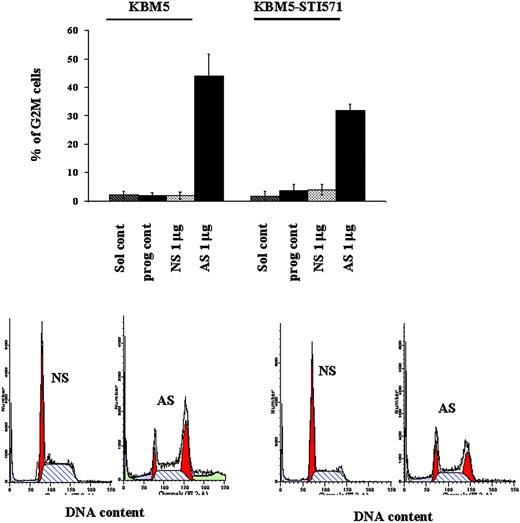
To better understand the mechanisms of cell-growth arrest in these cells, in light of the dual function of survivin as a cell-proliferation and apoptosis regulator, we performed cell-cycle analysis and measured apoptotic-cell death. At 48 hours, 43.84% ± 7.9% and 31.76% ± 2.2% of Sur-AS-ODN–treated KBM5 and KBM5-STI571 cells, respectively, were blocked in G2M compared to only 1.79% ± 1.2% and 3.91% ± 1.8% of Sur-NS-ODN–treated KBM5 and KBM5-STI571 cells, respectively (Figure 5).
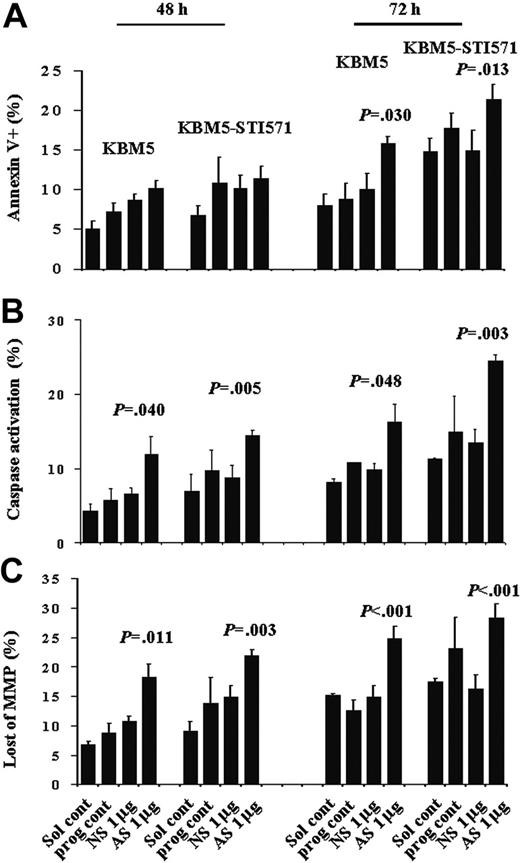
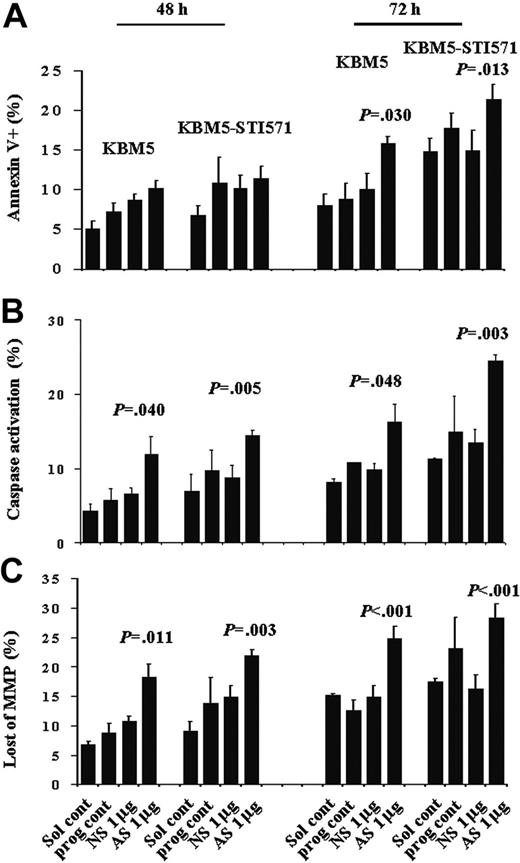
Even though there were increased numbers of cells with activated caspases and loss of matrix mitochondrial membrane potential (MMP), no significant increase in the number of apoptotic cells was detected at this time, as shown by annexin V staining (Figure 6). However, at 72 hours, although no further increase in the number of KBM5 or KBM5-STI571 cells blocked in G2M was observed, the proportion of annexin V+ cells had increased from 10.1% ± 2.0% to 15.8% ± 1.0% (P = .030) and from 14.9% ± 2.6% to 21.3% ± 1.9% (P = .013), caspase-activated cells from 9.8% ± 0.9% to 16.3% ± 2.4% (P = .048) and 13.4% ± 1.8% to 24.4% ± 0.8% (P = .003), and cells with depolarized MMP increased from 14.9% ± 2.0% to 24.8% ± 2.2% (P < .001), and from 16.2% ± 2.5% to 28.4% ± 2.3% (P < .001) in KBM5 and KBM5-STI571 cells, respectively (Figure 6). Our results therefore suggest that down-regulation of survivin expression induces G2M cell-cycle block and subsequent cell death in both KBM5 and KBM5-STI571 cells, in agreement with our previous findings in HL-60 cells, an AML cell line. In addition, this effect was independent of the cell response to imatinib.
Treatment with CI1040. Survivin expression and cell viability were determined by trypan blue exclusion at 48 hours in KBM5 and KBM5-STI571 cells treated with the MEK inhibitor, CI1040.
Treatment with CI1040. Survivin expression and cell viability were determined by trypan blue exclusion at 48 hours in KBM5 and KBM5-STI571 cells treated with the MEK inhibitor, CI1040.
Survivin expression and cell viability in KBM5 and KBM5-STI571 cells treated with Sur-AS-ODN. (A) Sur-AS-ODN induced a dose- and time-dependent down-regulation of survivin protein and (B) down-regulation of survivin expression resulted in decreases in the viability of both KBM5 and KBM5-STI571 cells. Sol cont indicates solution T control; prog cont, nucleofection program K17 control; NS, Sur-NS-ODN control; AS, Sur-AS-ODN.
Survivin expression and cell viability in KBM5 and KBM5-STI571 cells treated with Sur-AS-ODN. (A) Sur-AS-ODN induced a dose- and time-dependent down-regulation of survivin protein and (B) down-regulation of survivin expression resulted in decreases in the viability of both KBM5 and KBM5-STI571 cells. Sol cont indicates solution T control; prog cont, nucleofection program K17 control; NS, Sur-NS-ODN control; AS, Sur-AS-ODN.
Reduction of survivin expression enhances imatinib-induced cell death in KBM5 cells
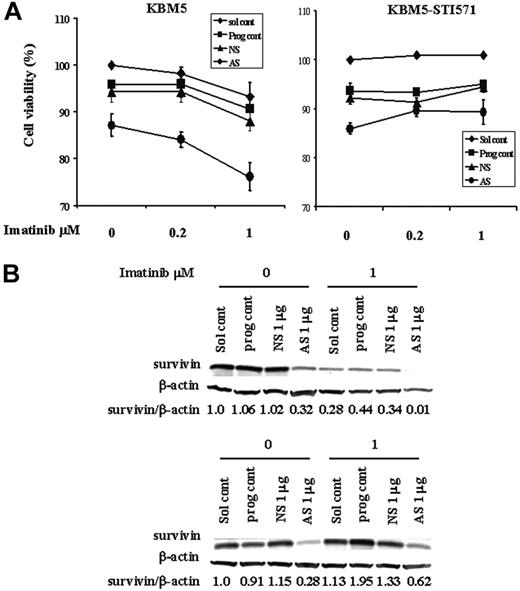
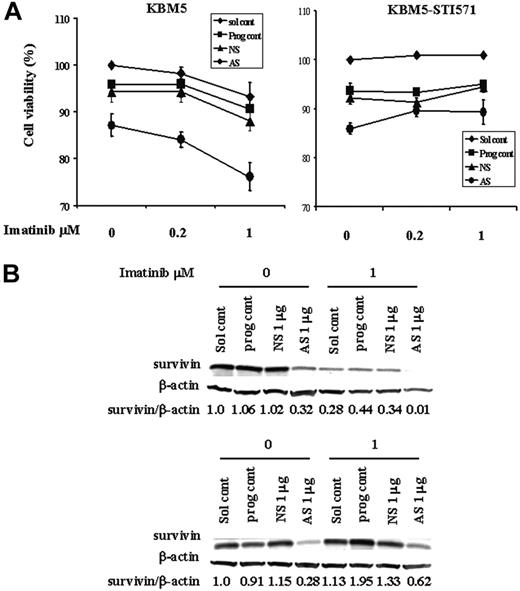
Because Sur-AS-ODN reduces survivin protein levels and imatinib inhibits cell growth and induces cell death through multiple mechanisms including down-regulation of survivin expression, we next examined whether this reduction by Sur-AS-ODN would enhance the effect of imatinib. To study this, we first electroporated Sur-AS-ODN into KBM5 and KBM5-STI571 cells and then added imatinib 24 hours later for additional 48 hours. As shown in Figure 7, the combination of Sur-AS-ODN and imatinib was more effective in reducing survivin levels and inducing cell death than was either agent alone in KBM5 cells. In contrast, imatinib did not enhance cell death in Sur-AS-ODN–treated KBM5-STI571 cells (Figure 7).
G2M cell-cycle block induced by survivin down-regulation in KBM5 and KBM5-STI571 cells. KBM5 and KBM5-STI571 cells were treated with Sur-AS-ODN and its control oligonucleotide Sur-NS-ODN. Cell-cycle distribution was analyzed at 48 hours after PI staining. Sol cont indicates solution T control; prog cont, nucleofection program K17 control; NS, Sur-NS-ODN control; AS, Sur-AS-ODN.
G2M cell-cycle block induced by survivin down-regulation in KBM5 and KBM5-STI571 cells. KBM5 and KBM5-STI571 cells were treated with Sur-AS-ODN and its control oligonucleotide Sur-NS-ODN. Cell-cycle distribution was analyzed at 48 hours after PI staining. Sol cont indicates solution T control; prog cont, nucleofection program K17 control; NS, Sur-NS-ODN control; AS, Sur-AS-ODN.
Apoptosis in KBM5 and KBM5-STI571 cells treated with Sur-AS-ODN. Apoptotic-cell death was measured by annexin V (A), CaspaTag (B), and CMXRos/MTGreen (C) staining at 48 and 72 hours. Sol cont indicates solution T control; prog cont, nucleofection program K17 control; NS, Sur-NS-ODN control; AS, Sur-AS-ODN.
Apoptosis in KBM5 and KBM5-STI571 cells treated with Sur-AS-ODN. Apoptotic-cell death was measured by annexin V (A), CaspaTag (B), and CMXRos/MTGreen (C) staining at 48 and 72 hours. Sol cont indicates solution T control; prog cont, nucleofection program K17 control; NS, Sur-NS-ODN control; AS, Sur-AS-ODN.
Combination treatment of KBM5 and KBM5-STI571 cells with Sur-AS-OND and imatinib. KBM5 and KBM5-STI571 cells were treated with Sur-AS-ODN for 24 hours. Imatinib was added for an additional 48 hours. (A) Cell death was analyzed by annexin V positivity. (B) Survivin protein levels were determined by Western blot analysis. Sol cont indicates solution T control; prog cont, nucleofection program K17 control; NS, Sur-NS-ODN control; AS, Sur-AS-ODN.
Combination treatment of KBM5 and KBM5-STI571 cells with Sur-AS-OND and imatinib. KBM5 and KBM5-STI571 cells were treated with Sur-AS-ODN for 24 hours. Imatinib was added for an additional 48 hours. (A) Cell death was analyzed by annexin V positivity. (B) Survivin protein levels were determined by Western blot analysis. Sol cont indicates solution T control; prog cont, nucleofection program K17 control; NS, Sur-NS-ODN control; AS, Sur-AS-ODN.
Down-regulation of survivin expression decreases BFUs of bone marrow blasts from blast crisis CML patient samples
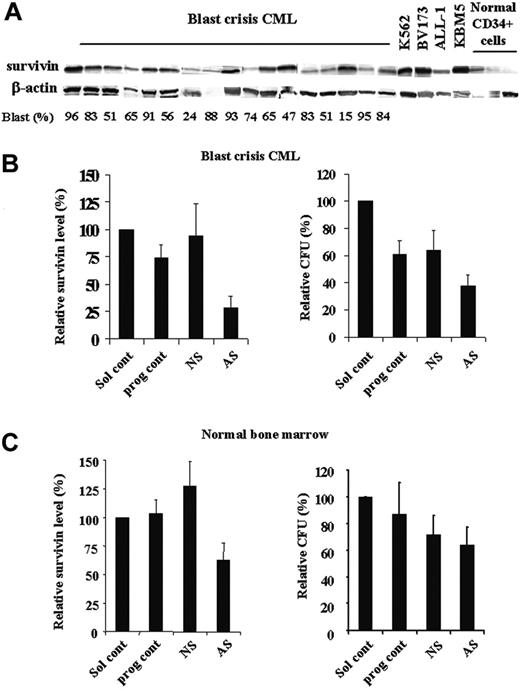
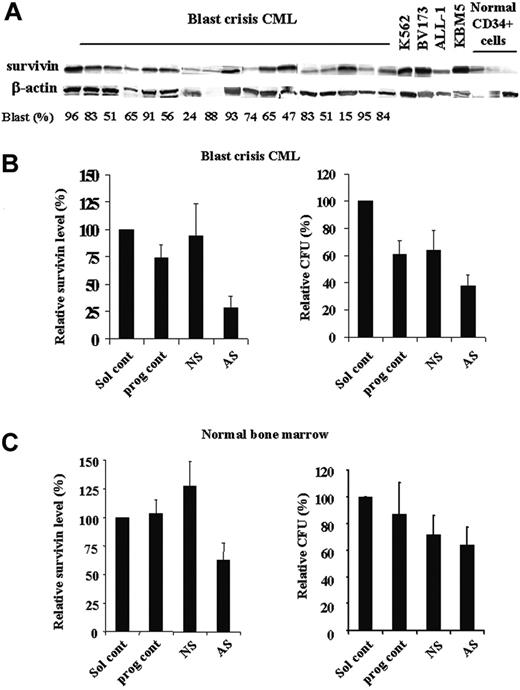
Recent study has shown increased survivin expression in Ph+ blast crisis CML patients by RT-PCR.10-12 We analyzed the levels of survivin protein in samples from blast crisis CML patients, Ph+ cell lines, and samples of CD34+ cells from normal bone marrow by Western blot. As shown in Figure 8A, most blast crisis CML samples expressed survivin protein, although at various levels. In addition to KBM5 cells, survivin protein was also highly expressed in other CML and Ph+ acute lymphoblastic leukemia (ALL) cell lines analyzed (K562, BV173, and ALL-1). Normal CD34+ cells showed low expression levels of survivin. Our previous study in AML also demonstrated that survivin is highly expressed in AML and survivin levels are significantly lower in normal CD34+ cells than those in blasts from AML patients.9
Because blasts from blast crisis CML bone marrow and normal bone morrow cells cycle very slowly in cultures, we could not detect changes in cell viability in short-time cultures of Sur-AS-ODN–treated cells. Therefore, we assessed the effect of down-regulation of survivin on bone marrow cells using colony assays. The characteristics of the CML patients used in this study and the patients' responses to Sur-AS-ODN are shown in Table 1. Five CML and 3 normal bone marrow samples were treated ex vivo with Sur-AS-ODN and its control Sur-NS-ODN. Survivin levels were decreased at 48 hours in all 5 patient samples treated with Sur-AS-ODN compared with those of Sur-NS-ODN (ranging from 54.9% to 82.7% decrease, P < .001; Figure 8B; Table 1). Significant decreases of CFUs were observed in 4 patients (patients 1, 2, 3, and 5, ranging from 38.2% to 51.9% decrease, P < .001; Table 1). One patient (patient 4) showed slight reduction of CFUs (7.8%, Table 1). Patients 1, 2, and 4 were treated with imatinib previously and they were either not responding or later developed resistance to imatinib. Patients 3 and 5 were not exposed to imatinib prior to the sample collection and their responses to imatinib are not known. Our studies demonstrate that decreasing survivin expression could significantly reduce CFUs of CML bone marrow blasts (4 of 5 samples) independent of patients' responses to imatinib. Decrease in CFUs does not correlate with blast count and WBC count (Table 1). Although we did not have samples from all the patients, initial baseline survivin levels do not seem to correlate with the patients' responses to Sur-AS-ODN (Table 1). Interestingly, although there were decreased survivin levels (52.2% ± 11.8% at 48 hours compared with Sur-NS-ODN, P = .016; Figure 8C) in all 3 normal bone marrow cells, down-regulation of survivin had a minimal effect on CFUs (88.9% ± 18.1% of Sur-NS-ODN; Figure 8C).
Discussion
Formation of the BCR-ABL fusion gene is the hallmark of CML and the basis for imatinib-targeted therapy. However, the high rate of patients with residual disease in chronic-phase CML, the limited clinical activity in patients with advanced CML, and the development of resistance to imatinib point to limitations of imatinib's efficacy in the treatment of CML. Survivin, a member of the IAP family of proteins, is highly expressed in many cancers. Studies from our and other laboratories have shown that survivin is essential for cell-cycle progression and cell proliferation. This was demonstrated by the finding that a reduction in survivin levels resulted in cell-proliferation defect, polyploidization, and cell death.41,51-58 Recently, survivin was shown to be low or undetectable in chronic phase CML and expressed in Ph+ CML patients in blast crisis but much reduced in Ph- CML.10-12
Decreases of BFUs of bone marrow blasts from BC CML patient samples by survivin down-regulation. Expression of survivin in bone marrow samples of blast crisis CML, Ph+ cell lines, and normal CD34+ cells by Western blot (A). Survivin expression (48 hours) by Western blot and CFUs of CML bone marrow (n = 5) (B) and normal bone marrow (n = 3) (C) treated with Sur-AS-ODN. Sol cont indicates solution T control; prog cont, nucleofection program K17 control; NS, Sur-NS-ODN control; AS, Sur-AS-ODN.
Decreases of BFUs of bone marrow blasts from BC CML patient samples by survivin down-regulation. Expression of survivin in bone marrow samples of blast crisis CML, Ph+ cell lines, and normal CD34+ cells by Western blot (A). Survivin expression (48 hours) by Western blot and CFUs of CML bone marrow (n = 5) (B) and normal bone marrow (n = 3) (C) treated with Sur-AS-ODN. Sol cont indicates solution T control; prog cont, nucleofection program K17 control; NS, Sur-NS-ODN control; AS, Sur-AS-ODN.
We demonstrate in the present study that survivin is highly expressed in KBM5 cells, which are derived from a CML patient in blast crisis, in imatinib-resistant KBM5 cells, and in blast crisis CML samples, and that its expression is regulated through the Bcr-Abl/MAPK signal transduction cascade. To overcome resistance to imatinib and enhance the effect of imatinib on blast crisis CML cells, we targeted survivin expression in both imatinib-sensitive KBM5 cells and imatinib-resistant KBM5-STI571 cells. The Sur-AS-ODN–induced inhibition of survivin expression resulted in G2M cell-cycle block and cell death in both KBM5 and KBM5-STI571 cells and decreased CFUs in 4 of 5 blast crisis CML blasts regardless of their response to imatinib. In addition, the combination of survivin down-regulation and imatinib treatment was more effective in inducing cell death in KBM5 cells than either approach alone. Targeting survivin thus both overcomes imatinib resistance in imatinib-resistant CML cells and increases imatinib efficacy in imatinib-responsive CML cells. Our study therefore suggests that survivin could be a good therapeutic target for patients with CML in blast crisis regardless of their response to imatinib.
Survivin expression is significantly inhibited by imatinib in imatinib-sensitive KBM5 cells, but much less so in imatinib-resistant KBM5-STI571 and not in Bcr-Abl- cells. Its expression is increased in HL-60 cells with ectopic expression of Bcr-Abl protein and is inhibited by Bcr-Abl inhibitor in these cells. All 3 imatinib-responsive chronic CML patients showed no detectable baseline survivin protein. Like KBM5-STI571 cells, survivin expression was partially inhibited by imatinb ex vivo in samples from 2 of 3 patients with accelerated/blast phase CML and all 4 patients chronic phase CML with detectable survivin who were resistant/relapsed to imatinib clinically (Figure 1). These findings suggest that survivin expression is driven by Bcr-Abl. A recent study by Conte et al also demonstrated that survivin expression correlated with the percentage of Ph+ cells and with Bcr-Abl expression levels in all CML patients.10 However, survivin is elevated mainly in blast crisis CML,10,12 perhaps because survivin expression is also regulated by other factors. For example, it is known that p53 negatively regulates survivin expression.59,60 Evolution of CML from chronic phase to blast crisis is often associated with additional chromosomal and molecular changes resulting in cells with enhanced proliferation, survival, and differentiation arrest and most of these genetic abnormalities have a direct or indirect effect on p53.61 Loss of p53 or other protein functions in blast crisis of CML will remove natural inhibitors of survivin expression, which then is overexpressed driven by activators such as Bcr-Abl. Imatinib, a tyrosine kinase inhibitor, should only affect Bcr-Abl tyrosine kinase activity, not its protein level. However, when we treated HL-60p185 cells with imatinib, a decrease in Bcl-Abl protein level was observed. Similar results were recently reported by Radujkovic et al62 in different Ph+ cell lines. The mechanism of this reduction is not understood at this time.
We also found that down-regulation of survivin expression by inhibition of Bcr-Abl occurs at both the RNA and posttranslation levels. The lack of inhibition of survivin protein levels by imatinib in the presence of proteasome inhibitor MG132 suggests that Bcr-Abl inhibition induces survivin protein degradation through ubiquitination-proteasome degradation pathways. However, even though MG132 blocked survivin degradation induced by imatinib, the MG132 itself induced a decrease in survivin protein levels (Figure 2). This could be due to the inactivation of an upstream regulator of survivin by MG132. One possible candidate is NF-κB because it is well known that proteasome inhibitors prevent NF-κB activation by inhibiting the proteasome degradation of IκB, the inhibitor of NF-κB.63,64 However, whereas other IAP members have been shown to be regulated by NF-κB,65-68 not many studies support that NF-κB regulates survivin. Nonetheless, a study of the mouse survivin promoter region demonstrated 3 NF-κB sites.69 A recent study in human coronary arteriolar endothelial cells showed that hypoxic preconditioning increased survivin levels and this increase was abrogated by the NF-κB antagonist SN50.70 In addition, a newly reported study demonstrated that human T-cell leukemia virus type I tax transcriptionally activates survivin expression through the NF-κB pathway.71 It is thus possible that MG132 stabilizes IκB, thereby inactivating NF-κB, which in turn down-regulates IAPs, including survivin.
The reduction in survivin levels by Sur-AS-ODN induced a G2M cell-cycle block in KBM5 and KBM5-STI571 cells by 24 hours with a maximal effect at 48 hours. However, even though caspase activation and mitochondrial depolarization were observed at 48 hours in Sur-AS-ODN–treated cells, a significant number of annexin V+ cells was not detected until 72 hours. Our results, therefore, indicate that survivin down-regulation results primarily in irreversible cell-cycle block, which results in cell death. This may also explain why no cell death was observed when survivin was down-regulated by Sur-AS-ODN in cells obtained from CML patient samples because the majority of these cells are not proliferating in ex vivo cultures. We were only able to detect the effect of Sur-AS-ODN on primary samples by long-term colony assay. We therefore believe that the effects of survivin inhibition on primary cells are mainly antiproliferative. This finding is in agreement with that from our previous study in HL-60 cells.41 The findings from the present study therefore further support the essential role of survivin in cell-cycle progression.
Inhibition of survivin expression significantly decreased colony formation in 4 of 5 blast crisis CML patient samples, independent of the patients' responses to imatinib treatment. One patient had a slight response (patient 4 in Table 1) although the survivin level was greatly inhibited by Sur-AS-ODN. This patient was treated with imatinib and the new Bcr-Abl tyrosine kinase inhibitor AMN107 clinically and had no responses. No mutation was found in the BCR-ABL gene of the patient. Therefore, like patient C in Figure 1B, this patient may have developed additional genomic abnormalities during disease progression, resulting in the activation of alternative signaling pathways, which rendered their blasts Bcr-Abl/survivin independent. Interestingly, all 3 normal bone marrow samples were more resistant to survivin inhibition. Survivin is overexpressed in most cancers including blast crisis CML and CML blasts are more sensitive to survivin inhibition than normal bone marrow cells suggesting that malignant cells may more than normal cells depend on survivin for survival and proliferation. Our studies in CML cell lines and patient samples provide the bases to select survivin as a therapeutic target for the treatment of both imatinib-sensitive and -resistant blast crisis CML patients.
With our increased understanding of the functions of apoptosis regulators and their regulation by signal transduction pathways in normal and pathologic conditions, the targeting of antiapoptotic proteins and their modulators may represent a particularly promising strategy for the treatment of leukemia and other cancers.
Prepublished online as Blood First Edition Paper, October 27, 2005; DOI 10.1182/blood-2004-12-4704.
Supported in part by grants from the National Institutes of Health (PO1 CA49639, PO1 CA55164, and CA16672) and the Paul and Mary Haas Chair in Genetics (M.A.) and the Elsa U. Pardee Foundation (B.Z.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr N. Dean from ISIS Pharmaceuticals for providing survivin antisense oligonucleotide and control oligonucleotide, Pfizer for the MEK inhibitor CI1040, and Rosemarie Lauzon for assistance in preparation of the manuscript.