Abstract
Recent insights into the molecular mechanisms of polycythemia vera (PV) and essential thrombocythemia (ET) are challenging the traditional diagnostic classification of these myeloproliferative disorders (MPDs). Clonality analysis using X-chromosome inactivation patterns has revealed apparent heterogeneity among the MPDs. The recently discovered single somatic activating point mutation in the JAK2 gene (JAK2-V617F) is found in the great majority of patients with PV, but also in many patients with phenotypically classified ET and other MPDs. In contrast to the acquired MPDs, mutations of the erythropoietin receptor and thrombopoietin receptor have been identified in familial forms of nonclonal erythrocytosis and thrombocytosis, respectively. The mechanisms of major clinical complications of PV and ET remain poorly understood. Quantitative or qualitative abnormalities of red cells and platelets do not provide clear explanations for the thrombotic and bleeding tendency in these MPDs, suggesting the need for entirely new lines of research in this area. Recently reported randomized clinical trials have demonstrated the efficacy and safety of low-dose aspirin in PV, and an excess rate of arterial thrombosis, major bleeding, and myelofibrotic transformation, but decreased venous thrombosis, in patients with ET treated with anagrelide plus aspirin compared to hydroxyurea plus aspirin.
Introduction
Polycythemia vera (PV) and essential thrombocythemia (ET) are among the classic chronic myeloproliferative disorders (MPDs)1 that also include chronic idiopathic myelofibrosis and chronic myelogenous leukemia (CML). Recent advances in molecular diagnostics and the new World Health Organization (WHO) classification of myeloid neoplasms2 have expanded the group of chronic MPDs by adding chronic neutrophilic leukemia, chronic eosinophilic leukemia (and the hypereosinophilic syndrome), systemic mast cell disease, and unclassifiable chronic MPD. These disorders share many features: genesis in a single, multipotent hematopoietic stem cell that assumes dominance over nontransformed progenitors; hypercellularity of the marrow, with apparently unstimulated overproduction of one or more of the formed elements of blood; and clinical features that include increased risk of thrombosis and bleeding, spontaneous conversion to acute leukemia, and marrow fibrosis.3
This review highlights recent breakthroughs in our understanding of the molecular basis of PV and ET, as well as other disorders of polycythemia and thrombocytosis, which will have an impact on the clinical diagnosis and treatment of the MPDs. It is not intended to be a comprehensive or balanced clinical review, but rather a critical analysis of the most important recent translational research advances in the field.
Clonality in PV and ET
Clonality in PV and ET was originally established in female patients who were coincidentally heterozygous for X-linked glucose-6-phosphate dehydrogenase (G6PD) deficiency. The circulating red cells, leukocytes, and platelets of these individuals all expressed exclusively the same G6PD isoenzyme, indicating their origin in a single transformed clone, whereas other somatic cells (eg, skin fibroblasts) exhibited a distribution of the 2 isoenzymes that would be expected to result from random inactivation of X chromosomes. Subsequently, other X-linked polymorphisms have been used to confirm the clonal nature of PV, ET, and the other MPDs by examining differential DNA methylation patterns of X-linked genes and the skewing of X-chromosome inactivation patterns in neutrophils in comparison with T lymphocytes. That these are clonal disorders affecting a common, pluripotent hematopoietic progenitor cell has also been determined by the finding of specific tyrosine kinase gene mutations in multiple blood cell lines, but not other somatic cells in the body: JAK2 in PV, ET, and myelofibrosis; bcr/abl in CML; c-kit in systemic mast cell disease4 ; FIP1L1-PDGFRA in hypereosinophilic syndrome.5
Clonality analyses have revealed apparent heterogeneity among the MPDs in that the circulating hematopoietic progenitor cells of some patients with PV and ET were unexpectedly found to be polyclonal in origin.6,7 Using X-chromosome inactivation patterns (XCIPs) to assess clonality, Harrison et al excluded from analysis 50% of patients who met the diagnostic criteria for ET as the results were considered uninterpretable due to constitutive or acquired, age-related XCIP skewing; 13 of the remaining 23 evaluable patients were found to have polyclonal myelopoiesis.8 Although such studies do raise the possibility that some patients with the classic clinical phenotype of MPDs may not have clonal disease, they also spotlight the potential pitfalls of currently available clonality assays.8,9 Notwithstanding the technical limitations of current methods of clonality analysis, the clinical and biologic relevance of apparent heterogeneity in clinically diagnosed MPDs merits further research.10
Erythropoietin and thrombopoietin regulation in PV and ET
Both PV and ET are characterized by increased sensitivity of committed hematopoietic cells to their respective primary humoral growth factors: erythroid precursors to erythropoietin (Epo) in PV and megakaryocytes to thrombopoietin (Tpo) in ET. The pathologic correlates of these in vitro observations are predominantly erythroid hyperplasia in PV and predominantly megakaryocytic hyperplasia in ET. In vitro, Epo-independent (endogenous) erythroid colony formation and also Tpo-independent megakaryocyte colony formation are found in both PV and ET.11
However, different regulatory mechanisms are operative in the involvement of Epo and Tpo and their respective cell surface receptors in these MPDs (Table 1). There is a generally reciprocal relationship between plasma Epo levels and red cell mass and erythrocytosis in PV. The receptor for Epo (Epo-R) is normally expressed and has normal affinity for Epo in PV. Therefore, clonal proliferation of the erythroid lineage in PV leads to suppression of renal Epo synthesis by a negative feedback mechanism. A similar negative feedback system between clonal proliferation of a hematopoietic lineage and the specific growth factor that controls its differentiation and proliferation is operative in CML, which is associated with decreased serum levels of granulocyte colony-stimulating factor.
In contrast, plasma Tpo levels in ET are elevated or inappropriately normal in the face of thrombocytosis. The circulating plasma level of free Tpo is normally modulated by binding to its receptors, referred to as Tpo-R (or c-Mpl), on platelet and megakaryocyte surfaces.12 Thus, as the platelet count drops, increased unbound Tpo is available to promote megakaryocyte differentiation and proliferation, leading to restoration of the platelet count; conversely, an increase in the platelet count makes less free Tpo available for megakaryocytopoiesis. In this way, the total mass of platelets (and megakaryocytes) can maintain platelet production in a steady state under normal conditions. The inappropriately elevated levels of plasma free Tpo encountered in ET are due at least in part to either a clonal defect in platelet/megakaryocyte expression or down-regulation of Tpo-R, causing impaired binding and clearance of Tpo.13,14 Despite the reduced binding of Tpo to megakaryocytes in ET, these progenitors are also markedly hypersensitive to the action of the hormone, leading to increased megakaryocytopoiesis and platelet production.15
Molecular mechanisms of PV and ET
In contrast to familial forms of nonclonal erythrocytosis and thrombocytosis (see “Familial and secondary forms of polycythemia and thrombocytosis”), no mutations of Epo and Tpo or their respective receptors, Epo-R and Tpo-R, have yet been identified in PV and ET. Furthermore, the classic MPDs, including PV and ET, are characterized by promiscuous hypersensitivity of progenitor cells to several different hematopoietic growth factors. These observations have suggested that PV and ET are caused by molecular abnormalities at cellular sites downstream from receptor engagement. The earlier recognition of dysregulated tyrosine kinases in other MPDs, including the bcr/abl oncogene product in CML, stimulated the notion that tyrosine kinase mutations might likewise be operative in PV and ET.
Hematopoietic growth factor responses mediated by the JAK/STAT pathway
The Janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathway16 plays a central role in initiating signal transduction from hematopoietic growth factor receptors; it therefore represented a logical target for identifying the molecular abnormalities of PV and ET. Furthermore, Epo-independent differentiation of erythroid progenitors in PV has been found to be repressed by inhibitors of JAK2,17 and constitutive activation of STAT3 has been reported in patients with PV.18 The work of several groups has recently converged to identify a consistent, single somatic activating mutation in the JAK2 gene of the majority of patients with PV and up to about half of all patients with ET and myelofibrosis/myeloid metaplasia19 (Table 2). Such a JAK2-activating mutation is consistent with the growth factor hypersensitivity exhibited by hematopoietic progenitor cells from patients with MPDs. It has also been recently shown that the Janus kinases, JAK2 and Tyk2, promote cell surface localization of Tpo-R and protect the receptor from proteasome degradation.20 Therefore, this point mutation in JAK2 might also contribute to the down-modulation of Tpo-R cell surface levels in the MPDs.
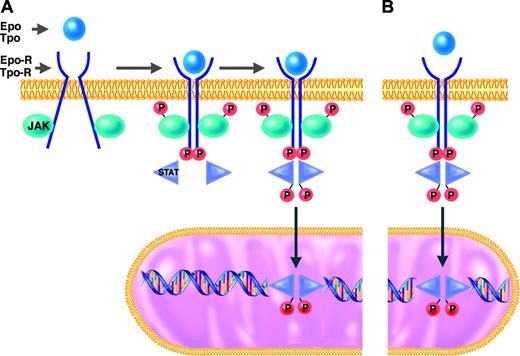
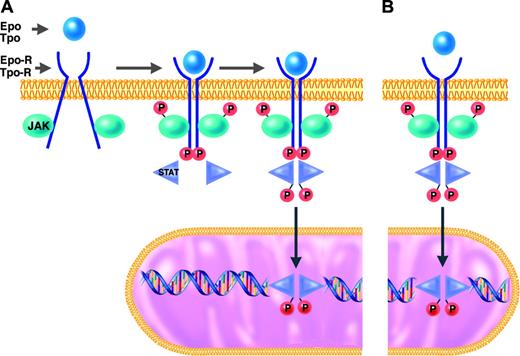
JAK-2 and the other members of the Janus kinase family are tyrosine kinases that function as intermediates between membrane receptors and intracellular signaling molecules.16,21 The JAK proteins are constitutively associated with the cytoplasmic domains of receptors. Cell activation normally occurs when the binding of a ligand (eg, Epo or Tpo) induces a shift in the conformation of preformed dimers of its receptor (eg, Epo-R or Tpo-R). JAK activation occurs on ligand-mediated change in receptor conformation because the 2 receptor-associated JAKs are brought into close proximity, allowing them to transphosphorylate each other. The activated and phosphorylated JAKs, in turn, phosphorylate the cytoplasmic domains of the receptors, which thereby become docking sites for downstream signaling molecules, particularly the STATs. STATs that are bound to phosphorylated tyrosine residues on the cytoplasmic domains of Epo-R or Tpo-R themselves become substrates for phosphorylation and activation by the activated JAKs. The activated STAT molecules then enter the nucleus, where they act as transcription factors by binding specific regulatory sequences to activate or repress the transcription of growth factor–responsive target genes (Figure 1).
JAK2 mutation in the MPDs
JAK2, like the other members of the JAK family, has an enzymatically active kinase domain (JH1) and a catalytically inactive pseudokinase domain (JH2). The JH2 domain has an autoinhibitory function that normally suppresses the kinase activity of JAK2.22 The mutation recently described in the classic MPDs by several groups23,28 is a guanine-to-thymine mutation encoding a valine-to-phenylalanine substitution at position 617 (V617F) in the JH2 domain of JAK2. It is a gain-of-function mutation in that it releases the autoinhibitory action of JH2 and thereby results in expression of a constitutively activated JAK2 tyrosine kinase. JAK2-V617F may thus bind to a receptor (eg, Epo-R or Tpo-R) and recruit STATs in the complete absence or in the presence of only trace quantities of hematopoietic growth factor (eg, Epo or Tpo)29 (Figure 1).
Several lines of evidence indicate that JAK2-V617F has a causal or contributory role in the pathogenesis of the MPDs and is not simply a passenger mutation that arose coincidentally in the same stem cell. A short interfering RNA(siRNA) to knockdown JAK2 expression blocked endogenous erythroid colony formation in cells from PV patients with the JAK2-V617F mutation.23 Expression of the mutated JAK2 (but not wild-type JAK2) induced Epo hypersensitivity and Epo-independent survival of cultured cell lines in vitro.23,24,26 The in vivo effect of the mutation was demonstrated by the development of erythrocytosis in mice that received transplants with bone marrow containing the JAK2-V617F mutation, but not wild-type JAK2.23
As shown in Table 2, all of the recently published studies showed that the majority of patients with PV (65%-97%) have the JAK2-V617F.23,28 In contrast, only 23% to 57% of patients with ET have the JAK2 mutation.23,26,28 Kralovics et al provided clinical correlations and reported that patients with JAK2-V617F had a significantly longer duration of disease, more treatment with cytoreductive agents, and higher rates of complications (myelofibrosis, thrombosis, and bleeding) than did those with wild-type JAK2.26
JAK/STAT signal transduction pathway in responses to hematopoietic growth factors. Under normal conditions (A), erythroid and megakaryocytic progenitors require binding of erythropoietin (Epo) or thrombopoietin (Tpo) to their respective receptors (Epo-R or Tpo-R) to initiate the intracellular sequence of phosphorylation and activation events leading to transcriptional activation of growth factor–responsive target genes (details presented in “Molecular mechanisms of PV and ET”). In hematopoietic progenitor cells of patients with MPDs who have the JAK2-V617F mutation (B), this JAK/STAT signal transduction pathway is constitutively activated in the absence of binding of Epo or Tpo to their respective receptors.
JAK/STAT signal transduction pathway in responses to hematopoietic growth factors. Under normal conditions (A), erythroid and megakaryocytic progenitors require binding of erythropoietin (Epo) or thrombopoietin (Tpo) to their respective receptors (Epo-R or Tpo-R) to initiate the intracellular sequence of phosphorylation and activation events leading to transcriptional activation of growth factor–responsive target genes (details presented in “Molecular mechanisms of PV and ET”). In hematopoietic progenitor cells of patients with MPDs who have the JAK2-V617F mutation (B), this JAK/STAT signal transduction pathway is constitutively activated in the absence of binding of Epo or Tpo to their respective receptors.
Several other biologic and epigenetic markers have been previously described in a variable number of patients with PV and ET. These include deregulated expression of Bcl-x, an inhibitor of apoptosis,30 overexpression of the PRV-1 and transcription factor NF-E2 genes,31,32 and impaired expression of Tpo-R.33 No mutations have yet been detected in these genes, and recent evidence indicates that altered expression of these markers is due to activation of the JAK/STAT pathway through the JAK2-V617F mutation.34 Therefore, abnormal expression of these molecules appears to be a secondary consequence of the primary JAK2 mutation.25
Molecular reclassification of the MPDs
The discovery of the JAK2-V617F mutation is a major breakthrough in understanding the molecular pathogenesis of the classic MPDs. However, it will be important to determine the reasons why (1) there is such phenotypic pleiotropy in patients with apparently different MPDs with the same disease allele and (2) not all patients demonstrate this mutation. Several explanations are possible. It is possible that the mutation arises only in specific subpopulations of primitive hematopoietic progenitor cells or has biochemical properties that restrict its activity to cells of a specific lineage(s).19 The recent observation that expression of JAK2-V617F in mouse bone marrow results in polycythemia in different strains, but that associated leukocytosis is strain-dependent, suggests that genetic modifiers affect the hematopoietic phenotype of the JAK2 mutation.35 Disease alleles other than JAK2-V617F might be involved, particularly involving other tyrosine kinases; however, Levine et al24 failed to identify any mutations by exon sequence analysis of the activating loops and autoinhibitory domains of other tyrosine kinases from granulocyte DNA samples from PV patients. Other mutations may occur36 in pathways that interact with JAK/STAT signaling or in other effector proteins, including adapter molecules that facilitate JAK/STAT pathway activation. Mutations in any one of several known negative regulators of the JAK/STAT pathway might be likewise operative in the other MPDs. For example, mutation of a hematopoietic cell phosphatase was previously reported to result in a MPD in mice,37 and numerous somatic mutations of protein tyrosine phosphatases have been identified in various human cancers.38
Finally, in view of the well-known phenotypic overlap and transitions that occur during the natural history of the classic MPDs, it is also possible that patients with clinically diagnosed ET or myelofibrosis who have the JAK2-V617F mutation actually represent variants of PV.39,40 This possibility is supported by the finding that up to 30% of patients with PV, but very few patients with ET, appear to be homozygous for JAK2-V617F.23,25,26 Transplantation of marrow containing the mutant JAK2 leads to predominantly erythrocytosis in at least one strain of mice.23,35 Therefore, mutation-positive patients with ET may, in fact, be individuals with “inapparent PV,” in whom variable degrees of plasma volume expansion might mask absolute increases in red cell mass unless these were directly measured.41 They may also represent early or late (spent) phases of PV. The specific genetic and physiologic modifiers that underlie such diagnostic distinctions have yet to be identified. The JAK-V617F mutation has been also found in a smaller minority of patients with chronic myelomonocytic leukemia, systemic mastocytosis, chronic neutrophilic leukemia, Philadelphia chromosome–negative CML, megakaryocytic acute myeloid leukemia (AML), and myelodysplastic syndromes (MDSs).42,43 The diagnostic classification of the MPDs is thus likely to be revised in the future, based on genotypic profiles of diagnostic molecular markers coupled with careful phenotypic correlations.
Clinical complications of PV and ET
Myelofibrosis, myelodysplasia, and acute leukemia
Both PV and ET can spontaneously convert to myeloid metaplasia with myelofibrosis (MMM) and acute leukemia or MDSs. The determination of the exact incidence of these transformations in the course of the natural histories of PV and ET is confounded by the frequent use of drugs that are themselves leukemogenic.
Myelofibrosis. The “spent phase” of PV, also referred to as “postpolycythemic myelofibrosis,” is characterized by clinical features that are similar to those of de novo primary MMM: development of anemia and other cytopenias, tear-drop poikilocytosis and other leukoerythroblastic changes in the peripheral smear, increasing splenomegaly, and slowly progressive bone marrow fibrosis. Survival after the onset of myelofibrosis and myeloid metaplasia in PV is shorter than that in primary MMM, and is characterized by a predominance of trisomy 1q, a rare abnormality in primary MMM.44 Post-polycythemic myelofibrosis develops in approximately 10% to 20% of patients with PV.45,46
In a recent series of 195 patients with ET followed for a median of 7.2 years, evolution into MMM occurred in 13 cases, a median of 8 years from diagnosis; 4 of these patients had not been exposed to treatment.47 The actuarial probability of this complication was 2.7% at 5 years, 8.3% at 10 years, and 15.3% at 15 years. Some apparent cases of ET actually represent the recently recognized entity of prefibrotic myelofibrosis, which evolves into overt myelofibrosis.48 Duration of disease and treatment with anagrelide increase the risk of conversion of ET to myelofibrosis.49
The proliferation of bone marrow fibroblasts in PV and ET is a reactive rather than a clonal process. The precise pathogenetic mechanism leading to marrow fibrosis in these disorders remains unclear, but the process involves abnormal megakaryocytes that synthesize and locally release fibrogenic cytokines, including platelet-derived growth factor, basic fibroblast growth factor, and transforming growth factor-β (TGF-β), in an autocrine fashion.50 Direct demonstration that TGF-β1 released by megakaryocytes plays a major role in the pathophysiology of myelofibrosis was reported in lethally irradiated mice repopulated with TGF-β1 null or wild-type stem cells engineered to overexpress Tpo. Severe fibrosis was observed in mice engrafted with wild-type cells, but no deposition of reticulin fibers was noted in those repopulated with TGF-β1 null cells.51
Acute leukemia and myelodysplasia. Recent analysis of 1638 patients enrolled in the European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study showed that acute myeloid leukemia/myelodysplastic syndrome (AML/MDS) developed in 22 patients after a median of 8.4 years from the diagnosis of PV.52 This figure was not far from those observed in earlier large clinical trials and observational studies, including the original PVSG-01 randomized trial in which the estimated incidence of acute leukemia in PV patients treated with phlebotomy alone was 1.5%.53 Exposure to P32, busulphan, and pipobroman, but not to hydroxyurea alone, was found to play an independent role in producing an excess risk of conversion to AML/MDS compared with treatment with phlebotomy.53 A consistent association of age has been observed with the risk of leukemia in PV.
ET rarely converts to acute leukemia or myelodysplasia in the absence of leukemogenic therapy.54,55 In one study with a median follow-up duration of 98 months, 4.5% of 357 patients with ET progressed to AML/MDS; all had received previous therapy with P32, alkylating agents, or hydroxyurea. A high proportion of cases with AML/MDS following hydroxyurea therapy had a 17p deletion.56
Bleeding and thrombosis
Bleeding, thrombotic, and vascular complications are the major causes of morbidity and mortality in PV and ET.57 Hemorrhagic problems are characteristically of the “platelet type,” involving spontaneous bleeding at superficial sites such as the skin (ecchymoses, purpura) and mucous membranes (gastrointestinal and genitourinary tract bleeding, epistaxis, hemoptysis). Risk of bleeding is increased in the MPDs by use of antiplatelet drugs. Counterintuitively, bleeding risk is also increased by extreme thrombocytosis (platelet count ≥ 1500 × 109/L [1.5 million/mm3]),58 possibly due to an acquired form of von Willebrand (see “Qualitative platelet abnormalities”).
Risk of thrombosis in PV and ET is increased with advanced age, prior history of thrombosis, and vascular risk factors (hypercholesterolemia, smoking).59 Although control of thrombocytosis does reduce the frequency of thrombotic and vascular complications in high-risk patients, particularly those with previous events, the degree of elevation of the platelet count does not generally correlate with the risk of thrombosis.54 Thrombotic episodes in PV and ET may involve the venous, arterial, or microvascular circulation.46,54,57,60,61
The most common venous events are deep vein thrombosis of the lower extremities and pulmonary embolism. Intra-abdominal venous thrombosis, notably hepatic vein (Budd-Chiari syndrome) or portal vein thrombosis, occurs particularly in younger patients with PV or ET.60 MPDs, which are sometimes clinically latent, are the leading causes of hepatic and portal vein thrombosis.62 Possible local factors contributing to the propensity for this anatomic localization of venous thrombosis in the MPDs might include hepatic extramedullary hematopoiesis, increased portal blood flow, and congestive splenomegaly.57
Large-vessel arterial thrombosis in PV and ET can involve the cerebrovascular, coronary, and peripheral arterial circulation, and accounts for most of the thrombosis-related morbidity and mortality in these disorders. Microcirculatory disturbances (including vasomotor symptoms) and microvascular thrombosis are more common in ET than in PV.61 Clinical manifestations include erythromelalgia, which may progress to digital ischemia and digit- or limb-threatening gangrene unless promptly treated with aspirin and cytoreduction.63 Transient neurologic and visual disturbances may also be caused by microvascular occlusion. Recurrent spontaneous abortion and fetal growth retardation complicate about 50% of pregnancies in women with ET.64 The major mechanism is thought to be multiple placental infarctions and consequent placental insufficiency caused by primarily platelet thrombosis.
Mechanism of bleeding and thrombosis
Erythrocytosis. The elevated hematocrit and resulting increased whole blood viscosity and decreased cerebral blood flow clearly contribute to the thrombotic tendency in PV because treatment to normalize the hematocrit reduces the risk of this complication. In addition to increased blood viscosity, the axial migration of red cells under normal in vivo flow conditions tends to displace circulating platelets toward the intimal surface of the vessel wall; erythrocytosis enhances platelet-vascular interactions, especially at the high shear rates found in arterioles and capillaries.65 The increased mass of red cells in PV may also contribute to heightened platelet activation.66 However, the hemorrheologic effects of erythrocytosis cannot be the only explanation for the thrombotic tendency in PV; comparable or even greater degrees of secondary erythrocytosis are not associated with thrombosis, and even normalization of the hematocrit in PV does not fully protect against the risk of thrombosis.
Thrombocytosis. Thrombocytosis may contribute to the hemostatic complications of ET or PV, particularly because platelet cytoreduction reduces the risk of recurrent thrombosis in high-risk patients. However, the lack of correlation between the degree of platelet count elevation and thrombosis in these disorders, and the lack of risk of hemostatic complications in patients with even extreme secondary (reactive) thrombocytosis, unless the underlying disease causing the thrombocytosis is itself a risk factor, makes it unlikely that thrombocytosis per se has a major causal relationship to hemostatic complications in ET and PV.
Qualitative platelet abnormalities. Acquired von Willebrand disease (aVWD) appears to be an important contributor to the bleeding tendency in many patients with thrombocytosis associated with MPDs, particularly ET.67 On multimer analysis with agarose gel electrophoresis, these individuals have a loss of large von Willebrand factor (VWF) multimers, similar to that seen in congenital type 2 VWD. aVWD is related to the degree of thrombocytosis and resolves with platelet cytoreduction. Possible mechanisms include increased clearance of large multimers of VWF by selective binding to platelets or enhanced platelet-dependent proteolysis of VWF, perhaps by exposure of VWF cleavage sites to ADAMTS13. Because aVWD has also been reported with reactive thrombocytosis,67 this defect is more likely to be a contributor than a cause of bleeding complications in patients with ET, particularly those with extreme thrombocytosis.
A variety of other structural and functional abnormalities of platelets have been reported in patients with MPDs, including decreased α-adrenergic receptor expression associated with loss of platelet responsiveness to epinephrine; abnormal expression of platelet membrane glycoproteins (Gp) Ib, IIb/IIIa, and IV; loss of prostaglandin D2 receptors; spontaneous platelet aggregation and circulating platelet aggregates; increased platelet microparticles; acquired storage pool disease; and abnormal arachidonic acid metabolism, with 12-lipoxygenase deficiency and increased thromboxane A2 synthesis.54,57,60 Unfortunately, none of these qualitative platelet defects has been convincingly demonstrated to be causally associated with either thrombotic or bleeding complications in PV and ET. The disappointing lack of persuasive clinical correlations could be attributed to the possibilities that different platelet abnormalities might develop during the natural history of a MPD, that several simultaneous platelet abnormalities in the same patient may have offsetting clinical consequences on hemostasis, or that these platelet abnormalities, even if clonal in nature, are unrelated to the clinical hemostatic complications in patients with PV and ET.
Other possible abnormalities of hemostasis. The inadequacy of explaining bleeding, thrombotic, and microvascular events in patients with MPDs simply on the basis of erythrocytosis, thrombocytosis, or qualitative platelet defects raises the likelihood that other, as yet poorly understood, mechanisms may be operative.
Because leukocytes are affected by the clonal abnormality of PV and ET, and are important participants in the pathogenesis of vascular ischemic syndromes, further research is needed to explore the possible role of qualitative leukocyte defects in the hemostatic complications of these disorders.68 In vivo polymorphonuclear leukocyte activation has been reported in PV and ET.69 Furthermore, increased platelet P-selectin expression and platelet-leukocyte conjugates have been noted in these disorders.70 Despite comparable levels of platelet cytoreduction, ET patients treated with the general myelosuppressive agent, hydroxyurea, had less arterial thrombosis and major hemorrhage than those treated with the more platelet/megakaryocyte-specific agent, anagrelide,49 perhaps indicating a salutary role for suppressing the leukocyte count to control hemostatic complications.
Circulating, bone marrow hematopoietic stem cell–derived endothelial progenitors can home to areas of vascular injury or ischemia and repopulate the intimal surfaces of the vessel wall as differentiated endothelial cells.71,72 It is plausible, therefore, that hemostatically defective endothelial cells, derived from abnormal hematopoietic stem cell clones in PV and ET, might also play a role in the clinical bleeding and thrombotic complications of these disorders.
Although Tpo alone does not induce the aggregation of normal platelets, it does prime platelets for enhanced response to platelet aggregating agonists. JAK2, which is constitutively activated in most patients with PV and ET (see “JAK2 mutation in the MPDs”), may be involved in signal transduction for Tpo-induced aggregation of platelets in these disorders.73 Therefore, it is possible that chronic, persistent hyperresponsiveness of platelets to Tpo associated with elevated plasma Tpo levels in ET and PV could contribute to the thrombotic tendency.
Epo levels are persistently decreased in PV, and this hormone is now recognized to have nonerythroid functions that are mediated by expression of Epo and its receptor in many other tissues, including the cardiovascular system.74,75 Exogenous or endogenous Epo reduces infarct volume in various animal models of stroke,74 so chronic “Epo deficiency” that occurs in both PV and many patients with ET might enhance cerebral infarction. Indeed, subnormal Epo concentration has been reported to be an independent risk factor for thromboembolic complications in ET.76 These and other hemostatic factors that are potentially operative in the MPDs merit new research.
Diagnosis of PV and ET
Until recently, the absence of specific diagnostic molecular markers of PV and ET has necessitated reliance on standardized diagnostic criteria. Although the original and revised PVSG criteria have been useful to identify subpopulations of patients with PV and ET for eligibility to participate in clinical trials, they have been too restrictive to define the entire spectrum of these MPDs in individual patients.
Laboratory tests for PV and ET
Guidelines for the diagnosis and investigation of polycythemia have been recently published.77 The central importance of directly measuring both red cell mass and plasma volume in the initial evaluation of erythrocytosis remains controversial. It has been considered reasonable to omit this test in cases where the hematocrit is greater than .60 (60%) in men and greater than .56 (56%) in women because values above these levels can be assumed to represent absolute erythrocytosis.77 However, many patients with PV present with lower levels of hemoglobin/hematocrit.78 Red cell mass and plasma volume measurements in these individuals have been considered by some to be mandatory in view of the variable degrees of plasma volume expansion that occur in PV (particularly in the presence of splenomegaly), which would tend to mask the polycythemia.79,80 In contrast, it has been argued that red cell mass measurements are rarely necessary today to distinguish apparent (relative) polycythemia from PV, and that the diagnosis of PV can usually be made on the basis of other clinical and laboratory tests.81,82
Serum Epo assays are useful in the initial evaluation of erythrocytosis, particularly when related to simultaneous hemoglobin/hematocrit determinations.83 Although PV remains a diagnostic possibility in the presence of low or normal serum Epo levels, erythrocytosis associated with increased Epo levels is usually due to secondary causes.81 Low Epo levels can also be seen in ET. Other biologic and epigenetic markers are found in variable numbers of PV patients. Initial experience indicates that mutation screening for JAK2-V617F can accurately distinguish between PV and secondary polycythemias.84 The commercial availability and validation of the diagnostic utility of the JAK2-V617F mutation in large-scale studies is anticipated.
Some patients with apparent ET have a Philadelphia chromosome or the BCR-ABL rearrangement, even in the absence of other features of CML; it has been suggested that it should be tested for in all cases of ET because of its potential therapeutic implications.85
Familial and secondary forms of polycythemia and thrombocytosis
The most crucial component of all diagnostic criteria for PV and ET has been the exclusion of secondary and familial forms of polycythemia and thrombocytosis (Table 3).
Familial polycythemia. The familial polycythemias include patients with (1) elevated or inappropriately high (ie, for the corresponding hemoglobin level) serum Epo concentrations, and (2) low or normal Epo levels86,87 (Table 3).
Those with inappropriately elevated Epo levels include individuals with inherited disorders of increased affinity of hemoglobin for oxygen, which leads to impaired delivery of oxygen to tissues, tissue hypoxia, and stimulation of Epo secretion. These patients have normal arterial oxygen saturation but an abnormally low partial O2 tension at which hemoglobin is 50% saturated with oxygen (P50). Most individuals in this category have an autosomal dominant high-O2-affinity hemoglobin or deficiency of 2,3-bisphosphoglycerate (2,3-BPG).
Inherited defects in oxygen sensing are associated with a normal P50 and normal arterial oxygen saturation. Chuvash polycythemia is due to homozygosity for a 598C>T missense mutation in the von Hippel-Lindau (VHL) gene. Oxygen homeostasis is normally regulated by the oxygen-sensing complex of VHL with the α subunit of hypoxia-inducible factor 1 (HIF-1α). The Chuvash polycythemia mutation in VHL impairs the interaction of VHL with HIF-1α, thereby enhancing the expression of Epo, a hypoxia-regulated gene.88 Other VHL mutations are now recognized as the causes of congenital polycythemias.86 In addition, familial polycythemia has been recently reported to result from a mutation in the HIF prolyl hydroxylase, PHD2 gene, which impairs hydroxylation of HIF and thereby increases Epo gene expression.89
Familial polycythemias with low/normal Epo levels include several nonsense and frameshift mutations in the Epo receptor gene (Epo-R), referred to as “primary familial and congenital polycythemias (PFCPs).” All of these mutations result in a premature stop codon, leading to the expression of an Epo-R that is truncated at its cytoplasmic carboxy terminus containing a negative regulatory domain.86,87 These autosomal dominant mutations thereby result in increased proliferation of erythroid precursors and hypersensitivity to Epo, as does the JAK2 mutation in PV.
Some patients with familial polycythemia, particularly those with Chuvash polycythemia, other defects in oxygen sensing, and the Epo-R mutations of PFCP, can be symptomatic (eg, headaches) and are at increased risk of cardiovascular, cerebrovascular, thrombotic, and bleeding complications.
Familial thrombocytosis. Most cases of familial thrombocytosis currently recognized are autosomal dominant disorders in which gain-of-function mutations in the 5′-untranslated region of the Tpo gene lead to overproduction of Tpo and marked elevation of plasma Tpo levels.90,91 These forms of familial thrombocytosis are examples of a distinctive genetic mechanism of disease that involves loss of translational repression leading to increased efficiency of mRNA translation.92 Other, Tpo-independent cases of familial thrombocytosis have been described, including one family with a dominant-positive activating mutation of the Tpo-R (c-Mpl) gene.93
Secondary polycythemia. The secondary polycythemias are generally driven by increased Epo production with normal responsiveness of erythroid progenitors to Epo and other growth factors. The secondary polycythemia may be physiologically appropriate, that is, stimulated by hypoxia. Alternatively, the secondary polycythemias may be physiologically inappropriate, that is, due to autonomous Epo production or administration of erythropoiesis-stimulating hormones. The specific secondary polycythemias are listed in Table 3 and have been reviewed elsewhere.94 Thrombotic complications may occur with extreme secondary polycythemias, presumably due to elevations in whole blood viscosity.
Secondary thrombocytosis. The most common cause of thrombocytosis, even with extreme elevations of the platelet count, is a secondary or reactive process.54 In one series of 732 consecutive medical and surgical patients with platelet counts 500 × 109/L (500 000/mm3) or higher, 88% had secondary thrombocytosis and only 12% had an MPD.95 In another series of 280 consecutive patients with platelet counts of 1000 × 109/L (1 000 000/mm3) or higher, 82% had secondary thrombocytosis, 14% had an MPD, and 4% had thrombocytosis of uncertain etiology.96
Secondary thrombocytosis is generally driven by increased production of Tpo or other cytokines and humoral mediators that directly or indirectly stimulate megakaryocytopoiesis. For example, IL-6, which is often elevated in reactive thrombocytosis and is an acute-phase reactant in various inflammatory and neoplastic diseases, up-regulates the expression of Tpo mRNA in the liver.97 In most patients with secondary thrombocytosis, the underlying disorder is clinically apparent. However, in some cases the cause is subclinical (eg, occult malignancy), and these have presented the most vexing challenges to clinicians in the absence of diagnostic markers of an MPD. Table 3 lists major causes of secondary thrombocytosis, which have been reviewed elsewhere.54
Treatment considerations
Aggressive phlebotomy therapy remains the mainstay of treatment of PV. The aim is to maintain the hematocrit below .45 (45%). It is reasonable to target an even lower hematocrit level (.42 [42%]) in women.81 Increased risk of thrombotic and vascular events was found to be progressively increased at hematocrits above .44 (44%) in one uncontrolled study using univariate analysis.98 Surprisingly, over 15% of hematologists still use a higher target hematocrit.99,100 In addition to phlebotomy, cytoreductive therapy is usually considered in high-risk patients with PV (age ≥ 60 years or previous history of thrombosis). Despite continued uncertainty about its leukemogenic potential,101 hydroxyurea remains the myelosuppressive agent of first choice.94,95 In younger high-risk patients, particularly in women of childbearing age (in whom hydroxyurea may be teratogenic), interferon alfa can be substituted.
The efficacy of platelet cytoreductive therapy in asymptomatic, low-risk patients with ET, irrespective of the degree of thrombocytosis, remains untested.54 One prospective, randomized, controlled trial showed sustained increase in thrombosis-free survival in high-risk patients with ET, that is, those 60 years or older or with a previous history of hemostatic complications.55,58 Anagrelide is a nonleukemogenic drug that relatively selectively inhibits megakaryocyte proliferation and differentiation,102 although it can also cause anemia with long-term therapy.103 It has become alternative first-line therapy to reduce the platelet count, when indicated, particularly in younger patients with ET. A randomized, multicenter trial in 809 patients with ET recently reported that, compared to hydroxyurea plus aspirin, anagrelide plus aspirin was associated with an excess rate of arterial thrombosis, major bleeding, and myelofibrotic transformation but decreased venous thrombosis, with a median follow-up of 39 months.49 These investigators concluded that hydroxyurea should remain first-line therapy in ET patients at high risk for vascular events. The notion that ET patients who have the JAK2-V617F mutation represent a biologically distinct subtype of the disease, which resembles the PV phenotype, is supported by the observation that mutation-positive individuals are more sensitive to hydroxyurea, requiring lower doses to control platelet counts and to reduce the hemoglobin and white cell counts than mutation-negative ET patients, a pattern not observed with anagrelide.39
Aspirin may be highly effective in conjunction with cytoreductive therapy in patients with PV and ET who have recurrent thrombotic and vascular complications, particularly those with cerebrovascular or digital ischemia. However, antiplatelet therapy must be used with caution in other patients with PV and ET because it can also cause serious bleeding.104 The risk of bleeding with aspirin use in patients with ET is increased when the platelet count is more than 1000 × 109/L (1 000 000/mm3).105 The recently reported double-blind, placebo-controlled, randomized ECLAP study in patients with PV who did not have contraindications to aspirin showed that low-dose aspirin (100 mg daily) is effective in preventing thrombotic complications without increasing the risk of major bleeding.106
Hematopoietic stem cell transplantation can be considered for highly selected younger patients with PV or ET who have advanced, complicated disease.107 Allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning has been found to prolong survival in high-risk patients with myelofibrosis/myeloid metaplasia, including those who had underlying PV and ET.108 With the recent recognition of the JAK (V617F) mutation in most patients with PV and many with ET, the prospect of molecularly targeted therapy is clearly on the horizon.
Prepublished online as Blood First Edition Paper, February 16, 2006; DOI 10.1182/blood-2005-08-3526.
I am indebted to Dr Josef T. Prchal for helpful discussions in preparation of this manuscript.