Abstract
The λ interferons (IFN-λs), also known as IL-28 and IL-29, are coexpressed with IFN-β after Toll-like–receptor (TLR) stimulation in human monocyte–derived dendritic cells (DCs). IFN-λ shares with type I IFNs an intracellular signaling pathway that drives the expression of a common set of genes. However, IFN-λ signaling is initiated through a membrane receptor system distinct from that of type I IFNs. Because IFNs produced by DCs in response to TLR stimulation are critical in the differentiation and maturation of DCs, we sought to investigate whether IFN-λ exhibits specific effects on DC differentiation. In this work, we show that DCs acquire IFN-λ responsiveness through the expression of the specific IFN-λ receptor chain during their differentiation from monocytes. IFN-λ–treated DCs express high levels of major histocompatibility complex class I (MHC class I) and MHC class II but low levels of costimulatory molecules. However, they express CCR7 and acquire the ability to migrate to lymph nodes when intravenously injected into SCID/Bg mice. In mixed lymphocyte reaction (MLR) cultures, IFN-λ–treated DCs specifically induced IL-2–dependent proliferation of a CD4+CD25+Foxp3+ T-cell subset with contact-dependent suppressive activity on T-cell proliferation initiated by fully mature DCs. IFN-λs are thus able to generate tolerogenic DCs, an activity that could thwart IFN-β functions.
Introduction
The function of type I interferons (IFNs) in the regulation of the innate immune response is clearly established.1-3 More recently, the importance of the type I IFN family (several α subtypes and one β subtype) in the generation of T cell–mediated adaptive immunity has emerged.4,5 The fact that IFN-β is produced by dendritic cells (DCs) upon Toll-like–receptor (TLR) stimulation and can act as a differentiation and maturation factor on DCs has led to the concept that IFN-β is a cytokine linking innate and adaptive immunity.6 However, type I IFNs are never produced alone: TLR-3 and TLR-4 stimulation of human monocyte–derived DCs, by their respective ligands, also induce the production of IFN-β and IFN-λs.7
The IFN-λ family was recently described.8-10 It has 3 members: λ1, λ2, and λ3, also known as IL-29, IL-28A, and IL-28B, respectively. IFN-λs exhibit several common features with type I IFNs, including the ability to establish an antiviral state in sensitive cell lines, probably because IFN-λs share with type I IFNs an intracellular signaling pathway that drives the expression of a common set of IFN-stimulated genes (ISGs).11,12 However, IFN-λ signaling is initiated through a membrane receptor system distinct from that of type I IFN and composed of a unique IFN-LR1 subunit and the IL-10R2 chain, which is also part of the IL-10, IL-22, and IL-26 receptors.13 The different receptor use of type I IFNs and IFN-λs could give rise to unique signaling pathways in addition to recruitment of Stat1 and Stat2, both of which are activated by type I IFN and IFN-λs through the Tyk2 and Jak1 kinases. Another important difference between type I IFN and IFN-λs is the regulation of their receptor expression. Whereas IFN-AR1 and IFN-AR2, the 2 chains of the type I IFN receptor, and IL-10R2, the second chain of the IFN-λ receptor, are ubiquitously expressed, IFN-LR1 expression is cell-type dependent. Indeed, a limited variety of tissues are responsive to IFN-λ,13,14 and we show here that IFN-LR1, while not expressed in monocytes, is up-regulated during the DC differentiation program initiated in human monocytes by granulocyte macrophage–colony-stimulating factor (GM-CSF) and IL-4. Immature DCs (iDCs) are thus fully responsive to IFN-λ.
DCs play several key functions in immunity. iDCs reside in peripheral tissues, where they capture antigens. Signals such as TLR stimulation or type I IFNs, acting in a paracrine or an autocrine manner, induce DCs to mature in that they acquire the ability to migrate to lymphoid organs and to present antigens to naive T cells through major histocompatibility complex (MHC) classes I and II. Depending on their maturation state, which correlates with MHC level and costimulatory molecule expression, DCs can drive the expansion and proliferation of effector or regulatory T cells.15,16
Experiments presented in this paper were undertaken to define the role of IFN-λ in the maturation of human monocyte–derived DCs. We show that IFN-λ promotes the generation of partially mature DCs, which display a tolerogenic phenotype: IFN-λ–matured DCs express high levels of MHC classes I and II but low levels of costimulatory molecules, and they acquire the ability to migrate to lymph nodes when injected intravenously into immunodeficient mice. In allogeneic cultures, IFN-λ–matured DCs specifically induce IL-2–dependent proliferation of a CD4+CD25+Foxp3+ T-cell population with contact-dependent suppressive activity on T-cell proliferation. Thus, in contrast to type I IFNs that render iDCs potent inducers of TH1 cells,3,5,6 IFN-λ drives iDCs into a maturation stage that promotes regulatory T-cell expansion.
Materials and methods
Cell cultures and purification
Primary cells were isolated from fresh buffy coat derived from the blood of healthy donors (Établissement Français du Sang, Montpellier, France). Buffy coat preparations were fractionated over Ficoll-Histopaque 1077 (Sigma-Aldrich, St Louis, MO). CD14+ monocytes and CD25+ T cells were positively selected with a magnetically activated cell sorter (MACS) system (Miltenyi Biotec, Auburn, CA). Depending on the experiments, peripheral blood DCs or CD3+ or CD4+ T lymphocytes were negatively selected using the human blood dendritic cell isolation kit II, Pan T, or CD4+ T-cell isolation kits from Miltenyi Biotec. To derive DCs, CD14+ monocytes were cultured at 1 × 106 cells/mL in RPMI 1640, 100 g/mL penicillin, 100 g/mL streptomycin (Invitrogen, Carlsbad, CA), 10% FCS, and 1% nonessential amino acids (Sigma-Aldrich) (DC medium) supplemented with 50 ng/mL GM-CSF and 20 ng/mL IL-4 (PeproTech, Rocky Hill, NJ). Macrophages were obtained by culturing monocytes at 1 × 106 cells/mL in DC medium, supplemented with 50 ng/mL GM-CSF. Cells were incubated between 6 and 7 days at 37°C, 5% CO2. Medium was replaced every 2 or 3 days. To mature DCs, cells were treated for 24 hours with either 200 pM human IFN-β (from Dr Laura Runkel, Biogen, Cambridge, MA) or 50 ng/mL lipopolysaccharide (LPS) (Escherichia coli 0127:B8; Sigma-Aldrich). IFN-λ1 (IL-29) and IFN-λ2 (IL-28A) were from PeproTech. DCs were treated with IFN-λ for 24 hours. All experiments reported here were performed with 3 nM IFN-λ1. This concentration represents a large excess because MHC class I and class II up-regulation on DCs attained maximum level after treatment with 100 pM IFN-λ1, as shown in Figure S1 (available on the Blood website; see the Supplemental Figure link at the top of the online article). In addition, DCs treated with 100 pM IFN-λ1 behaved similarly to DCs treated with 3 nM IFN-λ1 in allogeneic reaction for their capacity to stimulate IL-2–dependent CD4+ T-cell proliferation (Figure S1). IFN-λ2 was found to be qualitatively equivalent to IFN-λ1.
FACS analysis
Purity of freshly purified blood monocytes, DCs, CD4+ T cells, and CD25+ T cells was evaluated by fluorescence-activated cell sorter (FACS) analysis with human anti-CD14 fluorescein isothiocyanate (FITC)–conjugated antibodies (M5E2), anti-CD11c allophycocyanin (APC)–conjugated (B-Ly6), anti-CD4 FITC-conjugated antibodies (RPA-T4; BD Pharmingen, San Diego, CA), and anti-CD25 PE-conjugated antibodies (Miltenyi Biotec), respectively. Cell purity was always 90% or greater for all positive and negative selections. The following commercial antibodies were used according to the manufacturer's instructions to access DC phenotype: anti–HLA-A, anti–HLA-B, anti–HLA-C FITC-conjugated (G46-2.6), anti-CD40 (5C3), anti-CD80 (L307.4), anti-CD83 (HB15e), anti-CD86 (2331 FUN-1), HLA-DR (G86-6), and CCR7 (3D12) PE-conjugated antibodies (BD Pharmingen). For antigen uptake assay in vitro, iDCs were left untreated or were treated with IFN-β, IFN-λ, or LPS for 24 hours. Cells were incubated for 30 minutes with 1 mg/mL FITC-dextran particles at either 37°C or 4°C. Dextran uptake was accessed by flow cytometry. Fluorescence acquisition was performed on FACSCalibur, and data analyses were performed with the use of CellQuest Pro software (BD Biosciences, San Jose, CA).
Lymphoproliferation
In mixed lymphocyte reaction (MLR) experiments, T-cell proliferation was determined by [3H]-thymidine incorporation. Briefly, 1 × 105 negatively sorted CD3+ T lymphocytes or CD4+ T cells from 2 healthy donors were cocultured with allogeneic irradiated DCs (15 Gy) at various DC/T ratios (from 1:10 to 1:500). Cells were grown for 7 to 9 days at 37°C in 96 U-well trays in DC medium. [3H]-Thymidine (1 μCi [0.037 MBq]/well; Amersham Pharmacia, Piscataway, NJ) was added for the last 18 hours of culture. Incorporated radioactivity in cells was counted using a liquid scintillation counter system (Packard TopCount, Meriden, CT). All proliferation assays were performed at least in quadruplicate. To access the regulatory effect of the CD4+CD25+ T lymphocytes, cells were positively selected from MLR cultures using anti-CD25 microbeads and were added in various numbers to control MLR reactions. Coculture experiments with filters were performed in 96-well plates with 0.2-μm Anopore filter membranes (Nunc, Naperville, IL). For MLR experiments using neutralizing anti–IL-2 antibodies, cells were cultured for 9 days at a 1:10 DC/T initial ratio in 96-well plates, as described, or in 24-well plates at 2 × 106 cells/mL for real-time reverse transcription–polymerase chain reaction (RT-PCR) analysis. Cells were cultured in DC medium in the presence of anti–IL-2 antibodies or normal rabbit control IgG (references 500-P22 and 500-P00; PeproTech) at 250 ng/mL final concentration.
IL-12 assays
iDCs were treated with IFNs, LPS, or medium for 48 hours or were used as stimulating cells in MLR culture after 24 hours of maturation for 8 days at a 1:10 DC/T-cell ratio. Human IL-12p70 release in culture supernatants was determined by ELISA following the manufacturer's instructions (OptEIA Kit; Becton Dickinson, San Jose, CA).
Real-time RT-PCR
Real-time RT-PCR using Light Cycler (Roche, Indianapolis, IN) for glyceraldehyde phosphate dehydrogenase (GAPDH) and 2′5′ oligo adenylate synthetase was performed as previously described.17 Sequences of primers used for Foxp3 and IFN-LR1 amplifications were as follows: Foxp3 forward, 5′CCAGGACAGGCCACATTCA 3′; Foxp3 reverse, 5′ CACTGGGATTTGGGAAGGTG 3′; IFN-LR1 forward, 5′ CCGGAAACAAGACCCTATT 3′; IFN-LR1 reverse, 5′ CAGGTGGGCTTAGAGAACTT 3′. The specificity of the primer pairs was validated by DNA sequencing of the PCR products All data are expressed as a ratio to the GAPDH level. Standard errors of the ratios were calculated using the Student t test (95% confidence limits).
In vivo migratory assays
iDCs were treated with IFNs, LPS, or medium for 24 hours, washed extensively, and injected intravenously (3 × 106 cells/mouse) into SCID/Bg immunodeficient mice. PBS injections were used as negative controls. Recipient mice were killed 8 hours or 24 hours after adoptive transfer, and organs were collected under sterile conditions. DNA extractions were performed using DNeasy Tissue KitAll (Qiagen, Valencia, CA). The presence of human alu repetitive sequence was analyzed by PCR on 7 ng DNA using alu Sp primer set: forward, 5′ACCCCGTCTCTACTAAAAAT 3′; reverse, 5′ AGTTTCGCTCTTGTTGC 3′. Amplifications were performed for 30 cycles with an annealing temperature of 60°C using Platinum Taq DNA Polymerase (Invitrogen) and were analyzed on an ethidium bromide–containing 2% agarose gel. Real-time PCR on the Light Cycler (Roche) was performed using the alu Sb8 primer set, as described.18 Data were standardized against the amount of murine DNA as quantified using a primer set detecting the intronless murine IFN-α4 gene: forward, 5′ CCTGTGTGATGCAGGAACC 3′; reverse, 5′ ATGAGTGGTGGTTGCAGGC 3′. Standard errors of the ratios were calculated using Student t test (95% confidence limits).
Results
Induction of IFN-LR1 expression and IFN-λ responsiveness during DC differentiation
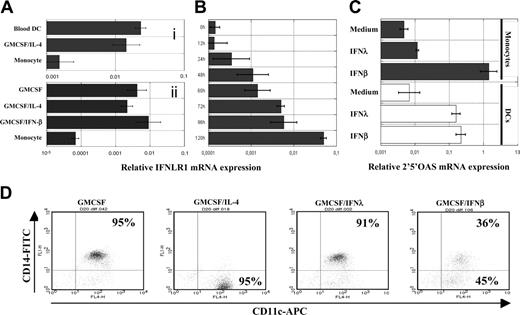
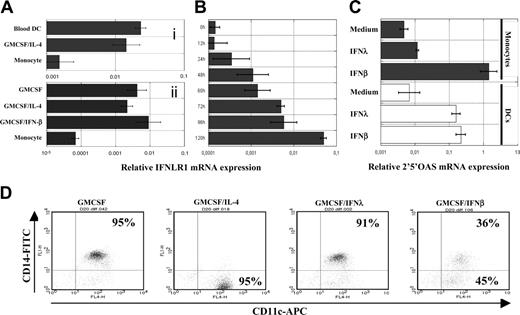
Unlike the ubiquitously expressed type I IFN receptor, expression of the IFN-λ receptor is cell-type specific, and it is thought that IFN-LR1 is not expressed in leukocytes.13 Accordingly, the transcript encoding IFN-LR1 was hardly detectable by real-time RT-PCR in freshly isolated human CD14+ monocytes. However, IFN-LR1 mRNA was detectable in circulating blood DCs and in DCs generated in vitro from monocytes treated with GM-CSF and IL-4 (Figure 1Ai). IFN-LR1 mRNA was also expressed by DCs and macrophages generated by culturing monocytes in the presence of GM-CSF and IFN-β or GM-CSF alone, respectively (Figure 1Aii). The IFN-LR1 mRNA level was gradually increased during DC differentiation, reaching its maximum level when monocytes were close to their full state of DC differentiation (Figure 1B). IL-10R2, the second chain of the IFN-λ receptor, was constitutively expressed by monocytes and DCs at any maturation states (data not shown). DCs, but not monocytes, were consequently responsive to IFN-λ, as shown in Figure 1C for the induction of the 2′5′ oligo adenylate synthetase gene, a classic ISG whose expression is a signature of type I IFN and IFN-λ early response. Monocytes were resistant to IFN-λ; hence, we observed that IFN-λ could not substitute IL-4, in contrast to IFN-β, which could drive the differentiation of monocytes to DCs (Figure 1D).5
Acquisition of IFN-λ responsiveness by monocytes during DC differentiation. (A) Quantification of IFN-LR1 mRNA expression in monocytes, monocyte-derived DCs, and freshly purified blood DCs (i) or in IFN-β DCs and macrophages (ii). Top and bottom panels represent experiments on 2 different blood donors. (B) Kinetics of IFN-LR1 mRNA expression by monocytes during their differentiation into DCs in the presence of GM-CSF and IL-4. (C) Quantification of 2′5′ oligo adenylate synthetase mRNA expression by monocytes and DCs from the same blood donors after 4 hours of IFN-β or IFN-λ treatment. Transcript levels were quantified by real time RT-PCR relative to GAPDH. (D) Monocytes were cultured for 4 days in the presence of GM-CSF alone or were supplemented with IFN-β, IFN-λ, or IL-4. Expression of the surface markers CD11c and CD14 were accessed by flow cytometry. For all experiments, similar results were obtained with cells from 2 to 3 blood donors.
Acquisition of IFN-λ responsiveness by monocytes during DC differentiation. (A) Quantification of IFN-LR1 mRNA expression in monocytes, monocyte-derived DCs, and freshly purified blood DCs (i) or in IFN-β DCs and macrophages (ii). Top and bottom panels represent experiments on 2 different blood donors. (B) Kinetics of IFN-LR1 mRNA expression by monocytes during their differentiation into DCs in the presence of GM-CSF and IL-4. (C) Quantification of 2′5′ oligo adenylate synthetase mRNA expression by monocytes and DCs from the same blood donors after 4 hours of IFN-β or IFN-λ treatment. Transcript levels were quantified by real time RT-PCR relative to GAPDH. (D) Monocytes were cultured for 4 days in the presence of GM-CSF alone or were supplemented with IFN-β, IFN-λ, or IL-4. Expression of the surface markers CD11c and CD14 were accessed by flow cytometry. For all experiments, similar results were obtained with cells from 2 to 3 blood donors.
IFN-λ–treated DCs efficiently migrate into lymphoid compartments in vivo
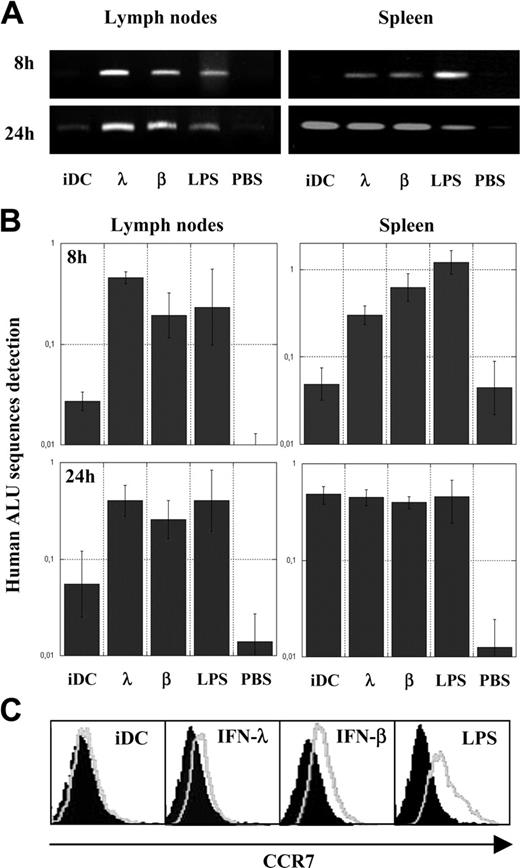
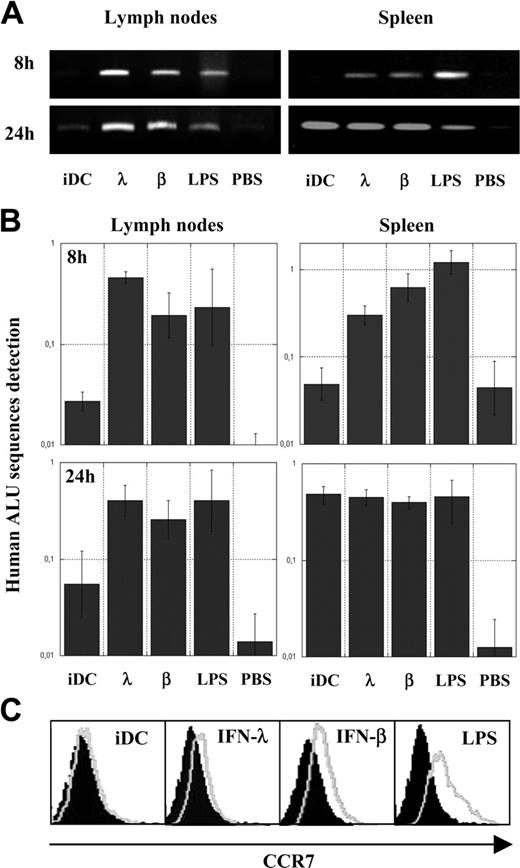
We evaluated the activity of IFN-λ on DC maturation. Because the most important DC maturation trait is the ability to migrate to T-cell areas of strategic lymphoid compartments, such as lymph nodes and spleen, to prime specific immune responses, we first performed in vivo migratory assays in SCID/Bg immunodeficient mice. Human iDCs were treated for 24 hours with IFN-λ or with conventional maturing agents, such as IFN-β or LPS. SCID/Bg mice were then given intravenous injections of IFN-λ–treated DCs, IFN-β, or LPS-matured DCs as positive controls or with iDCs and PBS as negative controls. Eight hours and 24 hours after injection, mice were killed and DNA from spleen or pooled mesenteric, inguinal, brachial, axillary, and superficial cervical lymph nodes were purified. To assess the presence of human DCs in these murine organs, the presence of human DNA was detected by real-time PCR or classic PCR using, respectively, alu Sp– and alu Yb8–specific primer sets, both amplifying human alu–repeated sequences. Figure 2A-B shows that 8 hours after their adoptive transfer, IFN-λ–treated DCs and IFN-β or LPS-matured DCs were present in lymph nodes and spleens, whereas human DNA was not detectable or was barely detectable in mice given injections of PBS or iDCs. Twenty-four hours after adoptive transfer, iDCs were detected at low levels in lymph nodes compared with IFN-λ, IFN-β, and LPS-treated cells, confirming the lower migration abilities of iDCs. Nevertheless, all DC types were present at similar levels in spleens at that time point. These results indicate that IFN-λ, as the other conventional maturing agent, enables DCs to reach lymphoid organs.
Acquisition of in vivo migratory abilities and CCR7 expression by IFN-λ–treated DCs. Control PBS, untreated iDCs, or iDCs treated for 24 hours with IFN-β, IFN-λ, or LPS were intravenously injected into SCID/Bg mice; DNA from lymph nodes and spleens were extracted after 8 or 24 hours. Human alu repetitive sequences were analyzed qualitatively by PCR (A) and quantitatively by real-time PCR (B) as a ratio with the quantification of a murine gene (see “Materials and methods”). Results are the means (95% confidence limits) of triplicate experiments performed on a single mouse for each condition tested. (C) Analysis of chemokine receptor CCR7 expression by flow cytometry. iDCs were treated for 24 hours with IFNs or LPS (gray lines); isotopic controls are indicated by filled surfaces. Results presented are from a single blood donor and are representative of results obtained with cells from 7 blood donors.
Acquisition of in vivo migratory abilities and CCR7 expression by IFN-λ–treated DCs. Control PBS, untreated iDCs, or iDCs treated for 24 hours with IFN-β, IFN-λ, or LPS were intravenously injected into SCID/Bg mice; DNA from lymph nodes and spleens were extracted after 8 or 24 hours. Human alu repetitive sequences were analyzed qualitatively by PCR (A) and quantitatively by real-time PCR (B) as a ratio with the quantification of a murine gene (see “Materials and methods”). Results are the means (95% confidence limits) of triplicate experiments performed on a single mouse for each condition tested. (C) Analysis of chemokine receptor CCR7 expression by flow cytometry. iDCs were treated for 24 hours with IFNs or LPS (gray lines); isotopic controls are indicated by filled surfaces. Results presented are from a single blood donor and are representative of results obtained with cells from 7 blood donors.
Given that the chemokine receptor CCR7 is a well-characterized cell-surface marker associated with the acquisition of migrating abilities of DCs,19 we use FACS to compare the expression of CCR7 in iDC and in IFN-λ, IFN-β, or LPS-treated DCs. Figure 2C shows that IFN-λ, as well as IFN-β and LPS, did indeed promote substantial CCR7 surface expression and that induction by IFN-λ was lower than that by IFN-β or LPS. These results were reproducible using cells from 7 blood donors. Compared with iDCs, CCR7 expression in IFN-λ–treated DCs was significantly increased because 2-tailed P value was less than .02 for the nonparametric Wilcoxon-matched pairs test and the parametric paired t test.
IFN-λ–treated DCs display a partially matured phenotype
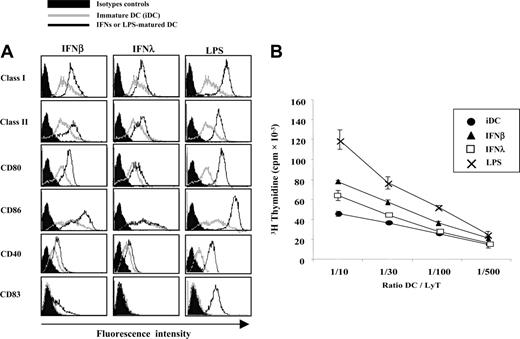
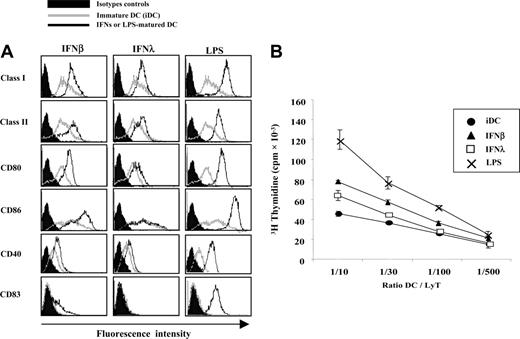
Cell-surface expression levels of MHC class I, MHC class II, and some costimulatory molecules were evaluated by FACS in IFN-λ–treated DCs and compared on the one hand with iDCs and on the other hand with LPS or IFN-β–matured DCs (Figure 3A). As expected, IFN-β– and LPS-matured DCs showed increased expression of MHC class I, MHC class II, CD80, CD86, and CD40. As reported elsewere,5 IFN-β did not induce full expression of the maturation factor CD83. MHC class I and class II were clearly up-regulated by IFN-λ. However, CD80 and CD40 were only weakly up-regulated. Nonparametric statistical analysis, performed on DCs from the 18 blood donors tested, showed a nonsignificant variation for these markers. CD86 and CD83 expression was unchanged by IFN-λ treatment.
We then tested the ability of the IFN-λ–treated DCs to induce T-cell proliferation in MLR culture. iDCs and DCs treated with IFN-λ, IFN-β, or LPS were extensively washed and incubated with allogeneic T lymphocytes for 7 to 9 days. Figure 3B shows that the ability of IFN-λ–treated DCs to induce T-lymphocyte proliferation was lower than it was for LPS or IFN-β-matured DCs.
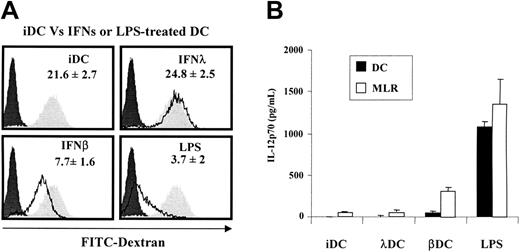
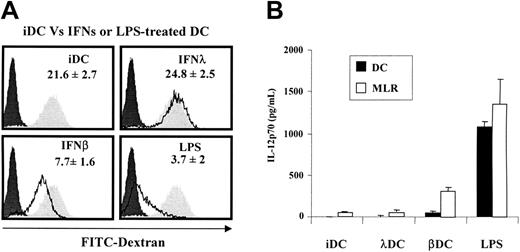
Full maturation of DCs is associated with loss of their ability to take up antigens. Using an FITC-labeled dextran uptake assay, we could show that in this respect IFN-λ–treated DCs were immature because they retained their phagocytic ability (Figure 4A). Together, these data indicate that IFN-λ induces only a subset of DC maturation markers, which include CCR7 expression, migratory ability, and MHC class I and class II cell-surface expression. For all other maturation markers tested, IFN-λ–treated DCs remained mainly immature. Accordingly, IFN-λ–treated DCs poorly induced T-cell proliferation in allogeneic reaction. They have a partially matured phenotype reminiscent of the tolerogenic DC phenotype.15,16
Phenotype and T-cell priming abilities of IFN-λ–treated DCs. iDCs were left untreated or were treated with IFN-β, IFN-λ, or LPS for 24 hours. (A) Surface expression of MHC class I, MHC class II, CD80, CD86, CD40, and CD83 were accessed by flow cytometry. IFN- or LPS-treated cells (black lines) were compared with iDCs (gray lines) and isotopic control conditions (filled areas). Histograms are representative of results obtained with cells from 18 blood donors. (B) DCs were tested for their ability to stimulate allogeneic CD4+ T-cell proliferation in vitro. MLR culture assays were performed at least in quadruplicate with various DC/T ratios. Similar results were obtained in 6 independent experiments.
Phenotype and T-cell priming abilities of IFN-λ–treated DCs. iDCs were left untreated or were treated with IFN-β, IFN-λ, or LPS for 24 hours. (A) Surface expression of MHC class I, MHC class II, CD80, CD86, CD40, and CD83 were accessed by flow cytometry. IFN- or LPS-treated cells (black lines) were compared with iDCs (gray lines) and isotopic control conditions (filled areas). Histograms are representative of results obtained with cells from 18 blood donors. (B) DCs were tested for their ability to stimulate allogeneic CD4+ T-cell proliferation in vitro. MLR culture assays were performed at least in quadruplicate with various DC/T ratios. Similar results were obtained in 6 independent experiments.
IFN-λ–treated DCs keep their antigen uptake abilities and do not release IL-12p70 in vitro. (A) iDCs were left untreated or were treated with IFN-β, IFN-λ, or LPS for 24 hours. Cells were incubated for 30 minutes with 1 mg/mL FITC-dextran particles at 37°C or at 4°C. DC uptake ability was accessed by flow cytometry. Dextran uptake at 37°C by IFNs or LPS-treated DCs (black line) were compared with iDCs (filled gray areas) and 4°C control conditions (filled black areas). Experiments were performed in duplicate and are representative of mean results obtained with cells from 5 blood donors. (B). The release of IL-12p70 by DCs in culture supernatant was determined after 48 hours of maturation by IFNs, LPS, or medium (▪) or after 8 days of MLR culture at a 1:10 DC/T ratio (□). Results are the mean ± SEM of 3 to 4 independent experiments performed on different blood donors.
IFN-λ–treated DCs keep their antigen uptake abilities and do not release IL-12p70 in vitro. (A) iDCs were left untreated or were treated with IFN-β, IFN-λ, or LPS for 24 hours. Cells were incubated for 30 minutes with 1 mg/mL FITC-dextran particles at 37°C or at 4°C. DC uptake ability was accessed by flow cytometry. Dextran uptake at 37°C by IFNs or LPS-treated DCs (black line) were compared with iDCs (filled gray areas) and 4°C control conditions (filled black areas). Experiments were performed in duplicate and are representative of mean results obtained with cells from 5 blood donors. (B). The release of IL-12p70 by DCs in culture supernatant was determined after 48 hours of maturation by IFNs, LPS, or medium (▪) or after 8 days of MLR culture at a 1:10 DC/T ratio (□). Results are the mean ± SEM of 3 to 4 independent experiments performed on different blood donors.
A sine qua non condition for DC tolerogenic activity is the low production, or the absence of production, of IL-12.20 Accordingly, in the absence of a secondary signal such as CD40 ligation or TLR stimulation, IL-12p70 was barely detectable in IFN-λ–treated DC culture medium, whereas it was massively released by LPS-matured DCs and at a lower level by IFN-β–treated cells. In allogeneic reaction, IL-12p70 was released at a high level by LPS-matured DCs and IFN-β–treated cells, but it was maintained at a low level in culture supernatant of MLR stimulated by iDCs and IFN-λ–treated DCs (Figure 4B).
IFN-λ–treated DCs specifically induce the proliferation of CD4+CD25+ T cells
We analyzed by flow cytometry the phenotype of the T-cell population present at days 7 to 9 after MLR performed with IFN-λ–treated DCs. CD4+ T lymphocytes were assessed for expression of the regulatory marker CD25. Representative analysis is shown in Figure 5A. Among the CD4+ T-cell population, cells were found in 3 subsets—CD25–, CD25+lo, and CD25+hi. IFN-λ–treated DCs generated a high proportion of CD25+hi T lymphocytes, whereas IFN-β–treated cells induced a low level of CD25+hi T cells. LPS-treated cells induced mostly the generation of CD25+lo cells and a low proportion of CD25+hi cells. Proportions of CD25+hi T cells among the CD4+ population present in the MLR cultures from 3 independent experiments are summarized in Figure 5B. It is remarkable that IFN-λ–treated DCs induced the highest proportion of CD4+ CD25+hi T cells in MLR cultures.
To establish whether IFN-λ–treated DCs could specifically induce the proliferation of the CD4+CD25+ T-cell subpopulation, freshly purified CD4+ T cells were separated for their expression of the surface marker CD25 (Figure 5C). IFN-treated DCs were cultured with CD4+CD25+ or with CD25– T cells in MLR. Of note, the CD25+ population used for this experiment was a pool of all CD25-expressing CD4+ T cells positively selected, including high- and low-expressing cells. We found that IFN-λ–treated DCs and iDCs preferentially induced CD4+CD25+ cell proliferation, whereas LPS-treated DCs and IFN-β–treated DCs were the most potent inducers of the CD25– subtype proliferation (Figure 5D). Among all conditions tested in MLR cultures, LPS-matured DCs were the highest inducers of CD25–CD4+ T-cell proliferation, whereas IFN-λ–treated DCs were the most efficient stimulating cells for CD25+CD4+ T-cell proliferation. After 7 days of MLR culture, the responding CD25– T-cell population was still negative for the expression of the CD25, and the CD25+ population showed similar or greater expression of this marker (data not shown).
Profile of responding T lymphocytes primed by stimulating DCs in allogeneic reactions. iDCs or IFN-β, IFN-λ, or LPS-treated DCs were used for 24 hours to stimulate allogeneic T-cell proliferation in vitro at a 1:10 DC/T-cell ratio. [3H]-Thymidine was added for the last 18 hours of culture. (A) CD4+ T lymphocytes present in MLR reactions were analyzed for their expression level of CD25 by flow cytometry. (B) Percentage of CD4+ CD25+hi T cells in MLR cultures. Mean ± SEM of 3 experiments performed with different blood donors. (C) Purity analysis of CD4+ T cells separated for the expression of CD25 by immunomagnetic selection into CD4+CD25– (i) and CD4+CD25+ T cells (ii). (D) Proliferation of CD4+CD25+ or CD25– T lymphocytes primed by DCs. Mean ± SEM of 3 independent experiments performed on different blood donors.
Profile of responding T lymphocytes primed by stimulating DCs in allogeneic reactions. iDCs or IFN-β, IFN-λ, or LPS-treated DCs were used for 24 hours to stimulate allogeneic T-cell proliferation in vitro at a 1:10 DC/T-cell ratio. [3H]-Thymidine was added for the last 18 hours of culture. (A) CD4+ T lymphocytes present in MLR reactions were analyzed for their expression level of CD25 by flow cytometry. (B) Percentage of CD4+ CD25+hi T cells in MLR cultures. Mean ± SEM of 3 experiments performed with different blood donors. (C) Purity analysis of CD4+ T cells separated for the expression of CD25 by immunomagnetic selection into CD4+CD25– (i) and CD4+CD25+ T cells (ii). (D) Proliferation of CD4+CD25+ or CD25– T lymphocytes primed by DCs. Mean ± SEM of 3 independent experiments performed on different blood donors.
Regulatory ability and Foxp3 expression of CD4+CD25+ T cells generated in vitro by IFN-λ–treated DCs. (A) CD4+CD25+ T cells generated in vitro by IFN-λ–treated DCs were purified from MLR culture reaction and assayed for their suppressive activity on CD4+ T-cell proliferation in MLR cultures. CD4+ responding T cells were stimulated with LPS-treated DCs at a 1:10 DC/T ratio. Cellular [3H]-thymidine incorporation found for this control MLR is indicated by the dotted line. Various numbers of CD4+CD25+ T cells were directly added either to the MLR culture reaction (□) or to permeable filters to avoid cell–cell contact interactions (▨). Data are the mean ± SD of 2 independent experiments performed in 6 replicates with cells from different blood donors. (B) Quantification of Foxp3 mRNA expression by real-time PCR in CD4+ T cells purified from MLR cultures mixing the indicated DCs and CD4+ T cells. For comparison, Foxp3 levels were quantified in freshly purified CD25+ or CD25–CD4+ T cells derived from a same donor. Results are expressed relative to GAPDH level. The experiment was performed twice on 2 blood donors with similar results.
Regulatory ability and Foxp3 expression of CD4+CD25+ T cells generated in vitro by IFN-λ–treated DCs. (A) CD4+CD25+ T cells generated in vitro by IFN-λ–treated DCs were purified from MLR culture reaction and assayed for their suppressive activity on CD4+ T-cell proliferation in MLR cultures. CD4+ responding T cells were stimulated with LPS-treated DCs at a 1:10 DC/T ratio. Cellular [3H]-thymidine incorporation found for this control MLR is indicated by the dotted line. Various numbers of CD4+CD25+ T cells were directly added either to the MLR culture reaction (□) or to permeable filters to avoid cell–cell contact interactions (▨). Data are the mean ± SD of 2 independent experiments performed in 6 replicates with cells from different blood donors. (B) Quantification of Foxp3 mRNA expression by real-time PCR in CD4+ T cells purified from MLR cultures mixing the indicated DCs and CD4+ T cells. For comparison, Foxp3 levels were quantified in freshly purified CD25+ or CD25–CD4+ T cells derived from a same donor. Results are expressed relative to GAPDH level. The experiment was performed twice on 2 blood donors with similar results.
CD4+CD25+ T cells generated by IFN-λ–treated DCs are naturally occurring regulatory T cells
If the CD4+CD25+ T cells generated in MLR primed with IFN-λ–treated DCs are representative of the naturally occurring regulatory T cells, they must match 2 criteria: (1) cell contact–dependent suppressive activity on T-cell proliferation induced by fully mature DCs and (2) expression of Foxp3, a transcription factor identified as a master switch in CD4+CD25+ Treg cell differentiation and function.21
CD4+CD25+ T cells generated by IFN-λ–treated DCs were selectively purified and added to an MLR culture performed with responding allogeneic CD4+ T cells and stimulating LPS-matured DCs at a 1:10 DC/T ratio. Potential regulatory CD4+CD25+ T cells were added at various numbers to the culture reaction. Strikingly, the CD4+CD25+ T cells generated by IFN-λ–treated DCs inhibited T-cell proliferation in MLR culture stimulated by fully mature allogeneic DCs (Figure 6A, white bars). T-cell proliferation was inhibited by up to 60% when the CD4+CD25+ T cells were added at a 1:2 ratio to responding T cells. We observed approximately 30% of inhibition at a 1:5 ratio and approximately 10% of inhibition at a 1:20 ratio compared with the control MLR reaction. In contrast, by using a permeable filter system that allowed secreted molecules to diffuse within the culture reaction but prevented direct contact between effector cells, the inhibition process was clearly blocked (Figure 6A, hatched bars). These results show that in the absence of cell–cell interactions, CD4+CD25+ T cells generated by IFN-λ–treated DCs were unable to exert their suppressive activity.
Tolerogenic functions of IFN-λ–treated DCs are IL-2 dependent. iDCs or IFN-β, IFN-λ, or LPS-treated DCs were used for stimulating allogeneic CD4+ T-cell proliferation in vitro at a 1:10 DC/T-cell ratio. Neutralizing anti–IL-2 or control IgG was added to the cultures as indicated. (A) CD4+ T-cell proliferation was determined by [3H]-thymidine incorporation after 8 days of culture under stimulating iDC or λDC conditions. (B) Quantification of Foxp3 mRNA expression by real-time PCR in MLR cultures of DCs and CD4+ T cells as indicated. Experiments were performed on a pool of T cells from 3 blood donors. Results are expressed relative to GAPDH level.
Tolerogenic functions of IFN-λ–treated DCs are IL-2 dependent. iDCs or IFN-β, IFN-λ, or LPS-treated DCs were used for stimulating allogeneic CD4+ T-cell proliferation in vitro at a 1:10 DC/T-cell ratio. Neutralizing anti–IL-2 or control IgG was added to the cultures as indicated. (A) CD4+ T-cell proliferation was determined by [3H]-thymidine incorporation after 8 days of culture under stimulating iDC or λDC conditions. (B) Quantification of Foxp3 mRNA expression by real-time PCR in MLR cultures of DCs and CD4+ T cells as indicated. Experiments were performed on a pool of T cells from 3 blood donors. Results are expressed relative to GAPDH level.
Analysis of Foxp3 expression was performed by real-time RT-PCR on RNA extracted from CD4+ T cells purified from MLR cultures with different DCs and compared with the levels of Foxp3 in CD4+CD25+ or CD4+CD25– T cells freshly isolated from blood (Figure 6B). A 10- to 30-fold higher Foxp3 expression was found in CD4+ T cells primed with IFN-λ–treated DCs than in cells stimulated with IFN-β–treated DCs. Interestingly, the levels of Foxp3 expression found in CD4+ T cells primed with IFN-λ– and IFN-β–treated DCs were comparable to the Foxp3 levels found in freshly isolated CD4+CD25+ and CD4+CD25 – T cells, respectively. IFN-λ–treated DCs were inefficient at inducing Foxp3 expression in CD4+CD25– T cells.
IL-2 is required for the generation of CD4+CD25+ T cells by IFN-λ–treated DCs
IL-2 was recently described in mice as a component essential to initiate in vivo the survival and expansion of Foxp3 CD25+ peripheral T cells.22-24 Based on this finding, we evaluated the role of IL-2 in the induction of CD4+CD25+ T-cell proliferation by IFN-λ–treated DCs in vitro. Neutralizing anti–IL-2 antibodies added to cultures clearly blocked the ability of IFN-λ–treated DCs to induce T-cell proliferation in allogeneic reaction (Figure 7A). Accordingly, Foxp3 mRNA expression in MLR cultures stimulated by IFN-λ–treated DCs was dramatically decreased in the presence of neutralizing anti–IL-2 (Figure 7B).
Taken together, these data indicate that IFN-λ–treated DCs are capable, through a mechanism that involves IL-2, to specifically stimulate the proliferation of a regulatory T-cell population that meets all the criteria of naturally arising regulatory T cells.
Discussion
Little is known of the biologic role of the IFN-λ family. Since their first description in 2003, several reports have pointed to functional similarities between IFN-λ and type I IFNs, including their signaling through the Jak/Stat signaling pathway.9-11,14 In this study, we show that IFN-β and IFN-λ exert radically different activities on monocyte-derived DC maturation. IFN-β and IFN-α have 2 main functions in DC biology. They can promote DC differentiation from precursor cells and act as efficient maturing agents.6 In vivo, DCs that are differentiated or matured in the presence of type I IFNs enhance the antibody response and promote switching to all subclasses of IgG.25 In contrast, this report shows that IFN-λ has no effect on monocytes because these DC precursors do not express IFN-LR1, an essential component of the IFN-λ receptor. IFN-LR1 is, however, expressed by DCs that thus become responsive to IFN-λ. In contrast to type I IFNs, IFN-λ instructs iDCs toward a developmental program associated with the capacity to trigger the proliferation of a Foxp3-expressing CD4+CD25+ T-cell subset. These cells, known as naturally occurring regulatory T cells, have the ability to suppress T-cell proliferation induced by fully mature DCs in a cell–cell contact manner. Naturally occurring regulatory T cells were shown to be critical in normal immunohomeostasis for maintaining self-tolerance and for negatively regulating immune responses.21,26
Several studies have demonstrated that, depending on their maturation state, DCs play a key role in the induction of immune responses or in the induction and maintenance of immune tolerance. For instance, exposure of naive CD4+ T cells to allogeneic iDCs generates Tr1 cells.27 Tr1 cells are Foxp3– regulatory T cells that suppress T-cell responses in an MHC-restricted manner and through an IL-10– and a TGF-β–dependent mechanism. On the other hand, Foxp3+ naturally occurring regulatory T cells, while requiring TCR stimulation for their activation, exert their suppressive activities toward a large population of bystander cells in a non–MHC-restricted manner.28 Both in vitro and in vivo, Foxp3+CD4+CD25+ Treg cells are found in a state of anergy. In mice, mature DCs expressing high levels of CD86 were shown to be capable of breaking this anergy and triggering the proliferation of Foxp3+CD4+CD25+ cells, suggesting that the expansion of these Treg cells is a natural consequence of immune responses.29,30 We report here that IFN-λ–treated DCs were the best inducers of CD4+CD25+ T-cell proliferation, whereas LPS or IFN-β–matured DCs preferentially induced the proliferation of the CD25– subset (Figure 5). Recent work in murine models demonstrated that the cytokine IL-2 is required for the expansion of Foxp3+ regulatory T cells in vivo.22-24 Our data clearly show that IL-2 is also required for the generation of Foxp3+CD4+CD25+ T cells in our in vitro human model.
Naturally occurring regulatory T cells are considered a separate lineage that develops in the thymus.21 However, in particular circumstances, CD25–CD4+ T cells can be converted to CD25+Foxp3+ T cells.31,32 Our data indicate that IFN-λ–treated DCs stimulate the proliferation of pre-existing CD25+Foxp3+ T cells rather than drive their generation de novo because Foxp3 expression was not increased in allogeneic cultures in which CD25– T cells and IFN-λ–treated DCs were mixed (Figure 6B). These observations suggest that IFN-λ–treated DCs amplify a regulatory T-cell population from a pool of CD4+ T cells that express Foxp3 and CD25.
Recently, the study of a mouse in which a fluorescent reporter was knocked into the Foxp3 locus has allowed us to localize Foxp3-expressing cells into lymphoid organs, mainly in peripheral lymph nodes and in the bone marrow.33 It is thus essential for stimulating DCs to come into the proximity of T cells in lymphoid organs. Our work provides evidence that IFN-λ–treated DCs up-regulate CCR7 and migrate more efficiently than iDCs to the lymph nodes upon intravenous injection into SCID/Bg mice (Figure 2). Thus, it is likely that the unique ability of IFN-λ–treated DCs to initiate the proliferation of Foxp3+ cells in vitro may have biologic relevance in vivo. The 3 IFN-λ subtypes were found to be coexpressed with IFN-β in iDCs stimulated with TLR3 and TLR4 agonists or to be coexpressed with all type I IFN subtypes in virally infected DCs.7 Interestingly, it was shown that TLR3 or TLR4 stimulation or Newcastle disease virus infection in mice unresponsive to type I IFNs affects the maturation but not the migration of DCs.2
Because type I IFNs and IFN-λs appear to exert opposite effects on DC function and given the tolerogenic activity of IFN-λ, it should be viewed as an element of the cytokine network that acts coordinately to ensure a safe immune response.34 On the other hand, it cannot be excluded that some physiologic or pathologic situations may lead to selective IFN-λ production. Interestingly, the phenotype of DCs that have ingested early-phase apoptotic cells resembles that of the IFN-λ–treated DCs described here.35 Because the removal of dying cells by macrophages and DCs is a natural process that induces T-cell tolerance,36 it would be interesting to monitor the induction of IFN-λ during the phagocytosis of apoptotic bodies. Beyond the physiologic role of IFN-λ, our study also suggests that IFN-λ could be instrumental in re-establishing self-tolerance in autoimmune diseases.
Prepublished online as Blood First Edition Paper, February 14, 2006; DOI 10.1182/blood-2005-10-4129.
Supported by grant RGP60/2002 from the Human Frontier Science Program.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank S. Pellegrini, M. Albert, O. Acuto E. Coccia, and J. Van der Heyden for critical reading of the manuscript, M. Perreau and D. Monneron for technical help, and PeproTech for the gift of IFN-λs.

![Figure 5. Profile of responding T lymphocytes primed by stimulating DCs in allogeneic reactions. iDCs or IFN-β, IFN-λ, or LPS-treated DCs were used for 24 hours to stimulate allogeneic T-cell proliferation in vitro at a 1:10 DC/T-cell ratio. [3H]-Thymidine was added for the last 18 hours of culture. (A) CD4+ T lymphocytes present in MLR reactions were analyzed for their expression level of CD25 by flow cytometry. (B) Percentage of CD4+ CD25+hi T cells in MLR cultures. Mean ± SEM of 3 experiments performed with different blood donors. (C) Purity analysis of CD4+ T cells separated for the expression of CD25 by immunomagnetic selection into CD4+CD25– (i) and CD4+CD25+ T cells (ii). (D) Proliferation of CD4+CD25+ or CD25– T lymphocytes primed by DCs. Mean ± SEM of 3 independent experiments performed on different blood donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/11/10.1182_blood-2005-10-4129/4/m_zh80110696450005.jpeg?Expires=1765055890&Signature=rFG2a-eFd4epnW5Sb16mPdyVyEba52LDR09y85OccQI3vSb1kIZ1lt0646BO5JGjCh~SRR4c9K~b60BcBpFmzeRZKZhYXFPR5vOHMUsBqJDDJ~L6vsICeTchXJi2o7Q1VNs4CdsCE0iG82DhtV6FS3pkxVmUJpXCphhdFkdz7t9vV6klNt02zHatJq0ehms3w3g6FIpb1mBAac2-4LlhppCWnCRJdKiHY6V2zu0dc4fS9gTuBXwtZlhmj3OsYJ3ygHjKlSvGudawEGZQDLcytZ6t1271WA8BYI8-HqpAdFaWslOE3S3lRSGXyiUOHWTzA9SgkJga9Nflejo1S0Kp1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Regulatory ability and Foxp3 expression of CD4+CD25+ T cells generated in vitro by IFN-λ–treated DCs. (A) CD4+CD25+ T cells generated in vitro by IFN-λ–treated DCs were purified from MLR culture reaction and assayed for their suppressive activity on CD4+ T-cell proliferation in MLR cultures. CD4+ responding T cells were stimulated with LPS-treated DCs at a 1:10 DC/T ratio. Cellular [3H]-thymidine incorporation found for this control MLR is indicated by the dotted line. Various numbers of CD4+CD25+ T cells were directly added either to the MLR culture reaction (□) or to permeable filters to avoid cell–cell contact interactions (▨). Data are the mean ± SD of 2 independent experiments performed in 6 replicates with cells from different blood donors. (B) Quantification of Foxp3 mRNA expression by real-time PCR in CD4+ T cells purified from MLR cultures mixing the indicated DCs and CD4+ T cells. For comparison, Foxp3 levels were quantified in freshly purified CD25+ or CD25–CD4+ T cells derived from a same donor. Results are expressed relative to GAPDH level. The experiment was performed twice on 2 blood donors with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/11/10.1182_blood-2005-10-4129/4/m_zh80110696450006.jpeg?Expires=1765055890&Signature=tbbyIbDL7Ny9-4MychrbIK5H1~hd4JRLHCwfD7fFU~LSudO9-Xvg9DSiEkh2b0bersJXdQCczKfZRwzJSOoGxRr-ZezMwFj9Mh9js6ke~EYZ9jQKGP4KwQhKWa2KeAuaSkDmV0ghVQunFywoifHuGLhW5klJNzzHr0AZexdHXRGhWAeX339YPb5v1hJu1NUuFDX8eY9skftZm6VenR0RAyIPWKznwE-3xUPCXo3w-4VhSEoSpsEjMczlKMGa6FL4K3PJb8SbLOL3zZ21AvtlM1iYPq4hJJx-KOgdccQY-ErFTiBD5I8uQX9giaMA0~Nlz82H4CwwkbSktPv7jXFMAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Tolerogenic functions of IFN-λ–treated DCs are IL-2 dependent. iDCs or IFN-β, IFN-λ, or LPS-treated DCs were used for stimulating allogeneic CD4+ T-cell proliferation in vitro at a 1:10 DC/T-cell ratio. Neutralizing anti–IL-2 or control IgG was added to the cultures as indicated. (A) CD4+ T-cell proliferation was determined by [3H]-thymidine incorporation after 8 days of culture under stimulating iDC or λDC conditions. (B) Quantification of Foxp3 mRNA expression by real-time PCR in MLR cultures of DCs and CD4+ T cells as indicated. Experiments were performed on a pool of T cells from 3 blood donors. Results are expressed relative to GAPDH level.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/11/10.1182_blood-2005-10-4129/4/m_zh80110696450007.jpeg?Expires=1765055890&Signature=XRJC9Z50D9ut0qB1D300Q-b1cibG8~Kd~5xGZuDSD01y8ALOO~LVnxV9N2WT-rgranhQAdcIXhSg12qy7b9OogKikWwjPXmcqpz8d8zVU1~sNN3rreq4-c4XBwBOo~~UU1aBCFy~KZlOcoO8aiXTYGY1Q1A361DEFbIdG-A~GC2UgmzVFqMf1NbI0d9c8X-e2rGVW8JDZ9FQS8y7ROuhtEcT1DlJu-qfyURfETGVzy7BXRYpzwRmCqest4EsCp6FWlSBp2VcGYt3CGSAPr5cpVVww5ajMSamTPbTTa18oUINOAdYlVlJIcgmV4ewkprpLJ9RQQK2W05TX03jJR0mMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Profile of responding T lymphocytes primed by stimulating DCs in allogeneic reactions. iDCs or IFN-β, IFN-λ, or LPS-treated DCs were used for 24 hours to stimulate allogeneic T-cell proliferation in vitro at a 1:10 DC/T-cell ratio. [3H]-Thymidine was added for the last 18 hours of culture. (A) CD4+ T lymphocytes present in MLR reactions were analyzed for their expression level of CD25 by flow cytometry. (B) Percentage of CD4+ CD25+hi T cells in MLR cultures. Mean ± SEM of 3 experiments performed with different blood donors. (C) Purity analysis of CD4+ T cells separated for the expression of CD25 by immunomagnetic selection into CD4+CD25– (i) and CD4+CD25+ T cells (ii). (D) Proliferation of CD4+CD25+ or CD25– T lymphocytes primed by DCs. Mean ± SEM of 3 independent experiments performed on different blood donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/11/10.1182_blood-2005-10-4129/4/m_zh80110696450005.jpeg?Expires=1765091396&Signature=ugfB1HyONi-iJHareaEC~axFDPEDV1qmdOwOD3uNXoJcvWm~1WfPybc6X1vk~8n8CjoldJT-NGY9BHHDWIFj1KIlzqs-yRPoc2gsAf~iU68cMLFu3UIVBpk3PhEuWdtVc0QN1aAoIMWmu2HKY~IyeadKM4QCKhUVDMKeYrHGnhTaHt0ZNxK4w3hCP7~BigOTqeeojgI9glpaIcTINKgoGuLUfW6hBph4caMDXrp5ArqvTpL10xL0JaC59kswv5ptVABAWz7OVVnvzqq09aHhkv28nEShrA2c~1foJrd6uwUGSYJvH8A3xT5T~Dg~fM399VHeLkAlLQ4XpNyieVAM6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Regulatory ability and Foxp3 expression of CD4+CD25+ T cells generated in vitro by IFN-λ–treated DCs. (A) CD4+CD25+ T cells generated in vitro by IFN-λ–treated DCs were purified from MLR culture reaction and assayed for their suppressive activity on CD4+ T-cell proliferation in MLR cultures. CD4+ responding T cells were stimulated with LPS-treated DCs at a 1:10 DC/T ratio. Cellular [3H]-thymidine incorporation found for this control MLR is indicated by the dotted line. Various numbers of CD4+CD25+ T cells were directly added either to the MLR culture reaction (□) or to permeable filters to avoid cell–cell contact interactions (▨). Data are the mean ± SD of 2 independent experiments performed in 6 replicates with cells from different blood donors. (B) Quantification of Foxp3 mRNA expression by real-time PCR in CD4+ T cells purified from MLR cultures mixing the indicated DCs and CD4+ T cells. For comparison, Foxp3 levels were quantified in freshly purified CD25+ or CD25–CD4+ T cells derived from a same donor. Results are expressed relative to GAPDH level. The experiment was performed twice on 2 blood donors with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/11/10.1182_blood-2005-10-4129/4/m_zh80110696450006.jpeg?Expires=1765091396&Signature=XUJXWQoc3OVpFRbxfxcX05qMsOOAAIPkjE7EZi3GRjLU6baXvFU9qIq9c6UBWi3peC2VR8PZJfs5B-FwPUHhGZhnDaWoH7FaPVfoZo7fMYvZa8iV8EEnp11kb1SmgFncXAfoinQQ89lrkJOfunQsrLJoV6M2FepvBbfVYjTzHxLFruCep523yfYr8cdqrtl4BYNUD5EgJREHIciGeBWLI3NYskFVm97PMX2E0Ob2t8IBfHYEpvY4JEXeDvEVexlgtw60hYDn856RQaY0b~~UI780jEu~kucG~Cle9cHVSunlnUqlnJzZMjSgtqqB997UYJmHCRBx3-bcOC9zhP53lg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Tolerogenic functions of IFN-λ–treated DCs are IL-2 dependent. iDCs or IFN-β, IFN-λ, or LPS-treated DCs were used for stimulating allogeneic CD4+ T-cell proliferation in vitro at a 1:10 DC/T-cell ratio. Neutralizing anti–IL-2 or control IgG was added to the cultures as indicated. (A) CD4+ T-cell proliferation was determined by [3H]-thymidine incorporation after 8 days of culture under stimulating iDC or λDC conditions. (B) Quantification of Foxp3 mRNA expression by real-time PCR in MLR cultures of DCs and CD4+ T cells as indicated. Experiments were performed on a pool of T cells from 3 blood donors. Results are expressed relative to GAPDH level.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/11/10.1182_blood-2005-10-4129/4/m_zh80110696450007.jpeg?Expires=1765091396&Signature=xYkfZmVZUSvcJp6C0iVszA8n8HEw2Zjdiv4HpsSTHyQoWLaLDM2hAPEiuVv~hbCipFKkuIcTTAiSOdZaEFOTN~9oZibL2CUqxPbyX8tvF6bt4jkrc0K8OrFMjRlQoEJq8GC884roiVo~Lf3mVaYk7b-3oaxgYeJvItb21OIbViFDLtC~1IksfHDR7g9dBsGb4-jIxd7Iim94gFwDItAgRnfOXoNeixSiaTIv8G9SOYnVxGSai~OWVDsOE~k-qVoDd7emGn57Fl2cVylQl3dCccpfQoD88kV7FxCqmODIImRz1nydicjYjOCZKTbemjihBRbe1XhIiae7VcDBuDyVtA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)