Abstract
Vascular endothelial growth factor-A (VEGF-A) and its 2 transmembrane tyrosine-kinase receptors, VEGFR-1 and VEGFR-2, constitute a ligand-receptor signaling system that is crucial for developmental angiogenesis. VEGF-B and placental growth factor (PlGF) activate VEGFR-1 selectively, however, mice lacking either ligand display only minor developmental defects. We hypothesized that the relative contributions of VEGF-B and PlGF to VEGFR-1 signaling may be masked in the presence of VEGF-A, which is abundantly expressed during postnatal development. To test this hypothesis, neonatal or adult mice were treated with a monoclonal antibody (G6-23-IgG) blocking murine VEGF-A or a soluble VEGFR-1 receptor IgG chimeric construct [mFlt(1-3)-IgG], which neutralizes VEGF-A, VEGF-B, and PlGF. Both compounds attenuated growth and survival of neonatal mice to similar extents and the pathophysiologic alterations, including a reduction in organ size and vascularization, changes in gene expression, and hematologic end points, were essentially indistinguishable. In adult mice, we observed only minor changes in response to treatment, which were similar between both anti-VEGF compounds. In conclusion, our findings suggest that PlGF and VEGF-B do not compensate during conditions of VEGF-A blockade, suggesting a minor role for compensatory VEGFR-1 signaling during postnatal development and vascular homeostasis in adults. The absence of compensatory VEGFR-1 signaling by VEGF-B and PlGF may have important implications for the development of anticancer strategies targeting the VEGF ligand/receptor system.
Introduction
VEGF-A is a member of the VEGF platelet-derived growth factor (Pdgf) gene family and is among the most potent positive regulators of angiogenesis.1 Other family members include VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF) and display varying degrees of homology with VEGF-A (reviewed in Ferrara2 ). Among them, VEGF-B and PlGF bind selectively to VEGFR-1.3,4 In contrast to VEGFR-2, which is mainly expressed on endothelial cells,5 VEGFR-1 expression was found on myeloid cells such as monocytes/macrophages and neutrophils6-8 and other nonendothelial cells 9,10 (reviewed in Autiero et al11 ). Evidence for the key regulatory role for the VEGF receptor/ligand system was provided from conventional gene knock-out experiments in mice. Mice lacking one VEGF allele or both VEGFR-2 alleles had almost no endothelial and hematopoietic cells and died on embryonic days 10.5 and 9.5, respectively.1,12,13 Mice lacking VEGFR-1 died about embryonic day 8.5 due to increased hemangioblast and endothelial cell formation and severe disorganization of the vascular system.14,15 Conditional Vegf gene ablation experiments and biochemical interference with VEGF activity in neonatal mice resulted in reduced endothelial cell survival, disrupted organ angiogenesis, and multiple organ failure and death.16
Increased VEGFR-1 expression was reported on endothelial cells during development17 and on pathologic vasculature. Combined with Flt1 gene knock-out and receptor-blocking studies, these data suggest that the VEGFR-1 signaling system may have important regulatory roles during postnatal development.11 However, the relative contributions of VEGF-B and PlGF to VEGFR-1–mediated signaling during conditions of selective VEGF-A blockade have not been investigated. One reason for this incomplete understanding is the confounding effect resulting from incomplete Vegf gene ablation in lung, kidney, and other organs in the conditional knock-out studies.16 Other reasons include the multi-ligand neutralizing activity of the mFlt(1-3)-IgG compound used,16 which did not distinguish between ligands, and the abundant expression of VEGF-A during developmental processes, which may mask potential angiogenic functions of VEGF-B and PlGF.
In this report, the neonatal mouse growth assay16 was employed to test potential compensatory angiogenic and hematopoietic roles for VEGF-B and PlGF during conditions of VEGF-A blockade. Because of the rapid bodily growth associated with neoangiogenesis in all organ systems during the first 2 postnatal weeks, this assay is well suited to monitor differences in pharmacologic efficacies between different antiangiogenic compounds. To block murine VEGF-A selectively, we employed a novel monoclonal antibody generated by phage display technology (G6-23-IgG)18 and compared its efficacy to interfere with angiogenesis with the multi-ligand blocker mFlt(1-3)-IgG. Both compounds induced similar pathophysiologic changes in most organs during development. Our findings suggest a lack of compensatory signaling of VEGFR-1 during conditions of VEGF-A blockade and may have implications for the development of therapeutic compounds, targeting different elements within this signaling pathway.
Materials and methods
Materials
Tetramethylbenzidine (TMB) substrate was obtained from Kirkegaard and Perry Laboratories (Gaithersburg, MD). Anti-CD3, -B220, –Gr-1, –Mac-1, -NK1.1, –Ter-119, –ScaI, and –CD16/CD32 antibodies (clones: 145-2C11, RA3-6B2, RB6-8C5, M1/70, PK136, TER-119, D7, and 2.4 G2, respectively) were obtained from BD Pharmingen (San Jose, CA). Fluorescein isothiocyanate (FITC)–conjugated rat anti–mouse F4/80 antibody (clone = CI: A3-1) was obtained from Serotec (Raleigh, NC). Rat anti–mouse VEGFR-2 antibody (MALK-1) was used for immunohistochemistry as previously described.16 Recombinant murine VEGF-B and PlGF were purchased from R&D Systems (Minneapolis, MN). [125I]–rhVEGF-A was purchased from Amersham Biosciences (Piscataway, NJ). Recombinant human and mouse VEGF-A forms were supplied by Genentech (South San Francisco, CA). The fusion protein mFlt(1-3)-IgG is composed of the first 3 Ig domains of murine VEGFR-1 fused to murine Fc (γ2B).19 G6-23-IgG is an antibody that binds to and neutralizes murine and human forms of VEGF-A. G6-23-IgG is derived using phage display technology, with the IgG portion consisting of murine isotype IgG2a. Isotype-matched control antibodies for mFlt(1-3)-IgG and G6-23-IgG were mouse IgG2b and mouse anti–human ragweed IgG2a, respectively. The Statview Program was used to perform statistical analysis (analysis of variance [ANOVA]; SAS Institute, Cary, NC).
Optimizing pharmacologic activity of G6-23-IgG and mFlt(1-3)-IgG and pharmacokinetics in neonatal and adult mice
Pilot experiments determined the dosing regimens inducing optimal pharmacodynamic effects of mFlt(1-3)-IgG and G6-23-IgG in neonatal CD1 mice (Figure S1; see the Supplemental Figures link at the top of the online article, at the Blood website) and C57/Bl6 mice (Gerber et al16 ; data not shown). Initial weights were taken the day of delivery, determined as day 1, and administration of all compounds occurred on the next day following delivery of neonate mice, determined as day 2. Administration of mFlt-(1-3)-IgG at 25 mg/kg and 50 mg/kg induced comparable changes in body weights and survival of neonatal mice (Figure S1C-D). Similarly, G6-23-IgG administered at 10 mg/kg and 25 mg/kg resulted in comparable changes in both of these end points (Figure S1A-B). These findings suggest that both compounds, when administered at 25 mg/kg daily, induced maximal pharmacologic effect in neonatal mice. In addition, optimal dosing regimens inducing maximal inhibition of tumor xenograft growth were determined for both compounds in beige nude, X-linked immunodeficiency (XID) mice. From these experiments we concluded that G6-23-IgG at 10 mg/kg twice weekly induced maximal pharmacodynamic effects (A.K.M. and H.-P.G., manuscript in preparation).
Enzyme-linked immunosorbent assay (ELISA) using plates coated with recombinant human VEGF-A was used to determine serum levels of G6-23-IgG and mFlt(1-3)-IgG. Serum was harvested from nonfasted mice by bleeding the retro-orbital sinus 2 days after administering the compounds to nude mice. The levels determined at 10 mg/kg/d intraperitoneally ranged from 59 μg/mL to 129 μg/mL (data not shown) and were comparable to levels in mice treated with a murine antibody binding to human VEGF-A (A4.6.1).20 Given serum levels of less than 80 pg/mL of free VEGF-A routinely measured in adult mice (data not shown), we expect a greater than 100 000-fold molar excess of compound/free VEGF-A in the serum of anti-VEGF–treated mice. The free serum levels for VEGF-B or PlGF in adult mice are unknown at this time. The severe reduction in blood volume precluded determination of steady-state levels of G6-23-IgG and mFlt(1-3)-IgG in the serum of anti-VEGF–treated neonatal mice.
Endothelial cell proliferation assay
Pooled dermal human microvascular endothelial cells (Cambrex, Walkersville, MD) were seeded in 0.1% gelatin-coated 6-well culture dishes (Primaria; Becton Dickinson, Heidelberg, Germany) at a density of 7000 cells/well in 3 mL of Clonetics (Cambrex). The endothelial cell medium was composed of endothelial cell growth medium (EGM-2) basal medium supplemented with a bullet kit containing growth factors (Cambrex). On day 2, cells were washed and media were replaced with Clonetics (EGM-2 basal medium) supplemented with 0.5% fetal bovine serum and 30 ng/mL of human VEGF or murine VEGF-A. G6-23-IgG or mFlt(1-3)-IgG were added at concentrations shown in Figure S1. On day 7, cells were trypsinized and resuspended in isotonic buffer solution and counted at 1:10 dilution on a Z2 Coulter Particle and Size Analyzer (Beckman Coulter, Fullerton, CA). The 50% inhibitory concentration (IC50) values were obtained using KaleidaGraph software (Synergy Software, Reading, PA).
Treatment of mice with mFlt(1-3)-IgG or G6-23-IgG and histopathologic studies of tissues and organs
C57/Bl6 neonatal mice aged 1 day were injected daily with G6-23-IgG 25 mg/kg/d intraperitoneally, isotype control IgG, or 25 mg/kg/d intraperitoneal mFlt(1-3)-IgG. Body weights were monitored daily. On day 5, 1 group of neonatal mice was killed and individual organs were weighed. Various organs were also sectioned and analyzed by histology (hematoxylin and eosin [H&E] staining) and by immunohistochemistry using MALK-1 [G6-23-IgG, n = 3; antiragweed, n = 2; mFlt(1-3)-IgG, n = 2]. Young or adult C57BL/6 mice were treated with mFlt(1-3)-IgG 25 mg/kg every 2 days or G6-23-IgG 10 mg/kg twice weekly to study the effects of anti-VEGF treatment on hematologic parameters. After 1 or 2 weeks of treatment, peripheral blood was collected by retro-orbital bleed for fluorescence-activated cell sorter (FACS) or blood cell counts.
Vascular density was assessed in MALK-1–stained histologic sections of the liver, ventricular wall, and renal glomeruli as previously described.16 Inhibition of glomerular maturation was assessed in digital images of H&E-stained sections by manually outlining nephrogenic precursors (including distal ureteric bud epithelium and comma- and S-shaped bodies); pixels within outlined areas were summed and normalized per unit length of renal cortex. Microscopy and photography were conducted using a Leica DMRB fluorescence microscope (Leica, Allendale, NJ) fitted with a Nikon DXM-1200 camera (Nikon, Melville, NY) using a 10 ×/0.30 numeric aperture objective. When indicated, pixels of stained vessels were selected in Adobe Photoshop and vessel surface area was calculated as described in Samson et al.47
Flow cytometry and blood cell count
Bone marrow mononuclear cells were isolated by flushing femurs with phosphate-buffered saline containing 2% heat-inactivated fetal bovine serum and 2 mM EDTA (ethylenediaminetetra-acetate) using a 22-gauge needle. Red blood cells were removed from the bone marrow cell isolates by lysis on ice for 5 to 10 minutes in 10 mM NH4Cl made in 10 mM Tris (pH 7.2). Peripheral blood was collected by retro-orbital bleed. A total of 40 μL of peripheral blood or 1 × 106 bone marrow mononuclear cells was pretreated with CD16/CD32 antibodies for 5 minutes to block Fc receptors and then incubated with lineage-specific monoclonal antibodies. Bone marrow mononuclear cells were washed once with phosphate-buffered saline containing 2% heat-inactivated fetal bovine serum and analyzed on a Coulter Elite XL FACS analyzer (Beckman Coulter). All fluorescein isothiocyanate– or phycoerythrin-conjugated antibodies were used at 1 μg or 0.4 μg, respectively, except for Gr-1 and Mac-1, which were used at 5- or 25-fold lower amounts, respectively. An aliquot of whole blood was analyzed on an automatic blood cell counter, according to manufacturer's instructions (Baker System 9118+cp; Biochem Immunosystem, Montreal, QC, Canada), to determine leukocyte, red blood cell, platelet, and lymphocyte cell counts; hematocrit; and hemoglobin level.
ELISA binding assay
G6-23-IgG Fab or mFlt(1-3)-IgG were immobilized on a breakaway immunoabsorbent assay plate and binding to murine VEGF, PlGF-1, and VEGF-B were measured by ELISA as described previously.19 Data were analyzed and IC50 values were determined using a 4-parameter non–linear curve–fitting program (Kaleidagraph; Abelbeck Software, Reading, PA). Each experiment was performed in duplicate or triplicate.
Real-time quantitative RT-PCR (TaqMan) analysis
Total RNA was isolated from frozen tissues using the STAT 60 method (TEL-TEST B, Friendswood, TX) and purified on RNeasy quick-spin columns (Qiagen, Valencia, CA). Real-time–polymerase chain reaction (RT-PCR) reactions were performed in 96-well plates on an ABI 7700 Sequence Detector and results were analyzed using SDS1.6.1 software (PE Applied Biosystems, Foster City, CA). RT-PCR conditions were 30 minutes at 48°C, 10 minutes at 95°C, and 40 cycles of 30 seconds at 95°C and 90 seconds at 60°C. Five-fold dilutions (100 ng to 0.16 ng) of normal control RNA (a 1:1:1:1 mixture of total RNAs from normal mouse spleen, liver, lung, and heart; Clontech, Palo Alto, CA) were run with glyceraldehydes-3-phosphate dehydrogenase (GAPDH) probe/primers to create a standard curve. A total of 100 ng of RNA was analyzed per reaction. Relative RNA equivalents for each sample were obtained by comparing control values with a GAPDH standard curve. Expression levels of specific genes are indicated as RNA units relative to GAPDH levels. The sequences of TaqMan primer/probes have been described previously.21
Results
Biochemical analysis of G6-23-IgG and mFlt(1-3)-IgG binding to VEGF-A, VEGF-B, and PlGF and interference with endothelial cell proliferation in vitro
G6-23-IgG is a murine monoclonal antibody originally identified by screening a phage display library for binding human heavy-chain fragments to mouse VEGF-A.18 Both compounds, G6-23-IgG and mFlt(1-3)-IgG, were tested for their selectivity and binding affinities for VEGF-A, VEGF-B, and PlGF by competitive immunoabsorbent assay (ELISA) with 125IVEGF-A (Table 1). As expected, G6-23-IgG bound to murine VEGF-A with an IC50 value of 1.09 ± 0.15 ng/mL but failed to bind to PlGF and VEGF-B. The mFlt(1-3)-IgG construct bound to murine VEGF-A, PlGF, and VEGF-B with IC50 values of 2.13 ± 0.49, 15.81 ± 3.57, and 4.66 ± 1.37 ng/mL, respectively. The affinities compare with previous reports examining the binding of full-length VEGFR-1 (Table 1).19,22
To determine the potency of each anti-VEGF compound to interfere with VEGF-A–induced endothelial cell proliferation,23 human microvascular endothelial cells were cultured with murine VEGF and increasing amounts of G6-23-IgG or mFlt(1-3)-IgG. The results showed that G6-23-IgG and mFlt(1-3)-IgG neutralized murine VEGF-induced human microvascular endothelial cell proliferation with comparable IC50 values of 0.26 ± 0.027 nM and 0.50 ± 0.21 nM, respectively (Figure S2). In conclusion, both compounds bind and neutralize murine VEGF-A and inhibit VEGF-A–induced endothelial cell proliferation with comparable potencies.
mFlt(1-3)-IgG and G6-23-IgG induce similar pathophysiologic changes during postnatal development in mice
To test both VEGF-blocking compounds for their effects on angiogenesis during postnatal development in C57/Bl6 mice, G6-23-IgG and mFlt(1-3)-IgG were administered intraperitoneally and their effects on body growth were compared with control IgG–treated mice. G6-23-IgG or mFlt(1-3)-IgG intraperitoneally inhibited growth to similar extents (Figure 1A) and induced almost identical decreases in the survival rate of neonatal mice (Figure 1B). Starting on day 5, neonatal mice treated with either G6-23-IgG or mFlt(1-3)-IgG did not gain weight and lost weight thereafter. Survival in anti-VEGF–treated mice decreased from day 3, and most mice died between days 6 and 9 (Figure 1B). There were no consistent differences between mortality rates in mice treated with mFlt(1-3)-IgG or G6-23-IgG. Mortality in both groups reached 100% on day 10. The average body weight of mice treated with G6-23-IgG or mFlt(1-3)-IgG on day 5 was 55.1% and 56.8%, respectively, compared with controls. Weights of all organs were significantly reduced on day 5 (Figure 1C). There was no statistically significant difference between mFlt(1-3)-IgG and G6-23-IgG treatment in both total body weights and organ size (Figure 1C). There was a more pronounced inhibition in growth of the heart relative to other organs, which is consistent with the role of VEGF as a key regulator during cardiovascular development.24 In summary, the data demonstrate that both compounds reduce organ weight and survival to similar extents, indicating comparable degrees of interference with angiogenesis and growth of all major organs.
Because VEGF-A, VEGFR-1, and VEGFR-2 are involved in hematopoietic stem cell survival, the study investigated whether neutralizing different subsets of VEGF ligands during postnatal development may differentially affect hematologic parameters in peripheral blood and bone marrow. Similar to the findings in neonatal mice treated with mFlt(1-3)-IgG,16 G6-23-IgG treatment significantly increased the levels of white blood cells, neutrophils, and red blood cells and reduced circulatory levels of lymphocytes and eosinophils (Figure 1D) on day 5 of treatment. No significant differences were detected between mFlt(1-3)-IgG and G6-23-IgG treatment in any of the hematologic end points analyzed.
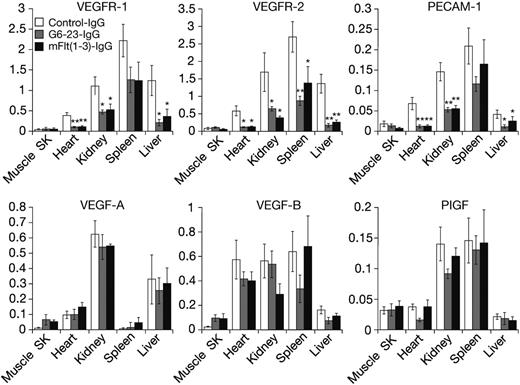
Expression level of some VEGF ligands and receptors can be altered in response to changes in environmental conditions such as hypoxia and hypoglycemia,25 both of which are induced in tumors during anti-VEGF treatment.21 Thus, we wanted to determine the expression levels of VEGF ligands and receptors in neonatal mice following anti-VEGF treatment. Messenger RNA from liver, kidney, heart, spleen, and skeletal muscle was isolated and analyzed by real-time–polymerase-chain reaction (RT-PCR). On day 5, anti-VEGF treatment induced significant and robust reductions in the expression levels of VEGFR-1, VEGFR-2, and CD31/platelet endothelial cell adhesion molecules (PECAMs), a marker on the vascular endothelium (Figure 2). With the exception of skeletal muscle tissues, all 3 genes were significantly reduced by anti-VEGF treatment in all organs analyzed. There was no statistically significant change in expression levels for all genes tested between mFlt(1-3)-IgG and G6-23-IgG treatment, indicating similar interference in developmental processes by either compound.
In contrast to the significant reduction of genes expressed by the vascular endothelium, changes in VEGF-A, VEGF-B, and PlGF expression in the lung, kidney, liver, and spleen were insignificant. There was a trend toward increased expression of VEGF-A [n = 4, P = .21 for mFlt(1-3)-IgG; and P = .11 for G6-23-IgG] and VEGF-B [n = 4, P = .15 for mFlt(1-3)-IgG; and P = .12 for G6-23-IgG] in response to anti-VEGF treatment in skeletal muscle. In summary, these data suggest a lack of a general compensatory change in gene expression of VEGFR-1 ligands in neonatal mice treated with anti-VEGF compounds.
Effect of G6-23-IgG or mFlt(1-3)-IgG on postnatal body growth, organ weight, and survival of neonatal mice. (A) Ten C57BL/6 neonatal mice per group were treated for 10 days with either compound at a dose of 25 mg/kg intraperitoneally. Body weights were assessed daily. Data represent mean ± standard deviation. (B) Kaplan-Meier survival curves of C57BL/6 neonatal mice treated with either mFlt(1-3)-IgG, G6-23-IgG, or an isotype-matched control antibody. Both compounds induced a similar increase in death. (C) Percent body and organ weights from anti-VEGF–treated C57BL6 neonatal mice relative to controls on treatment day 5. Each group consisted of 5 specimens, representing mean ± standard deviation. Data are from 1 representative of 2 independent experiments. (D) Changes in hematologic parameters in neonatal mice aged 5 days treated with anti-VEGF [G6-23-IgG, mFlt(1-3)-IgG] or a vehicle control (phosphate-buffered saline; PBS). Whole blood was collected at necropsy and analyzed by automated hematologic analysis of white blood cell count (WBC), neutrophil count (NEU), lymphocyte count (LYM), eosinophil count (EOS), basophil count (BAS), red blood cell count (RBC), hemoglobin level (HGB), hematocrit (HCT), and platelet count (PLT). Data represent mean ± SD.
Effect of G6-23-IgG or mFlt(1-3)-IgG on postnatal body growth, organ weight, and survival of neonatal mice. (A) Ten C57BL/6 neonatal mice per group were treated for 10 days with either compound at a dose of 25 mg/kg intraperitoneally. Body weights were assessed daily. Data represent mean ± standard deviation. (B) Kaplan-Meier survival curves of C57BL/6 neonatal mice treated with either mFlt(1-3)-IgG, G6-23-IgG, or an isotype-matched control antibody. Both compounds induced a similar increase in death. (C) Percent body and organ weights from anti-VEGF–treated C57BL6 neonatal mice relative to controls on treatment day 5. Each group consisted of 5 specimens, representing mean ± standard deviation. Data are from 1 representative of 2 independent experiments. (D) Changes in hematologic parameters in neonatal mice aged 5 days treated with anti-VEGF [G6-23-IgG, mFlt(1-3)-IgG] or a vehicle control (phosphate-buffered saline; PBS). Whole blood was collected at necropsy and analyzed by automated hematologic analysis of white blood cell count (WBC), neutrophil count (NEU), lymphocyte count (LYM), eosinophil count (EOS), basophil count (BAS), red blood cell count (RBC), hemoglobin level (HGB), hematocrit (HCT), and platelet count (PLT). Data represent mean ± SD.
Similar histopathologic alterations in neonatal mice treated with G6-23-IgG or mFlt(1-3)-IgG
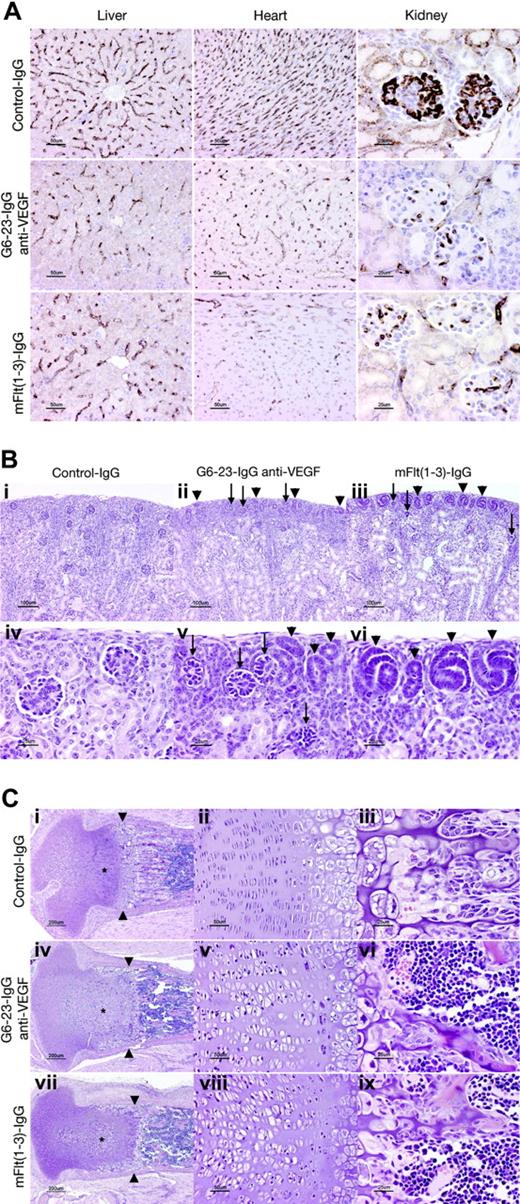
The effects of G6-23-IgG and mFlt(1-3)-IgG on tissue vascularity or vascular surface density (area of vessel surface per unit volume of tissue) in liver, kidney, and heart sections were assessed by immunohistochemical staining using a rat anti–mouse VEGFR-2 monoclonal antibody (MALK-1; Figure 3A). Vascular surface density was significantly decreased both in anti-VEGF–(G6-23-IgG) and mFlt(1-3)-IgG–treated animals in liver (≈50% of control value), heart (35%-40% of control value), and renal glomeruli (≈25% of control values; Table 2). In conclusion, there was no significant difference in vascular surface density between the mFlt(1-3)-IgG and G6-23-IgG treatment groups in all organs analyzed (Figure 3A; Table 2). We conclude that in agreement with our observations from endothelial cell proliferation studies in vitro (Figure S2), both compounds reduced survival and/or proliferation of endothelial cells in liver, heart, and kidney to similar extents.
Despite the similarity in the reduction of the vascular surface area in glomeruli of mFlt(1-3)-IgG– and G6-23-IgG–treated mice, there are subtle differences in the numbers of nephrogenic precursors at the cortical margin of kidneys between treatment groups. The outer cortex in both groups contained many abnormal glomerular “rosettes” composed of podocytes surrounding essentially avascular mesenchymal cores (Figure 3Bii-iii,v arrows). Glomeruli in the inner half of the cortex showed capillary surface densities were reduced more than 70% relative to control animals (Figure 3A; Table 2). In both treatment groups, glomerular maturation was delayed so that the peripheral cortex contained increased numbers of nephrogenic precursors (ureteric tubular and glomerular precursors; Figure 3Bii-iii,v-vi arrows). Interestingly, animals treated with mFlt(1-3)-IgG showed a more pronounced delay in peripheral cortical maturation than G6-23-IgG–treated animals. In representative histologic sections, the area composed of nephrogenic precursors in mFlt(1-3)-IgG–treated animals was approximately 2-fold higher than in G6-23-IgG–treated animals (21.2 vs 11.5 μm2 of nephrogenic precursors/μmof cortical margin); the result was marginally significant (t test, P = .039). The findings may indicate a role for PlGF or VEGF-B activity mediated by VEGFR-1 during kidney development. It is, however, unclear whether these differential changes are caused by impaired vascular functions of VEGFR-1 in treated kidneys or by other nonendothelial effector functions of the VEGFR-1 receptor in these tissues.
Significant changes were also noted in the cartilaginous growth zones of long bones in animals treated with G6-23-IgG and mFlt(1-3)-IgG (Figure 3C). Similar to previous studies in older animals treated with mFlt(1-3)-IgG,26 the length and number of primary bony trabeculae decreased in mFlt(1-3)-IgG– and G6-23-IgG–treated animals and secondary trabeculae were thicker than controls (Figure 3Ciii,vi,ix). Other alterations in the growth zone of neonatal animals were qualitatively different than previously described changes in mice treated from postnatal day 24 onwards.26 In the current study, the secondary center of ossification in the epiphysis is not yet formed; rather, the proximal tibial growth plate is contained within the mushroom-shaped cartilaginous end of the tibia (Figure 3Ci,iv,vii). Administering G6-23-IgG or mFlt(1-3)-IgG to animals caused the growth zone to elongate to 600 to 700 μm from the base of the cartilaginous “cap” to the provisional ossification zone compared with approximately 500 microns in control animals. Expansion of the growth zone was associated with an increase in the size of proliferating chondrocytes (see asterisks in Figure 3C), which was most pronounced in the axial (central) part of the growth plate. In control animals, proliferating chondrocytes appear in thin lacunae (≈5 μm) forming well-ordered stacks (Figure 3Ciii). In anti-VEGF–treated animals, the same cells appear in plump elliptical lacunae up to 20 μm in the long axis of the bone (Figure 3Cv,viii). In anti-VEGF–treated animals, the zone of hypertrophic chondrocytes (between proliferating chondrocytes and primary bony trabeculae) is generally narrower (≈150 μm, rather than 200-250 μm in control animals). At the metaphysis in G6-23-IgG– or mFlt(1-3)-IgG–treated animals, a peripheral collar of hypertrophic chondrocytes line the endosteal surface of the bony ring of Ranvier, extending 300 to 400 μm from the zone of ossification toward the joint space (Figure 3Ci,iv,vii). Combined, these findings indicate that administering G6-23-IgG and mFlt(1-3)-IgG resulted in similar histologic changes in the growth plate regions of long bones during mouse development.
Real-time polymerase chain reaction gene expression analysis using TaqMan of RNA isolated from tissues of neonatal mice treated for 5 days with mFlt(1-3)-IgG, G6-23-IgG, or control-IgG, respectively. Genes analyzed include VEGFR-1, VEGFR-2, and PECAM/CD31 and VEGF-A, VEGF-B, and PlGF for the ligands. Data represent mean ± standard deviation of 5 RNA isolates per treatment group. Probe/primer sequences and relative RNA units (RRUs) for murine GAPDH are calculated as described previously.21 One relative RNA unit corresponds with expression levels in pooled RNA from the liver, lung, and kidney. The analysis of variance (ANOVA) between groups program was used for statistical analysis. SK indicates skeletal. *P < .05, **P < .005 relative to control treatment.
Real-time polymerase chain reaction gene expression analysis using TaqMan of RNA isolated from tissues of neonatal mice treated for 5 days with mFlt(1-3)-IgG, G6-23-IgG, or control-IgG, respectively. Genes analyzed include VEGFR-1, VEGFR-2, and PECAM/CD31 and VEGF-A, VEGF-B, and PlGF for the ligands. Data represent mean ± standard deviation of 5 RNA isolates per treatment group. Probe/primer sequences and relative RNA units (RRUs) for murine GAPDH are calculated as described previously.21 One relative RNA unit corresponds with expression levels in pooled RNA from the liver, lung, and kidney. The analysis of variance (ANOVA) between groups program was used for statistical analysis. SK indicates skeletal. *P < .05, **P < .005 relative to control treatment.
Histopathologic analysis of C57/Bl6 neonatal mice treated for 5 days with G6-23-IgG, mFlt(1-3)-IgG, or control IgG. (A) Assessed vascular density in liver, heart, and kidney. Representative sections stained with rat anti–mouse VEGFR-2 antibody from each treatment group are illustrated. Vessel density was reduced to a similar extent by mFlt(1-3)-IgG and G6-23-IgG in all organs (see Table 2 for quantitative assessment). (B) Delayed and abnormal glomerular maturation in anti-VEGF–treated kidneys. Control glomeruli (iv) all have identifiable capillary loops supported by mesangial cells. Anti-VEGF treatment results in delayed glomerular maturation; persistent nephrogenic precursors including ureteric tubules and glomerular precursors are indicated with arrowheads (ii, iii, v, vi). Maturation appears more delayed in mFlt(1-3)-IgG– than G6-23-IgG–treated animals. Abnormal glomerular “rosettes” (ii, iii, v; arrows) are composed of podocytes surrounding largely avascular mesangial cores. (C) Alterations in the cartilaginous growth plate of anti-VEGF–treated animals. G6-23-IgG and mFlt(1-3)-IgG treatment results in expansion of proliferating chondrocytes (asterisks; compare control subpanel i with panels iv and vii). Subpanels ii, v, and viii show details of the proliferating chondrocyte zone, illustrating expansion of the lacunae of individual cells. Width of hypertrophic chondrocyte zone (arrowheads) is reduced in anti-VEGF–treated animals.
Histopathologic analysis of C57/Bl6 neonatal mice treated for 5 days with G6-23-IgG, mFlt(1-3)-IgG, or control IgG. (A) Assessed vascular density in liver, heart, and kidney. Representative sections stained with rat anti–mouse VEGFR-2 antibody from each treatment group are illustrated. Vessel density was reduced to a similar extent by mFlt(1-3)-IgG and G6-23-IgG in all organs (see Table 2 for quantitative assessment). (B) Delayed and abnormal glomerular maturation in anti-VEGF–treated kidneys. Control glomeruli (iv) all have identifiable capillary loops supported by mesangial cells. Anti-VEGF treatment results in delayed glomerular maturation; persistent nephrogenic precursors including ureteric tubules and glomerular precursors are indicated with arrowheads (ii, iii, v, vi). Maturation appears more delayed in mFlt(1-3)-IgG– than G6-23-IgG–treated animals. Abnormal glomerular “rosettes” (ii, iii, v; arrows) are composed of podocytes surrounding largely avascular mesangial cores. (C) Alterations in the cartilaginous growth plate of anti-VEGF–treated animals. G6-23-IgG and mFlt(1-3)-IgG treatment results in expansion of proliferating chondrocytes (asterisks; compare control subpanel i with panels iv and vii). Subpanels ii, v, and viii show details of the proliferating chondrocyte zone, illustrating expansion of the lacunae of individual cells. Width of hypertrophic chondrocyte zone (arrowheads) is reduced in anti-VEGF–treated animals.
Changes in organ size, hematologic end points, and blood chemistry in adult mice treated with anti-VEGF
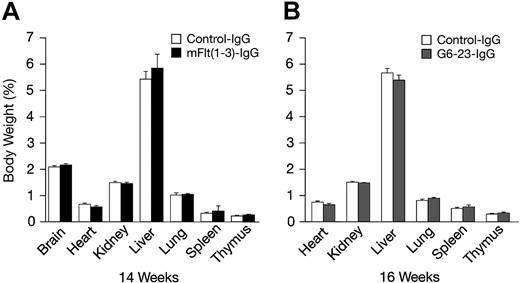
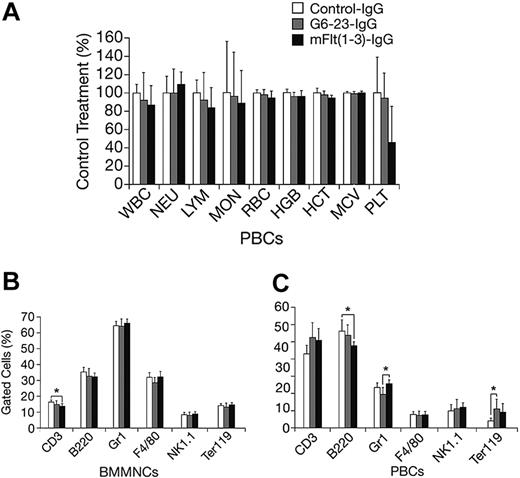
The effects of mFlt(1-3)-IgG or G6-23-IgG on organ size were analyzed in C57/Bl6 mice aged 14 and 16 weeks, respectively (Figure 4A-B). There were no significant changes in organ weights of adult mice treated for 2 weeks with either compound relative to controls. To detect potential changes in liver and kidney function, blood chemistry levels in mice aged 16 weeks were analyzed following 2 weeks of treatment with G6-23-IgG. Similar to previous findings in mFlt(1-3)-IgG–treated mice, there were no significant alterations in the blood chemistry of anti-VEGF–treated animals (Table 3). Changes in the levels of peripheral blood cells or bone marrow mononuclear cells (BMMNCs) were then analyzed by either automated analysis of whole blood (Figure 5A) or FACS (Figure 5B-C) following 2 weeks of anti-VEGF treatment.16 No significant alterations were observed in response to treatment relative to control groups in any of the peripheral blood cell populations (Figure 5A). FACS was used to determine the effects of G6-23-IgG and mFlt(1-3)-IgG administration on bone marrow mononuclear cells (BMMNCs; Figure 5B). BMMNCs were isolated from the femurs of C57BL/6 mice and the percentage of T cells, B cells, granulocytes, macrophages, natural killer (NK) cells, or erythroid cells was determined by using CD3 (T cells), B220 (B cells), Gr1 (granulocytes), F4/80 (macrophages), NK1.1 cells, and Ter119 (erythroid progenitors)–specific monoclonal antibodies, respectively. BMMNC population was not significantly altered by systemic anti-VEGF treatment in mice aged16 weeks when compared with control IgG–treated mice. Finally, FACS analysis of peripheral blood cells revealed a mild but significant reduction in B220-positive cell populations (B cells) in response to treatment with either compound and a 1.2-fold reduction in Gr1-positive cells in mice treated with G6-23-IgG but not mFlt(1-3)-IgG. In conclusion, the data suggest that anti–VEGF-A treatment induces only minimal changes on physiologic hematopoiesis in healthy adult mice after 2 weeks of anti-VEGF treatment.
Percent body and organ weights in adult C57BL/6 mice following 2 weeks of anti-VEGF treatment relative to control-treated groups. (A) Percent organ and total body weights of C57BL/6 mice aged 14 weeks treated with mFlt(1-3)-IgG (n = 7). (B) C57BL/6 mice aged 16 weeks treated with G6-23-IgG (n = 5). Data represents mean ± standard deviation. Data are from 1 representative of 2 independent experiments.
Percent body and organ weights in adult C57BL/6 mice following 2 weeks of anti-VEGF treatment relative to control-treated groups. (A) Percent organ and total body weights of C57BL/6 mice aged 14 weeks treated with mFlt(1-3)-IgG (n = 7). (B) C57BL/6 mice aged 16 weeks treated with G6-23-IgG (n = 5). Data represents mean ± standard deviation. Data are from 1 representative of 2 independent experiments.
Changes in hematologic parameters in adult mice treated with anti-VEGF. (A) Changes in C57BL/6 adult mice aged 16 weeks following 2 weeks of anti-VEGF or control IgG treatment. Whole blood was collected at necropsy and analyzed by automated hematologic analysis. MON indicates monocyte count; MCV, mean corpuscular volume. (B) Fluorescence-activated cell scan of BMMNCs isolated from C57BL/6 adult mice treated with the anti-VEGF or control compounds is indicated. There were no significant changes in the cell population of the group aged 16 weeks that were treated for 2 weeks. Data are mean ± standard deviation. (C) Fluorescence-activated cell scan of peripheral blood cells (PBCs) collected from G6-23-IgG–treated C57BL/6 adult mice (n = 7) following 2 weeks of treatment. Isolated cells were incubated with lineage-specific monoclonal antibodies and analyzed by flow cytometry. The relative percent of positively stained cells compared with total cells are displayed. There were minor changes in the B220, Gr1, and Ter119 population in some treatment groups relative to control.
Changes in hematologic parameters in adult mice treated with anti-VEGF. (A) Changes in C57BL/6 adult mice aged 16 weeks following 2 weeks of anti-VEGF or control IgG treatment. Whole blood was collected at necropsy and analyzed by automated hematologic analysis. MON indicates monocyte count; MCV, mean corpuscular volume. (B) Fluorescence-activated cell scan of BMMNCs isolated from C57BL/6 adult mice treated with the anti-VEGF or control compounds is indicated. There were no significant changes in the cell population of the group aged 16 weeks that were treated for 2 weeks. Data are mean ± standard deviation. (C) Fluorescence-activated cell scan of peripheral blood cells (PBCs) collected from G6-23-IgG–treated C57BL/6 adult mice (n = 7) following 2 weeks of treatment. Isolated cells were incubated with lineage-specific monoclonal antibodies and analyzed by flow cytometry. The relative percent of positively stained cells compared with total cells are displayed. There were minor changes in the B220, Gr1, and Ter119 population in some treatment groups relative to control.
Discussion
Different roles for VEGFR-1 during embryonic development and in adults
While VEGFR-2 is considered the main transducer of VEGF-A–mediated angiogenic signaling in endothelial cells, the role of VEGFR-1 on endothelial cells remains rather elusive. Recent evidence suggests different roles for VEGFR-1 during embryonic development and pathologic angiogenesis in adults.14,15,27 A VEGFR-1 blocking antibody was almost equally as efficient as a VEGFR-2 blocking antibody28,29 in inhibiting tumor growth and angiogenesis in xenograft experiments. VEGFR-1 expression is highly up-regulated on pathologic vasculature in adults,28,30,31 indicating that therapeutic effects of blocking VEGFR-1 signaling during tumor growth may be dependent on high expression levels of VEGFR-1 and its ligands.
VEGFR-1 is also expressed on hematopoietic stem/progenitor cells, the precursor cells that promote hematopoietic lineages, including endothelial progenitor cells.32 Evidence for a functional role for VEGFR-1 during bone marrow repopulation after bone marrow injury was provided by independent experiments in adult mice using function-blocking antibodies targeting VEGFR-1.33,34 Combined with other studies in adult mice,31,35,36 these findings suggest that VEGFR-1 may contribute to angiogenesis by regulating several different cell types including (1) endothelial cells, (2) smooth muscle cells and vessel maturation and stabilization, (3) inflammatory cells critical in the growth of collateral vessels, and (4) hematopoietic stem cells in the bone marrow.37 Thus, our analysis focused on the vascularization of the major organs and the effects on hematopoiesis and peripheral blood cells during mouse development. Selective neutralization of VEGF-A by G6-23-IgG induced similar pathophysiologic alterations to the multi-ligand blocking agent mFlt(1-3)-IgG, suggesting that VEGFR-1 signaling is redundant for postnatal development and vascular homeostasis in adult mice treated with anti–VEGF-A. Interestingly, we observed a difference in the onset and degree of lethality between gene ablation (38% lethality on day 7; Gerber et al16 ) and biochemical inactivation of VEGF-A in the current study (25% on day 7). However, the differences in the experimental conditions between both studies preclude any conclusions regarding the potential biologic relevance of the difference in onset of lethality.
The role of PlGF and VEGF-B during postnatal development and pathologic angiogenesis in adults
Gene targeting studies in mice revealed an almost 50% drop in vessel counts in the ovarian corpus luteum in female PlGF-deficient mice, however, adult mice did not display functional insufficiencies and reproduction was normal.38 When tested in models associated with pathologic angiogenesis including ischemia, inflammation, and cancer,28 PlGF-deficient mice experienced reduced disease severity. VEGF-B–deficient mice are viable, fertile, and do not exhibit any overt angiogenic deficiencies.39 However, adult knock-out mice exhibited an atrial conduction defect and reduced pathology and synovial angiogenesis in preclinical arthritis models.40 Combined, these data suggest that VEGF-B and PlGF fail to compensate during conditions of VEGF-A blockade but may help in the regulation of inflammatory events during pathologic angiogenesis in adults. Our findings further support the conclusions from single gene ablation experiments targeting Pgf and Vegfb in mice38,39 and extend the lack of compensatory signaling by both ligands via VEGFR-1 to postnatal development and homeostasis of adult vasculature during VEGF-A blockade. It will be interesting to test the potency and efficacy of both compounds to interfere with pathologic angiogenesis associated with tumor growth or inflammation in adult mice. In this context, VEGFR-1 expression was recently identified on several human tumor types where it may exert important cell autonomous functions regulating tumor cell survival and proliferation. These findings further support VEGFR-1 as a viable therapeutic strategy.41,42
One potential way to explain the failure of VEGFR-1 to compensate during VEGF-A blockade is that VEGFR-1 signaling and/or VEGF-B and PlGF activity are dependent on VEGF-A levels. However, our gene expression analysis of various tissues in neonatal mice revealed only minor interference between anti–VEGF-A treatment and expression of PlGF or VEGF-B, suggesting that endogenous levels of these ligands are not exclusively controlled by VEGF-A (Figure 2). In addition, previous reports suggest that VEGFR-1 signaling functions independently of VEGFR-2. For example, an anti–VEGFR-1–specific antibody was more effective when administered in combination with an anti–VEGFR-2–specific antibody,43 indicating the potential of VEGFR-1 to signal independently. Together, these findings suggest that PlGF and VEGF-B stimulate VEGFR-1 signaling independently and thus may potentially compensate during anti–VEGF-A blockade.
This study is the first to investigate the effects of selectively blocking VEGF-A in neonatal and adult mice. We were unable to detect significant compensatory effects induced by endogenous levels of PlGF and VEGF-B. Our findings may help to better comprehend some of the clinical data generated with various compounds interfering with VEGFR-1 and/or VEGFR-2 activity or other compounds targeting VEGF-A alone or in combination with VEGF-B and PlGF.44,45 Interestingly, most compounds tested clinically induced common types of “on-target” adverse events, including hypertension, mild proteinuria, and an increase in thromboembolic events. However, other side effects such as neutropenia or fatigue were more pronounced in patients treated with small-molecule inhibitors blocking VEGF-receptor signaling.46 The lack of compensatory VEGFR-1 signaling during VEGF-A blockade described in this report indirectly suggests that the differences in adverse events, efficacy, and tolerability between the 2 classes of compounds are unlikely to be caused by differences in their ability to interfere with VEGFR-1 signaling. More likely, the different adverse events may reflect the consequence of known or unknown “off-target effects” or differences in their pharmacokinetic properties.
Prepublished online as Blood First Edition Paper, September 27, 2005; DOI 10.1182/blood-2005-05-2047.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Special thanks to Leo DeGuzman, Jose Zavala-Solario, and Stuart Bunting for their help with animal models. Thanks to Marjie Van Hoy, Joel Morales, Laurie Leung, and Lorena Cabote for their pathology support.

![Figure 1. Effect of G6-23-IgG or mFlt(1-3)-IgG on postnatal body growth, organ weight, and survival of neonatal mice. (A) Ten C57BL/6 neonatal mice per group were treated for 10 days with either compound at a dose of 25 mg/kg intraperitoneally. Body weights were assessed daily. Data represent mean ± standard deviation. (B) Kaplan-Meier survival curves of C57BL/6 neonatal mice treated with either mFlt(1-3)-IgG, G6-23-IgG, or an isotype-matched control antibody. Both compounds induced a similar increase in death. (C) Percent body and organ weights from anti-VEGF–treated C57BL6 neonatal mice relative to controls on treatment day 5. Each group consisted of 5 specimens, representing mean ± standard deviation. Data are from 1 representative of 2 independent experiments. (D) Changes in hematologic parameters in neonatal mice aged 5 days treated with anti-VEGF [G6-23-IgG, mFlt(1-3)-IgG] or a vehicle control (phosphate-buffered saline; PBS). Whole blood was collected at necropsy and analyzed by automated hematologic analysis of white blood cell count (WBC), neutrophil count (NEU), lymphocyte count (LYM), eosinophil count (EOS), basophil count (BAS), red blood cell count (RBC), hemoglobin level (HGB), hematocrit (HCT), and platelet count (PLT). Data represent mean ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-05-2047/4/m_zh80020689670001.jpeg?Expires=1769542532&Signature=XJaFm~zwCM45FjbSVmpJUJ6k5FTgCYqN1zEO38p9aApLbv-DznnQ3ixZss8aF01rlXacNNNnCjgWcYa2KVOG~mGxaZd5mehJkCFwBW4PNiVagqWkoFJXgyRv8LuvqekOO7IGnd~uHQjz4D2nb-RsTmZeQ7xsTfQBVNflEhegbE4Uluf75wwPKNwpS0tYU9zaid~rbvi~NtLoHLgQbcxfFMi5gSQBQBvClOdV04Z2jjo7dF9x9JAC4UYvUSG8eKk6tU~evHos2IQi~CLFtZDALqPks876mg4CHFqxviCTCJP1Jji3b7cv~1qetnAJGosSRQuQzueYNZJjknNYOr00nA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)