Abstract
Patients with acute- or lymphoma-type adult T-cell leukemia (ATL) have a poor outcome because of the intrinsic drug resistance to chemotherapy. Protection from apoptosis is a common feature involved in multidrug-resistance of ATL. IAP (inhibitor of apoptosis) family proteins inhibit apoptosis induced by a variety of stimuli. In this study, we investigated the expression of IAP family members (survivin, cIAP1, cIAP2, and XIAP) in the primary leukemic cells from patients with ATL. We found that survivin was overexpressed in ATL, especially in acute-type ATL. Sodium arsenite was shown to down-regulate the expression of survivin at both the protein and RNA levels in a time- and dose-dependent manner, thus inhibiting cell growth, inducing apoptosis, and enhancing the caspase-3 activity in ATL cells. Nuclear factor-κB (NF-κB) enhances the transcriptional activity of survivin. Sodium arsenite suppressed the constitutive NF-κB activation by preventing the IκB-α degradation and the nuclear translocation of NF-κB. These findings suggest that survivin is an important antiapoptotic molecule that confers drug resistance on ATL cells. Sodium arsenite was shown to down-regulate the expression of survivin through the NF-κB pathway, thus inhibiting cell growth and promoting apoptosis of ATL cells.
Introduction
Adult T-cell leukemia (ATL) is an aggressive malignancy of CD4+ T cells associated with human T-cell leukemia virus type 1 (HTLV-1) infection.1 There is evidence suggesting that HTLV-1 viral Tax protein activates the expression of a number of cellular genes, such as growth factors, growth factor receptors, and oncogenes, through the induction of transcription factors, such as nuclear factor-κB (NF-κB),2 cyclic adenosine monophosphate (cAMP) response element binding protein (CREB)/AP-1 transcription factor (ATF),3 and serum response factor (SRF).4 However, the presence of tax gene expression has not yet been clearly established in freshly isolated ATL cells; moreover, defective viruses, which cannot produce Tax, have been observed in ATL cells, thus suggesting that the tax gene is necessary for the initial stages of leukemogenesis, but it is not essential for the late stage of leukemia.5 As suggested by the multistep model of tumorigenesis,6 mutations of various genes are considered to contribute to leukemogenesis.
ATL is divided into 4 clinical subtypes: acute, lymphoma, chronic, and smoldering.7 Chronic- and smoldering-type ATLs have a mild clinical course and do not require treatment with intensive chemotherapy. Acute- and lymphoma-type ATLs require intensive chemotherapy, and the median survival period is less than 1 year because of resistance to chemotherapy. Several mechanisms are involved in the multidrug resistance of ATL, including the overexpression of P-glycoprotein (P-gp),8 multidrug resistance protein 1 (MRP1),9 and lung resistance-related protein (LRP).10 Accumulating evidence suggests that the activation of NF-κB is a critical process in the inhibition of apoptosis and resistance to chemotherapy.11
Survivin, cIAP1, cIAP2, and XIAP belong to the IAP (inhibitor of apoptosis) family, which is defined by 1 or more repeats of a highly conserved 70–amino acid domain called the baculovirus IAP repeat (BIR) located at the amino-terminus. IAP family proteins directly bind and inhibit certain caspases and also inhibit apoptosis induced by a variety of stimuli.12 Previous studies have revealed that several IAPs, such as survivin, NAIP, and XIAP, were overexpressed in acutel myeloid leukemia (AML).13 Survivin was overexpressed in chronic lymphocytic leukemia (CLL) and ATL.14,15 The IAPs are probably involved in the drug resistance in leukemia. Survivin was also demonstrated to be an unfavorable prognostic factor in leukemia, oral squamous cell carcinoma, and bladder cancer.16-19 Among the known IAPs, survivin was prominently and consistently expressed in ATL and the expression level of the survivin mRNA has correlated with a shorter survival of the patients.15 However, the expression of other IAP members such as IAP1, IAP2, and XIAP in ATL is still uncertain. Ishitsuka and colleagues showed that arsenic trioxide (As2O3) has a therapeutic potential for the treatment of ATL.20 They suggested that As2O3 induced apoptosis of ATL cells by the destruction of the Bcl-2 protein and the enhancement of the Bak protein production.21
In the present study, we investigated the expression levels of the members of the IAP family in primary ATL cells. We thus found survivin to be overexpressed in ATL while sodium arsenite inhibited cell growth and apoptosis through the down-regulation of survivin.
Patients, materials, and methods
Patients
Between July 1999 and July 2003, we studied 38 patients with ATL (Table 1) consisting of 18 men and 20 women with a median age of 62 years (range, 21-87 years). According to previously reported diagnostic criteria,7 23 of these patients had acute-type ATL, 12 had chronic-type ATL, and 3 had smoldering-type ATL. The performance status (PS) was based on the 5-grade scale of the World Health Organization. Seventeen patients had a PS of 0; 8 patients had a PS of 1; 6 patients had a PS of 2; 3 patients had a PS of 3; and 4 patients had a PS of 4. During this study, we treated patients with acute ATL with combination chemotherapy regimens such as a response-oriented multidrug protocol; a cyclophosphamide, DOX, vincristine, and prednisone protocol; or an LSG15 protocol. None of the patients with chronic and smoldering ATL required treatment with intensive chemotherapy. Approval for this study was obtained from the Kagoshima University institutional review board. Informed consent was provided according to the Declaration of Helsinki.
Reagents and antibodies
RPMI 1640 was purchased from Nissui Seiyaku (Tokyo, Japan). Fetal calf serum (FCS) was obtained from Equitech-Bio (Kerrville, TX). Sodium arsenite (NaAsO2, AsIII) and Ac-DEVD-MCA (Ac-Asp-Glu-Val-Asp-MCA) were obtained from Wako Pure Chemical Industries (Osaka, Japan). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) was obtained from Sigma Chemical (St Louis, MO).
Antibodies against NF-κB (polyclonal antibody p65 and monoclonal antibody p50) and monoclonal antibodies against Bcl-2, PARP, and IκB-α were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). A monoclonal antibody against α-tubulin was from Oncogene (Boston, MA). A polyclonal antibody against survivin, MP001, was prepared in our laboratory. MRPm6, a monoclonal antibody against MRP1, was purchased from Progen Biotechnick (Heidelberg, Germany).
Cell lines and cell cultures
The human ATL cell line MT2 is derived from normal human leucocytes transformed by the leukemic T cells of a patient with ATL; S1T comes from HTLV-1–infected CD4+ T cells with no Tax expression that are derived from a patient with ATL. The acute T-cell lymphoma cell line Jurkat was used as a control. The cell lines were grown in RPMI 1640 containing 10% FCS, 2 mM glutamine, and 100 U/mL penicillin at 37°C in a 5% CO2 humidified atmosphere.
cDNA synthesis
Peripheral blood mononuclear cells were separated by Ficoll-Conray (Immuno-Biological, Gunma, Japan) density gradient centrifugation and stored at –80°C until use for RNA or protein extraction. The total RNA from peripheral blood mononuclear cells and the cultured cells was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA (1 μg) was reverse transcribed using a first-strand cDNA synthesis kit (ReverTra Ace α; TOYOBO, Osaka, Japan).
Reverse transcription–PCR
The resulting first-strand cDNA (1 μL) was used for each polymerase chain reaction (PCR). The human survivin primers were as follows: forward, 5′-GAT TTG AAT CGC GGG ACC CGT TG-3′; and reverse, 5′-TCAAGA CAA AAC AGG AGC ACA GT-3′. The primers of β-actin for internal control were as follows: forward, 5′-CAG CTT CGG AAC AAG AGA CC-3′; and reverse, 5′-GTC CGA TGA TTC CTG CTG AT-3′.
The PCR amplification mixture was adjusted to a final volume of 20 μL. Twenty-five cycles were performed at 94°C for 30 seconds for denaturation, 58°C for 30 seconds for annealing, and 72°C for 1 minute for extension. The PCR products were separated on 1% agarose gel and then were stained with ethidium bromide.
Real-time reverse transcription–PCR quantification
The resulting first-strand cDNA (1 μL) was assayed by real-time reverse transcription (RT)–PCR (PRISM 7900HT; Applied Biosystems, Foster City, CA) according to a technical brochure of the company. The sets of primers and TaqMan probes were designed with the primer design software Primer Express version 2.0 (Applied Biosystems). The primers and TaqMan probes were as follows: The sequence of the forward primer for survivin mRNA was 5′-TTC AAG AAC TGG CCC TTC TTG-3′, and that of the reverse primer was 5′-TGG CTC CCA GCC TTC CA-3′; the TaqMan probe was FAM-CCT GCA CCC CGG AGC GGA T-TAMRA. For XIAP mRNA, the forward primer was 5′-GCC TTA GAC AGG CCA TCT GAG A-3′, and the reverse primer was 5′-TTC CTC GGG TAT ATG GTG TCT GAT-3′; the TaqMan probe was FAM-TGC AGA CTA TCT TTT GAG AAC TGG GCA GGT-TAMRA. For cIAP1 mRNA, the forward primer was 5′-CAG ACA CAT GCA GCT CGA ATG-3′, and the reverse primer was 5′-AAG CCA CCA TCA CAA CAA AAG-3′; the TaqMan probe was FAM-TGT TCC AGT TCA GCC TGA GCA GCT TG-TAMRA. The forward primer for GAPDH mRNA was 5′-GAA GGT GAA GGT CGG AGT-3′, and the reverse primer was 5′-GAA GAT GGT GAT GGG ATT TC-3′; the TaqMan probe was FAM-CAA GCT TCC CGT TCT CAG CC-TAMRA. The conditions for the 1-step RT-PCR were as follows: 2 minutes at 50°C and 10 minutes at 95°C, and then 40 cycles of amplification for TaqMan Universal PCR Master Mix (Roche, Branchburg, NJ) at 95°C for 15 seconds and annealing and extension at 60°C for 1 minute. Human GAPDH was used for normalization. Quantification of the target gene expression was done using the comparative cycle threshold method according to the instructions of the manufacturer (Applied Biosystems). All experiments were performed in triplicate for each data point. Each quantitation was performed with the standard curve method.
Protein extraction and Western blotting
After treatment with various concentrations of sodium arsenite, cells were harvested and lysed with RIPA buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 20 mM NaF, 100 mM Na3VO4, 0.5% Nonidet P-40 [NP-40], 1% Triton X-100, and 1 mM PMSF). The lysates were passed through a 21-gauge needle to break up the cell aggregates, and were cleared by centrifugation at 14 000g for 15 minutes at 4°C; the supernatant (total cell lysate) was immediately used or stored at –80°C until use.
For the detection of NF-κB, the cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in 400 μL lysis buffer (buffer A, containing 10 mM HEPES [pH 7.9], 10 mM KCl, 0.2 mM EDTA, 1 mM DTT, 0.5 mM PMSF, and 0.6% NP-40). The lysates were centrifuged at 250g for 10 minutes. The supernatant was collected as the cytosolic fraction. The pellets containing the nuclei were washed in buffer A without NP-40 and then were resuspended in 50 μL nuclear lysis buffer (buffer C, containing 20 mM HEPES [pH 7.9], 0.4 M NaCl, 2 mM EDTA, 1 mM DTT, and 1 mM PMSF), incubated for 30 minutes at 4°C, and centrifuged at 20 000g for 20 minutes. The supernatants were either used as nuclear fractions immediately or stored at –80°C until use. The protein concentration was determined by a Bio-Rad protein assay according to the manufacturer's protocol (Bio-Rad Laboratories, Hercules, CA).
Lysates containing 100 μg of protein were subjected to 12.5% or 9.4% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and then were transferred to Immobilon-P membrane (Millipore, Bedford, MA). The membrane was incubated with the primary antibody (dilution of 1:1000) overnight at 4°C and then with a peroxidase-linked secondary antibody (dilution of 1:2000) for 1 hour at room temperature, and proteins were visualized by enhanced chemiluminescence.
For detection of MRP1, 100 μg crude membranes prepared from Jurkat and S1T cells and 10 μg membrane vesicles prepared from KB/MRP, which was used as a positive control, were subjected to 7.5% SDS-PAGE, and an m6 monoclonal antibody against MRP1 was used for MRP1 detection. The density of the bands for MRP1 was quantified using a charge-coupled device (CCD) camera (Bio-Rad Laboratories-Segrate, Milan, Italy).
Cell survival by the MTT assay
MT2 (2 × 104/mL), S1T, and Jurkat cells (1 × 104/mL) were incubated either with or without various concentrations of sodium arsenite for the indicated time in 96-well plates. Ten μL MTT solution (5 μg/mL) was added to each well and then the plates were incubated for an additional 4 hours. The MTT formazan precipitate was dissolved in 100 μL isopropanol containing 0.04 N HCl. The plates were shaken for 5 minutes and read immediately at 570 nm using a model 550 Micro Plate Reader (Bio-Rad, Hercules, CA).
For chemosensitivity in vitro, MT2, S1T, and Jurkat cells (1 × 104/mL) were incubated in culture medium with various concentrations of indicated drugs at a final volume of 100 μL. After 3 days, 10 μL MTT was added to each well and the plates were incubated for an additional 4 hours. Optical density at 570 nm (OD570) was measured as described.
Apoptosis analysis by FACS
MT2, S1T, and Jurkat cells (1 × 106) were treated with various concentrations of sodium arsenite for various periods. The cells were harvested, washed once with PBS, suspended in 50 μL PBS, and mixed with 50 μL Coulter DNA-prep LRP (COULTER, Miami, FL), and then 1 mL Coulter DNA-prep Stain was added. The mixtures were then incubated for 15 minutes at room temperature. The sub-G1 fraction was determined using a Coulter FACSCAN (Becton Dickinson, San Jose, CA) as previously described.22
Caspase-3 activity assay
Enzymatic reactions were carried out in the mixture containing 50 μg cell lysate treated by various concentrations of sodium arsenite, interleukin-1 β-converting enzyme (ICE)–like enzyme assay buffer (100 mM HEPES [pH 7.5], 10% sucrose, and 0.1% CHAPS), and 10 mM DTT. The reaction mixtures were incubated at 30°C for 30 minutes. Next, the 50 μM synthetic fluorogenic substrate, Ac-DEVD-MCA (Ac-Asp-Glu-Val-Asp-MCA), was added and incubated at 30°C for another 60 minutes. The activity of caspase-3 was measured at an excitation wavelength of 360 nm and an emission wavelength of 460 nm using a FP750 microplate fluorescence reader (Jasco, Tokyo, Japan).
Statistical analysis
All statistical analyses were performed by the Statview 5.0 software package for Windows (SAS Institute, Cary, NC). For patients with ATL, differences in the numerical data between the 2 groups were evaluated using the Mann-Whitney U test. A P value of less than .05 was considered to be statistically significant.
For the MTT assay, the fluorescence-activated cell-sorting (FACS) analysis, and the caspase-3 activity, the quantitative data were expressed as the mean plus or minus standard deviation (SD). Statistical comparisons were performed using one-way analysis of variance (ANOVA) or the unpaired Student t test. Differences were regarded as significant when the probability values were less than .01.
Results
Expression of mRNA for IAP proteins determined by real-time quantitative PCR or RT-PCR
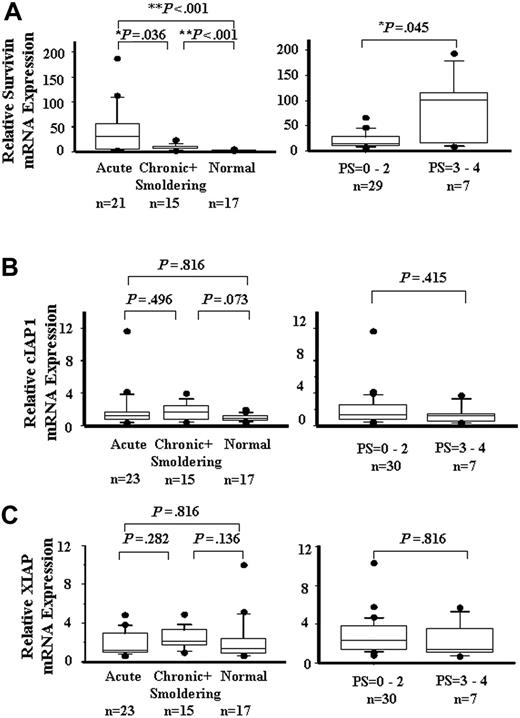
ATL's drug resistance is one of the main obstacles to successful chemotherapy. Kamihira et al15 reported that survivin was prominently and consistently expressed in ATL, thus correlating with the ATL prognosis. However, the role of other IAP members such as IAP1, IAP2, and XIAP in the resistance of ATL to chemotherapy is uncertain. To quantitate the mRNA expression levels of the IAP family in ATL cells, real-time quantitative RT-PCR was performed. As shown in Figure 1A, the expression of the survivin gene was significantly higher (P < .01) in acute, chronic, and smoldering ATL than that in healthy controls. The expression of the survivin gene in acute ATL was higher than that in chronic and smoldering ATL (P < .05). The expression of the survivin gene in PS3 to PS4 ATL was higher than that in PS0 to PS2 ATL (P < .05). The mRNA levels of cIAP1 and XIAP (Figure 1B-C) did not differ substantially among the subtypes of ATL and the healthy controls, and between PS0 to PS2 and PS3 to PS4 ATLs. The expression of cIAP2 was also detected by RT-PCR, but the expression level in ATL did not increase significantly more than that in the control (data not shown). The patients' age, sex, and white blood cell (WBC) count of ATL were not correlated with the expression of IAP proteins (data not shown). These results show that only the survivin gene was overexpressed in ATL, thereby showing a correlation with the PS of the patients.
Protein expression of survivin in ATL
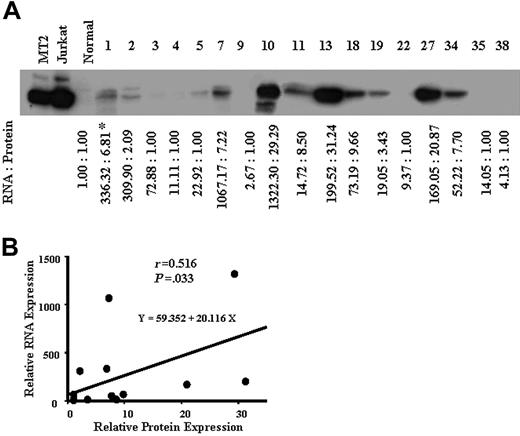
We next examined the expression levels of survivin in ATL cells from 17 patients by Western blotting (Figure 2A), and compared with the survivin mRNA levels in ATL cells of each patient. As shown in Figure 2B, the expression level of survivin mRNA was significantly correlated with that of the protein (r = 0.516, P = .033). These results suggest that survivin alone is overexpressed among the members of the IAP family in ATL, especially in acute ATL. The survivin expression level was correlated with the ATL progression (Figure 1A).
Down-regulation of survivin by sodium arsenite in ATL cells
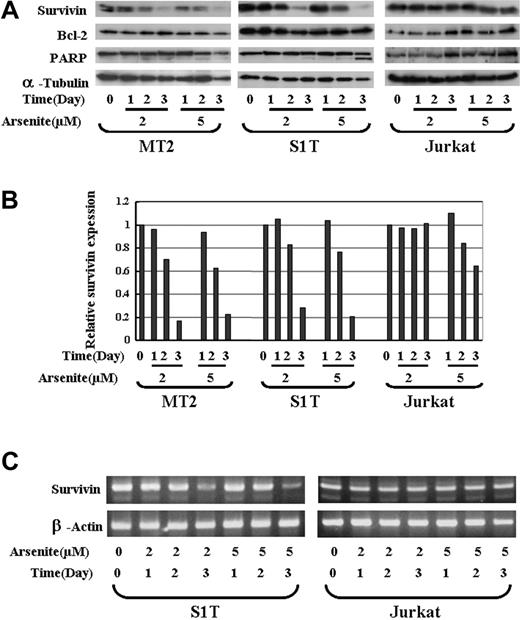
As2O3 was shown to have a therapeutic effect against acute promyelocytic leukemia (APL) and other types of haemotologic malignancies by decreasing the expression of Bcl-2 and inducing apoptosis. It was recently reported that As2O3 combined with interferon α (IFN-α) could also induce apoptosis in ATL cells. We thus investigated whether sodium arsenite can regulate the expression of survivin in ATL cells. MT2 (Tax-positive), S1T (Tax-negtive), and a T-cell lymphoma cell line, Jurkat (as a control) cells were treated with 0, 2, and 5 μM sodium arsenite for 1, 2, and 3 days, and the expression of survivin and BCL-2 was detected by Western blotting. α-tubulin was used as a control for protein loading. The expression of survivin in MT2 and S1T cells dramatically decreased from 2 days after treatment with 2 and 5 μM sodium arsenite (Figure 3A). The relative density of survivin compared with the untreated controls is shown in Figure 3B. However, no clear down-regulation of survivin in Jurkat cells was detected (Figure 3A-B). Sodium arsenite did not down-regulate Bcl-2 expression in all 3 cell lines used in this study. The RNA level of survivin was also decreased by sodium arsenite treatment in S1T cells, but not in Jurkat cells (Figure 3C). These data suggest that survivin is a target of sodium arsenite in ATL cells. Sodium arsenite could both dose- and time-dependently down-regulate the survivin expression at the RNA and protein levels in ATL cells.
IAP family mRNA expression. A quantitative RT-PCR analysis for survivin (A), cIAP1 (B), and XIAP (C) is shown in patients with acute, chronic, and smoldering ATL and in a healthy control (left), low PS and high PS (right). Each mRNA expression level was normalized on the basis of the GAPDH mRNA expression and expressed relative to the mRNA level in a healthy control. Boxes correspond to the interquartile range. Lines in the boxes represent median values. The vertical lines represent the 10th and 90th percentiles, and the circles represent the outliers. Differences were analyzed by Mann-Whitney's U test.
IAP family mRNA expression. A quantitative RT-PCR analysis for survivin (A), cIAP1 (B), and XIAP (C) is shown in patients with acute, chronic, and smoldering ATL and in a healthy control (left), low PS and high PS (right). Each mRNA expression level was normalized on the basis of the GAPDH mRNA expression and expressed relative to the mRNA level in a healthy control. Boxes correspond to the interquartile range. Lines in the boxes represent median values. The vertical lines represent the 10th and 90th percentiles, and the circles represent the outliers. Differences were analyzed by Mann-Whitney's U test.
Effects of sodium arsenite on cell proliferation
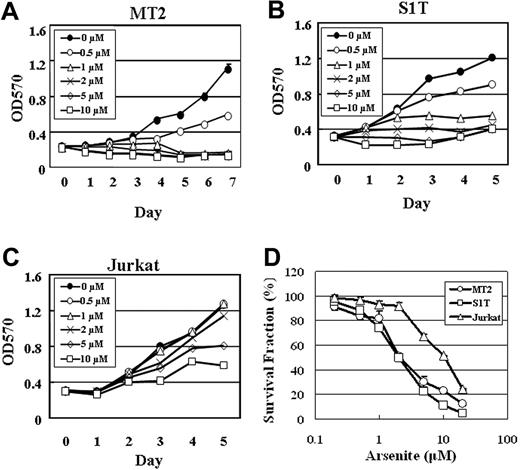
We next examined whether the growth inhibition of ATL cells by sodium arsenite correlates with the suppression of survivin expression. MT2, S1T, and Jurkat cells were treated with 0, 0.5, 1, 2, 5, and 10 μM sodium arsenite for 5 to 7 days, and cell proliferation was measured by an MTT assay. As shown in Figure 4, the growth of MT2 and S1T cells was inhibited at a dose of 0.5 μM sodium arsenite (Figure 4A-B). However, no significant growth suppression was observed at a dose of 2 μM sodium arsenite in Jurkat cells (Figure 4C).
The dose-response curves of sodium arsenite for S1T and MT2 cells were compared with that for Jurkat cells. As shown in Figure 4D, MT2 and S1T cells were more sensitive to sodium arsenite than Jurkat cells. IC50 values (the concentrations of sodium arsenite that inhibit 50% of cell growth) for MT2, S1T, and Jurkat cells were 2.17 μM ± 0.33 μM, 1.99 μM ± 0.03 μM, and 10.54 μM ± 0.17 μM, respectively. These results suggest that the ATL cell lines MT2 and S1T are sensitive to sodium arsenite even at low concentrations, and the suppression of survivin expression is, at least in part, involved in the growth inhibition of the ATL cells.
Effects of sodium arsenite on apoptosis
To investigate whether the inhibition of cell proliferation was due to enhanced apoptosis, the proportion of sub-G1 fraction was investigated in the presence of 0, 1, 2, 5, and 10 μM sodium arsenite for 1, 2, and 3 days. Sodium arsenite at 1 μM did not increase the proportion of sub-G1 fraction, but the proportion of sub-G1 fraction both dose- and time-dependently increased at 2, 5, and 10 μM in MT2 and S1T cells (Figure 5A-B). However, the increase in the proportion of the sub-G1 fraction of Jurkat cells treated with the same concentrations of sodium arsenite was less than that of the ATL cells (Figure 5C). Sub-G1 fraction in MT2 and S1T cells treated with various concentrations of sodium arsenite for 3 days was significantly higher than that in Jurkat cells (P < .01) (Figure 5D). This result suggests that sodium arsenite inhibits the growth of ATL cells by inducing apoptosis.
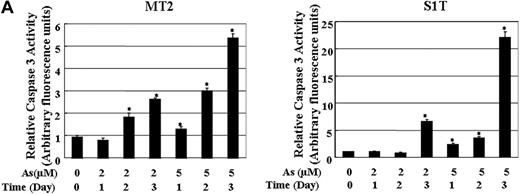
Effects of sodium arsenite on caspases activation in ATL cells
Survivin was shown to bind directly with caspase-3 to inhibit the caspase activity in human cells exposed to apoptotic stimuli. We investigated whether caspases-3 are activated in ATL cells by treatment with sodium arsenite. As shown in Figure 6, the caspases-3 activity increased in MT2 (Figure 6A) and S1T (Figure 6B) cells treated with 2 and 5 μM of sodium arsenite. The increased cleavage of PARP was also detected in MT2 and S1T cells treated with sodium arsenite (Figure 3A). These results indicate that the down-regulation of survivin by sodium arsenite thus causes caspase-3–dependent cell death.
Protein expression of survivin in ATL. Whole-cell lysates (50 μg protein) from patients with ATL were separated by 12.5% SDS-PAGE and then transferred to a PVDF membrane. The transferred proteins were reacted with antibody against survivin (A) as described in “Patients, materials, and methods.” *Concentrations of survivin protein and mRNA are expressed relative to the survivin protein and mRNA levels in control normal cells, which were assigned values of 1. (B) A comparison between the survivin mRNA level (y-axis) and the protein expression level (x-axis) in 17 patients with ATL. The survivin mRNA level correlated with the protein level (r = 0.516, P = .033).
Protein expression of survivin in ATL. Whole-cell lysates (50 μg protein) from patients with ATL were separated by 12.5% SDS-PAGE and then transferred to a PVDF membrane. The transferred proteins were reacted with antibody against survivin (A) as described in “Patients, materials, and methods.” *Concentrations of survivin protein and mRNA are expressed relative to the survivin protein and mRNA levels in control normal cells, which were assigned values of 1. (B) A comparison between the survivin mRNA level (y-axis) and the protein expression level (x-axis) in 17 patients with ATL. The survivin mRNA level correlated with the protein level (r = 0.516, P = .033).
The effects of sodium arsenite on the survivin expression in ATL cells. (A) MT2, S1T, and Jurkat cells were incubated in the presence of 2 or 5 μM arsenic trioxide for 24, 48, or 72 hours. Whole-cell lysates (100 μg protein) were prepared and separated by 12.5% SDS-PAGE and transferred to a PVDF membrane. The transferred proteins were reacted with the antibody against survivin, Bcl-2, or PARP as described in “Patients, materials, and methods.” As an internal control, α-tubulin expression was detected. (B) The quantification of the survivin levels in MT2, S1T, and Jurkat cells. The relative density of the bands for survivin obtained by a densitometric analysis and α-tubulin was used to normalize the respective intensities. (C) S1T and Jurkat cells were treated with sodium arsenite at the indicated concentration and time. Total RNA was then subjected to RT-PCR using primers specific for the amplification of survivin. β-actin expression was examined as an internal control to ensure the RNA integrity and proper amplification.
The effects of sodium arsenite on the survivin expression in ATL cells. (A) MT2, S1T, and Jurkat cells were incubated in the presence of 2 or 5 μM arsenic trioxide for 24, 48, or 72 hours. Whole-cell lysates (100 μg protein) were prepared and separated by 12.5% SDS-PAGE and transferred to a PVDF membrane. The transferred proteins were reacted with the antibody against survivin, Bcl-2, or PARP as described in “Patients, materials, and methods.” As an internal control, α-tubulin expression was detected. (B) The quantification of the survivin levels in MT2, S1T, and Jurkat cells. The relative density of the bands for survivin obtained by a densitometric analysis and α-tubulin was used to normalize the respective intensities. (C) S1T and Jurkat cells were treated with sodium arsenite at the indicated concentration and time. Total RNA was then subjected to RT-PCR using primers specific for the amplification of survivin. β-actin expression was examined as an internal control to ensure the RNA integrity and proper amplification.
Growth inhibition of MT2, S1T, and Jurkat cells by sodium arsenite. Proliferation of MT2 (A), S1T (B), and Jurkat (C) cells in the absence or presence of the indicated concentrations of sodium arsenite was assessed by MTT assay. (D) The sodium arsenite toxicity in MT2, S1T, and Jurkat cells was determined by an MTT assay. The points represent the means of triplicate determinations, while the bars show SD.
Growth inhibition of MT2, S1T, and Jurkat cells by sodium arsenite. Proliferation of MT2 (A), S1T (B), and Jurkat (C) cells in the absence or presence of the indicated concentrations of sodium arsenite was assessed by MTT assay. (D) The sodium arsenite toxicity in MT2, S1T, and Jurkat cells was determined by an MTT assay. The points represent the means of triplicate determinations, while the bars show SD.
Effects of sodium arsenite on the proportion of sub-G1 fraction of MT2, S1T, and Jurkat cells. MT2, S1T, and Jurkat cells were treated with 0, 1, 2, 5, or 10 μM sodium arsenite for 1, 2, or 3 days. The cells were then stained by propidium iodide (PI) and analyzed by flow cytometry. The proportion of sub-G1 fraction of MT2 (A) and S1T (B) was higher than that of Jurkat cells (C) under the indicated concentrations of sodium arsenite and the indicated time. Each column and bar represents the mean ± SD of 3 independent experiments. (D) The sub-G1 fraction of MT2, S1T, and Jurkat cells treated with sodium arsenite for 3 days was compared. Each column and bar represents the mean ± SD of 3 independent experiments. *P < .01.
Effects of sodium arsenite on the proportion of sub-G1 fraction of MT2, S1T, and Jurkat cells. MT2, S1T, and Jurkat cells were treated with 0, 1, 2, 5, or 10 μM sodium arsenite for 1, 2, or 3 days. The cells were then stained by propidium iodide (PI) and analyzed by flow cytometry. The proportion of sub-G1 fraction of MT2 (A) and S1T (B) was higher than that of Jurkat cells (C) under the indicated concentrations of sodium arsenite and the indicated time. Each column and bar represents the mean ± SD of 3 independent experiments. (D) The sub-G1 fraction of MT2, S1T, and Jurkat cells treated with sodium arsenite for 3 days was compared. Each column and bar represents the mean ± SD of 3 independent experiments. *P < .01.
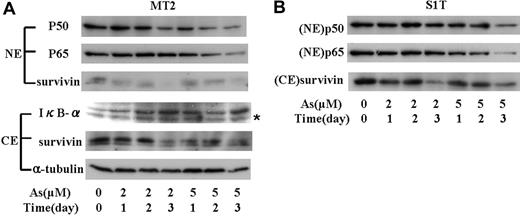
Decreased expression of survivin through the surpression of NF-κB activity by sodium arsenite
NF-κB is known to be constitutively activated in ATL. Either a Tax-dependent or Tax-independent mechanism of activation of the NF-κB pathway is crucial for the proliferation of malignant cells, protection from apoptosis, and drug resistance in ATL. Tax regulates the expression of survivin through NF-κB pathway, and the combination of As2O3 and IFN-α decreases the activation of NF-κB in ATL cells. We therefore investigated the levels of nuclear p50 and p65 in ATL cells treated with sodium arsenite. As shown in Figure 7A, sodium arsenite increased the level of IκB-α in MT2 cells. p50 and p65 in the nucleus of MT2 cells was decreased by sodium arsenite in a dose- and time-dependent manner. Cytosolic and nuclear survivin decreased in accordance with the decrease of nuclear p50 and p65. Although no significant decrease of p50 was seen in the nuclei of S1T cells, p65 in the nuclei dose-dependently decreased by sodium arsenite (Figure 7B). These results suggest that sodium arsenite suppress the expression of survivin by preventing IκB-α degradation and the translocation of NF-κB into the nuclei.
Expression of MRP1 and the resistance to sodium arsenite in Jurkat cells
To elucidate the molecular basis for the difference in sensitivity to sodium arsenite between Jurkat and S1T cells, we examined the expression levels of MRP1 in these cells. MRP1 is involved in arsenite resistance and supposed to transport arsenite conjugated with glutathione. As shown in Figure 8A, MRP1 was expressed in both Jurkat and S1T cells, but the expression level of MRP1 in Jurkat cells was about 3-fold higher than that in S1T cells. The higher molecular weight of MRP1 in S1T cells might be caused by a variation in glycosylation of MRP1 in the cells.
The activities of caspase-3 in MT2 and S1T cells in the absence or presence of sodium arsenite. The caspase-3 activity was measured in the cell lysates using the specific substrate Ac-DEVD-MCA. The data are expressed in arbitrary units. Each value represents the mean of 3 independent experiments. Bars indicate SD. *P < .01.
The activities of caspase-3 in MT2 and S1T cells in the absence or presence of sodium arsenite. The caspase-3 activity was measured in the cell lysates using the specific substrate Ac-DEVD-MCA. The data are expressed in arbitrary units. Each value represents the mean of 3 independent experiments. Bars indicate SD. *P < .01.
As shown in Figure 8B, Jurkat cells were about 4-fold more resistant to sodium arsenite than S1T cells (IC50 values of S1T and Jurkat cells for sodium arsenite were 1.99 μM ± 0.03 μM and 9.73 μM ± 0.71 μM, respectively). An inhibitor of MRP1, AG-A alone had no cytotoxic effect at 30 μM and decreased the IC50 value of Jurkat cells for sodium arsenite to 4.03 μM ± 0.04 μM. A specific inhibitor of MRP1, MK571 at 30 μM completely abolished the difference in sensitivity to sodium arsenite between the 2 cell lines. These findings suggest that the difference in sensitivity to sodium arsenite between Jurkat and S1T cells is attributed to the different expression levels of MRP1 in these cells.
Discussion
Patients with acute- or lymphoma-type ATL have a poor outcome, with a median survival of about 6 months for acute-type and 10 months for lymphoma-type; the expected 4-year survival is only about 5%. Combination chemotherapy regimens, especially those for the treatment of aggressive non-Hodgkin lymphoma or acute lymphoblastic leukemia, have little effect on ATL. The poor outcome might be due to multidrug resistance in ATL cells. Overexpression of P-gp, MRP-1, and LRP tend to be common features of the malignant cells, and they confer intrinsic and acquired drug resistance on the cells. Another important mechanism of the resistance to apoptosis-inducing anticancer agents is the failure to activate apoptosis. Members of the IAP family are also overexpressed in many tumors and leukemia, and they also inhibit apoptosis. In order to find out the specific target for ATL therapy, we investigated the expression levels of the members of the IAP family, the survivin gene, IAP1, IAP2, and XIAP, in ATL cells from patients using real-time PCR or RT-PCR. Among them, survivin alone was overexpressed in ATL, especially in acute-type ATL. The expression level of survivin in poor PS was higher than that in good PS.
The effect of sodium arsenite on the levels of IκB-α, NF-κB, and survivin in nuclear and cytosolic fraction from MT2 and S1T cells. (A) MT2 cells were treated with sodium arsenite at the indicated concentrations; nuclear and cytosolic fractions were prepared at the indicated time. Nuclear protein (50 μg) was subjected to Western blotting using the antibody against p50, p65, or survivin. Cytoplasmic fraction (100 μg of protein) was subjected to Western blotting using the specific antibody against IκB-α or survivin. α-tubulin expression was used as a loading control. NE indicates nuclear extract; CE, cytoplasmic extract. *Nonspecific band. (B) p50 and p65 in the nucleus and cytoplasmic survivin in S1T cells treated with sodium arsenite in the indicated concentration, and time was measured by Western blot as in panel A.
The effect of sodium arsenite on the levels of IκB-α, NF-κB, and survivin in nuclear and cytosolic fraction from MT2 and S1T cells. (A) MT2 cells were treated with sodium arsenite at the indicated concentrations; nuclear and cytosolic fractions were prepared at the indicated time. Nuclear protein (50 μg) was subjected to Western blotting using the antibody against p50, p65, or survivin. Cytoplasmic fraction (100 μg of protein) was subjected to Western blotting using the specific antibody against IκB-α or survivin. α-tubulin expression was used as a loading control. NE indicates nuclear extract; CE, cytoplasmic extract. *Nonspecific band. (B) p50 and p65 in the nucleus and cytoplasmic survivin in S1T cells treated with sodium arsenite in the indicated concentration, and time was measured by Western blot as in panel A.
Survivin is broadly expressed in embryonic and fetal organs,23 but it becomes undetectable in most terminally differentiated normal tissues.24 Survivin was overexpressed in the majority of human tumor types. In gene-profiling studies, survivin was identified as the fourth “transcriptome” expressed in the most common human cancers, but not in normal tissues.25 Retrospective trial studies suggest that the expression level of the survivin gene contributes to the clinical outcome of tumors, including an abbreviated overall survival, increased rates of recurrences, resistance to therapy, and reduced apoptotic index.26 Recent studies using real-time PCR showed that high expression levels of survivin mRNA were a risk factor for prognosis of ATL in the clinical setting.15 These results are consistent with our results in this study.
There are some reports regarding the relationship between survivin expression and sensitivity to anticancer agents. Forced overexpression of survivin increased the resistance to paclitaxel in prostate cancer cell lines. The inhibition of survivin sensitizes prostate cancer cells to paclitaxel-induced apoptosis through caspase-dependant mechanism in vitro and in vivo.27 Down-regulation of survivin expression with ribozyme or small interfering RNA (siRNA) increased sensitivity to topotecan and adriamycin in JR8 melanoma cell line and HL-60/ADR cells.28,29 We have knocked down survivin in KB-3-1 cells using siRNA and found that the cells with the decreased level of survivin were more sensitive to doxorubicin, etoposide, and sodium arsenite than were the control KB-3-1 cells (data not shown). Survivin is an attractive therapeutic target in cancer for its differential expression in tumors versus normal tissues, and for its role in maintaining cancer-cell viability.25 A survivin-based therapy would be effective in removing the general cell-viability machinery exploited by cancer cells and it is also expected to have less side effects since survivin is not detected in most normal tissues. Targeting survivin with antisense oligonucleotides,26 ribozymes,28 or expression of dominant-negative mutants30 resulted in caspase-dependent cell death and suppression of tumor growth in vivo. However, it remains difficult to apply these approaches to ATL therapy so far. In this study, we found, for the first time, that trivalent arsenite, sodium arsenite, could down-regulate the expression of survivin at RNA and protein level in ATL cells. Sodium arsenite may provide a new avenue to suppressing survivin, which is an attractive target for treatment of patients with ATL.
Expression of MRP1 and the resistance to sodium arsenite in Jurkat cells. (A) Crude membranes (100 μg protein) were prepared and separated by 7.5% SDS-PAGE and transferred to a PVDF membrane. The transferred proteins were reacted with an antibody against MRP1 as described in “Patients, materials, and methods.” KB/MRP membrane vesicles (10 μg) were used as a positive control. (B) Jurkat and S1T cells were incubated with the indicated concentrations of drugs for 72 hours, and cell viability was determined by the MTT assay. The points represent the means of triplicate determinations, and the bars show SD.
Expression of MRP1 and the resistance to sodium arsenite in Jurkat cells. (A) Crude membranes (100 μg protein) were prepared and separated by 7.5% SDS-PAGE and transferred to a PVDF membrane. The transferred proteins were reacted with an antibody against MRP1 as described in “Patients, materials, and methods.” KB/MRP membrane vesicles (10 μg) were used as a positive control. (B) Jurkat and S1T cells were incubated with the indicated concentrations of drugs for 72 hours, and cell viability was determined by the MTT assay. The points represent the means of triplicate determinations, and the bars show SD.
As2O3 is very effective in the treatment of APL, which carries the t(15;17) translocation involving the RAR-α and PML genes.31 As2O3 could also induce apoptosis in breast cancer, esophageal carcinoma, multiple myeloma, and ATL.32-35 It has recently been shown that As2O3 synergizes with IFN-α to induce cell-cycle arrest and apoptosis in cells infected with HTLV-1, and in the adult T-cell leukemia and lymphoma cells.36 Our study showed that sodium arsenite alone could suppress the growth and induce apoptosis in ATL cells. Sodium arsenite suppressed the growth of ATL cells at low concentrations and induced apoptosis of ATL cells at a relatively high concentration (2 μM), at which the expression of survivin was down-regulated.
Survivin plays an important role in the suppression of mitochondria-dependent apoptosis by either directly or indirectly inhibiting the activity of caspases. Complexes between survivin and caspase-9,37 caspase-3, or caspase-738 have been demonstrated. Survivin has also been shown to be associated with Smac/DIABLO,39 a proapoptotic protein that is released from mitochondria and it prevents the inhibitory effect of IAPs on caspase activation. Moreover, apoptosis induced by dominant-negative survivin mutants have the characteristics of mitochondria-dependent apoptosis with cytochrome c release and caspase-3 activation.40 These findings suggest that survivin protects apoptosis by interacting with Smac/DIABLO and caspase-9. In the present study, caspase-3 was activated by sodium arsenite. It is probable that sodium arsenite induced mitochondria-dependent apoptosis by decreasing the expression level of survivin in ATL cells.
NF-κB is known to be constitutively activated in ATL. The Tax-dependent or Tax-independent activation of the NF-κB pathway is crucial for proliferation, protection from apoptosis, and drug resistance in adult T-cell leukemia and lymphoma. The most common p50-RelA (p65) dimer, “specifically” known as NF-κB, is more abundant and controls the expression of more genes than any other heterodimers or homodimers. NF-κB exists as an inactive cytoplasmic complex, predominantly made up of p50-p65, and bound to IκB-α, an inhibitory protein of the NF-κB. Recently, El-Sabban et al41 showed that IFN-α/As2O3 treatment significantly, and As2O3 alone slightly decreased the expression of the viral transactivator protein Tax and repressed the activation of NF-κB pathway. As2O3 induced apoptosis of HL-60 cells by repressing the constitutive activation of NF-κB.42 Moreover, Tax induced survivin expression through NF-κB pathway.43 p50 and RelB were bound to the NF-κB binding site in the survivin promoter between –354 and –345.43 Tax transactivated the survivin promoter through this NF-κB binding site. Our results showed that the level of p65 and p50 in nuclei was decreased, while the level of cytoplasmic IκB-α was increased by treatment with sodium arsenite in Tax-expressing MT2 cells. This was consistent with the studies of El-Sabban et al41 and Kawakami et al.43 The NF-κB activity in the cells that were not infected with HTLV-1 was also repressed by As2O3.42 In accordance with this report, we found that sodium arsenite down-regulated the expression of survivin through the inhibition of NF-κB pathway in S1T cells that did not expess Tax. Sodium arsenite might suppress the NF-κB activity by repressing the p65 translocation to nuclei, but not through Tax.
Sodium arsenite down-regulated the expression of survivin, but not of Bcl-2. Since there are a number of genes besides survivin and Bcl-2 that are up-regulated by NF-κB and involved in facilitating tumor cell survival,44,45 further study is needed to elucidate whether the down-regulation of these genes is also involved in apoptosis and decreased cell growth of ATL cells caused by sodium arsenite.
In this study, the concentration of sodium arsenite required to inhibit the growth of ATL cells in vitro was more than 0.5 μM and the concentration required to induce apoptosis was more than 2 μM. These concentrations appear to be beneficial and safe for clinical use in patients with ATL. The plasma arsenic rapidly reached a mean maximum serum level of 6.85 μM (range, 5.54-7.30 μM) at 4 hours after intravenous injection of 10 mg As2O3.46 A recent phase 2 trial in 7 patients with a relapsed or refractory ATL has shown that the combination of IFN-α and As2O3 is a hopeful therapy for ATL.47 These preliminary results highlight that the treatment of ATL with As2O3 and IFN-α is feasible and has a clear antileukemic effect, even in patients with refractory disease. Further study is needed to clarify whether the combination of arsenite and IFN-α can down-regulate survivin more effectively, and whether arsenite can enhance the sensitivity of ATL cells to conventional anticancer agents.
T-cell leukemia Jurkat cells, which were used as a control, were more resistant to sodium arsenite than ATL cells. MRP1 is a member of the ATP binding cassette (ABC) superfamily of transport proteins and is involved in arsenite resistance. We have previously demonstrated the clinical significance of MRP1 expression and that high MRP1 expression correlated with short survival in patients with acute-type and lymphoma-type ATL.9 The expression level of MRP1 in Jurkat cells was about 3-fold higher than that in S1T cells, and MK571, a specific inhibitor of MRP1, abolished the difference in sensitivity to sodium arsenite between the 2 cell lines. Since MRP1 transported inorganic arsenic as a tri–glutathione (GSH) conjugate,48 we examined the GSH level in the cells and found that the level of GSH in Jurkat cells was similar to that in S1T cells (data not shown). These findings suggested that MRP1, at least in part, might be responsible for the decreased sensitivity to sodium arsenite of Jurkat cells.
In this study, we proved for the first time that arsenite could down-regulate survivin by repressing NF-κB activation in ATL cells regardless of the Tax expression. Our findings provide a rational basis for the new therapy for ATL using arsenite.
Prepublished online as Blood First Edition Paper, February 23, 2006; DOI 10.1182/blood-2005-08-3423.
Supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology, and grants from the Japan-China Medical Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Etsuko Sudou for excellent technical assistance and Ms Hiromi Kakura for valuable secretarial assistance.