The Tax oncoprotein plays a crucial role in the proliferation and transformation of human T-cell leukemia virus type I (HTLV-I)–infected T lymphocytes through various mechanisms, including activation of the nuclear factor (NF)–κB pathway. We found that cytoplasmic ubiquitylation of Tax C-terminal lysines is critical for Tax binding to the IkappaB kinase complex and subsequent nuclear translocation of RelA. Conversely, we demonstrate that the same lysines are sumoylated in the nucleus, an event required for the formation of RelA/p300-enriched Tax nuclear bodies and full NF-κB transcriptional activation. In contrast, Tax ubiquitylation and sumoylation are dispensable for its activation of cyclic adenosine monophosphate response element binding protein (CREB)–dependent genes. Thus, ubiquitylation and sumoylation of the same residues of Tax regulate 2 essential steps controlling NF-κB activation, demonstrating how these posttranslational modifications can cooperate to promote Tax-induced transformation.
Introduction
Human T-cell leukemia virus type I (HTLV-I)–induced T-cell transformation involves deregulation of multiple cellular processes, including activation of the nuclear factor (NF)–κB and cyclic adenosine monophosphate response element binding protein (CREB) pathways.1-3 Tax intervenes at multiple levels to initiate and maintain NF-κB activation.2 Tax is recruited to the IKKγ component of the IκB kinase (IKK) complex.4 This recruitment activates IKKα and IKKβ kinases, resulting in IκB degradation and nuclear translocation of RelA.5 Tax also colocalizes with RelA, p50, RNA polymerase II, and CBP/p300, in transcriptionally active Tax nuclear bodies.6
Regulation of protein functions by posttranslational modifications can be achieved through covalent attachment to ubiquitin or to small ubiquitin-related modifier (SUMO) molecules on internal lysine residues. Ubiquitylation of some transcription factors,7 or of the HIV-1 transactivator Tat,8 increases their transcriptional activity in a degradation-independent manner. Similarly, sumoylation plays an important role in subcellular localization, including nuclear protein import and protein targeting to nuclear bodies.9 Sumoylation of some transcription factors is associated with transcriptional repression.9
We and others have previously demonstrated that Tax is ubiquitylated.10,11 In this study, we investigated the contribution of ubiquitylation and sumoylation in Tax-induced NF-κB activation. We show that Tax ubiquitylation is critical for both Tax binding to the IKK complex and nuclear translocation of RelA. We also report that Tax sumoylation is critical for the formation of RelA-enriched Tax nuclear bodies and full transcriptional activation. Thus, ubiquitylation and sumoylation of Tax regulate 2 essential phases of NF-κB activation, demonstrating how a major function of this viral oncoprotein is under strict dependence of posttranslational modifications.
Materials and methods
Cell culture and transfection
HTLV-I–infected (HuT-102 and C8166) and HTLV-I–negative (Jurkat) T cells were grown in RPMI 1640, and HeLa and 293T cells were grown in Dulbecco modified Eagle medium. In all cases, the medium was supplemented with 10% fetal bovine serum (Gibco, Invitrogen, Paisley, United Kingdom). 293T cells were transfected by the calcium phosphate procedure as described.10 T-cell lines were transfected by DMRIE-c (Gibco) or Superfect (Qiagen, Courtaboeuf, France), and Hela cells by Lipofectamine 2000 (Gibco) or Effectene (Qiagen), according to the manufacturer's recommendations.
Drugs and antibodies
Tax was detected using either a pool of patient's sera or the monoclonal antibody (mab) 168-A51 (NIH AIDS Research and Reference Reagent Program, Germantown, MD). For immunostaining, goat anti–mouse fluorescein isothiocyanate (FITC; Molecular Probes, Eugene, OR), goat anti–mouse IgG-2a FITC (Southern Biotechnology, Birmingham, AL), and goat anti–rabbit IgG conjugated to Alexa Fluor 546 (Molecular Probes) or cyanine 3 (Jackson ImmunoResearch, Bar Harbor, ME), were used. HA-tagged proteins were revealed with the 12CA5 mab (Roche, Meylan, France), or a rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and poly-ubiquitylated products with the Fk2 mab (Tebu, Le Perrayen Yvelines, France). Antibodies against RelA/p65, p50, IKK-α, IKK-β, and IKK-γ, p300, tubulin, and lamin were obtained from Santa Cruz Biotechnology. The anti-GAPDH mab was obtained from Biogenesis (Stinford Fload, United Kingdom).
Plasmids and mutagenesis
Tax lysine-to-arginine mutants were produced by polymerase chain reaction (PCR) mutagenesis as described.10 All constructs were sequenced. Plasmids encoding M22 (T130A/L131S), or M47 (L319R/L320S) Tax mutants,12 HA-ubiquitin,10 HA-SUMO1, HA-SUMO2, and HA-SUMO3 expression plasmids (gift from T. Kamitani, Houston, TX) were also used. Tax-Ub and K4-8R-Ub plasmids (gift from F. Bex, Brussels, Belgium) were generated by the fusion of ubiquitin to the carboxy-terminus of wild-type Tax and K4-8R, respectively, with a mutation of the carboxy-terminal glycine residue of ubiquitin to alanine to prevent conjugation to substrates.13 Tax-SUMO and K6-8R-SUMO plasmids were generated by the fusion of SUMO1 to the carboxy-terminus of wild-type Tax and K6-8R, respectively. Tax mutants were used with or without an additional C-terminal 6 histidine tag. IKKα was produced from the pRC-HA-IKKα plasmid (gift from S.C. Sun, Hershey Medical Center, Hershey, PA).
Transactivation assays
Tax-induced transactivation of the CREB pathway was measured as described using the HTLV-LTR-Luc reporter.14 For NF-κB, Jurkat cells (106) were cotransfected with 500 ng of either pNF-κB-Luc (gift from G. Dbaibo, Beirut, Lebanon) or HIV-LTR-Luc reporter plasmid, 200 ng of the renilla luciferase (pRL-TK) plasmid (Promega), and 1 μg of either Tax plasmid. Total DNA was equalized in all samples using PSG-5 empty vector. Luciferase activity was quantified using the Dual Luciferase Assay System (Promega) and values were normalized with Renilla activity. C8166 cells (2 × 106 cells) were transfected with 1 μg of the HIV-LTR-lacZ or the HTLV-I-LTR-lacZ and with 0.3 μg, 1 μg, or 3 μg of either Tax plasmid. Beta-galactosidase activity was measured with the β-gal reporter assay (Roche).
Immunoprecipitations and immunoblots
Cells were lysed in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% IGEPAL CA-630, 0.5% Triton X-100, 1x protease inhibitor cocktail (Roche), with isopeptidase (10 mM N-ethylmaleimide) inhibitors (Sigma), on ice for 30 minutes. Protein concentration was determined using the colorimetric DC protein assay (Biorad, Hercules, CA). Proteins (50 μg) were loaded and resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) before transfer to nitrocellulose. Immunoblots were performed as described.10,15
Immunoprecipitation of Tax was performed using the anti-Tax mab. In the case of the Tax-Ub fusion proteins, whose level of expression is lower than that of wild-type Tax, the amount of transfected DNA was adjusted to obtain equal amounts of immunoprecipitated Tax proteins. Cell lysates were incubated 2 hours at 4°C, and antibody complexes were captured on protein A–agarose (Roche) overnight at 4°C. Agarose beads were then washed in lysis buffer before elution in laemli buffer. Products were separated by SDS-PAGE, transferred to nitrocellulose membranes, and blotted with specific antibodies.
Ni-NTA pull-down
Ni–Ni2+ nitrolotriacetate acid (NTA) pull-down was performed as described.10 Briefly, 36 hours after transfection cells were lysed in reducing and highly denaturing conditions and incubated with Ni-NTA beads. Beads were washed and bound proteins were eluted. Products were separated by SDS-PAGE, transferred to nitrocellulose membranes, and blotted with specific antibodies.
In vitro kinase activity
Cells were lysed and immunoprecipitated 24 hours after transfection with anti-IKKα antibody as described.15 Kinase activity was measured as described15 using γ-32P ATP (PerkinElmer, Shelton, CT) and recombinant GST-IκB (Santa Cruz Biotechnology) as substrate. Products were separated by SDS-PAGE and analyzed by autoradiography.
Immunofluorescence
At 24 hours after transfection, cells cultured on coverslips were fixed with methanol at –20°C, washed with phosphate-buffered saline (PBS), blocked in PBS containing 0.5% gelatin and 0.25% bovine serum albumin, and incubated with the primary antibody. The preparations were then washed with PBS, incubated with the secondary antibody, washed, then mounted with the Prolong Antifade Kit (Molecular Probes) and examined on a Zeiss LSM 410 fluorescence microscope (Zeiss, Gottingen, Germany) equipped with a 63×/1.25 oil-immersion objective lens. Images were captured with a Zeiss Axiocam, acquired with Zeiss proprietary software, and processed with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Electrophoretic mobility shift assay
Cells were transfected with Tax or Tax mutants and with an IKKα construct. At 24 hours after transfection, nuclear extracts were prepared and hybridization was performed as described16 using γ-32P ATP–labeled NF-κB oligonucleotides (Santa Cruz Biotechnology). Products were separated on a 5% nondenaturing polyacrylamide gel. Specific NF-κB bands were determined by competition experiments using a mutant oligonucleotide (Santa Cruz Biotechnology). Subunit specificity was determined by using specific antibodies to RelA and p50 in the incubation step, known to super-shift NF-κB complexes.
Cell fractionation
At 24 hours after transfection, HeLa cells were washed with ice-cold PBS and collected by centrifugation. The pellet was lysed by rapid freezing and thawed by resuspension in ice-cold buffer containing 10 mM Hepes, 10 mM KCl, 1.5 mM MgCl2, and 1 mM dithiothreitol and centrifuged at 2000g (3500 rpm) for 10 minutes at 4°C. The supernatant (cytoplasmic fraction) and the pellet (nuclear fraction) were lysed in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% IGEPAL CA-630, 0.5% Triton X-100, 1 × protease inhibitor cocktail (Roche) and 10 mM N-ethylmaleimide (Sigma), on ice for 30 minutes. Proteins (50 μg) were directly resolved on SDS-PAGE gel before blotting with antilamin or antitubulin antibodies. An additional 300 μg proteins were immunoprecipitated using anti-Tax antibodies.
Results
Carboxy-terminal lysines are major targets for Tax ubiquitylation
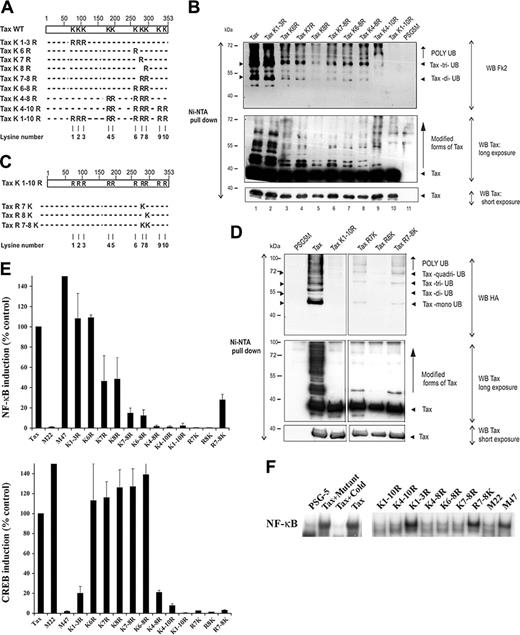
Tax sequence contains 10 lysines (K). Three of them (K1 to K3) are within the N-terminal half (residues 85, 88, and 111) while 7 (K4 to K10) are within the C-terminal part (residues 189, 197, 263, 280, 284, 324, and 346) (Figure 1A). We previously reported that Tax C-terminal lysines (K4 to K10) are the main target for ubiquitylation.10 To further define which of these lysines are involved, we produced additional lysine-to-arginine Tax-His mutants (Figure 1A). Tax-His proteins expressed in 293T cells were purified by Ni-NTA pull-down using fully denaturing conditions,10 ensuring that only ubiquitin molecules covalently attached to Tax were recovered. The abundance of ubiquitylated Tax species was evaluated by blotting with the Fk2 mab (Figure 1B; WB Fk2), specific for poly-ubiquitylated chains, and with anti-Tax antibodies, which allowed detection of mono-ubiquitylated Tax (Figure 1B; WB Tax). No species reacting with anti-Ub antibody were detected in the absence of Tax expression or when the lysineless form of Tax was produced (Figure 1B, upper panel). On the contrary, abundant Fk2-reactive species were detected in cells expressing wild-type Tax or Tax K1-3R mutated on the N-terminal lysines. Mutations of lysines 4 to 10 massively reduced monoubiquitylated (WB Tax) and poly-ubiquitylated Tax species (WB Fk2). Tax ubiquitylation was also massively reduced when lysines 6, 7, or 8 were mutated, either individually (K6R, K7R, or K8R), or in combination (K7-8R, K6-8R).
Tax C-terminal lysines are critical for NF-κB, but not CREB, activation. (A) Schematic representation of the lysine-to-arginine Tax mutants. (B) 293T cells were transfected with the various Tax-6His plasmids and proteins purified by denaturing Ni-NTA pull-down were blotted with mabs to poly-ubiquitin chains (top panel) or to Tax (middle and bottom panels). (C) Representation of Tax mutants in which lysines 7 and/or 8 were reintroduced to the lysineless protein K1-10R-6His. (D) 293T cells were transfected with Tax-6His, the lysineless Tax K1-10R-6His, or the indicated reverse mutants in the presence of an HA-Ub plasmid to favor Tax ubiquitylation. Proteins purified by denaturing Ni-NTA pull-down were blotted with mabs to HA (top panel) or to Tax (middle and bottom panels). (E) Jurkat cells were cotransfected with the NF-κB-Luc (top panel) or the HTLV-LTR-Luc (bottom panel) reporter plasmids, and the plasmids encoding for Tax or Tax mutants. For normalization, cells were cotransfected with renilla luciferase expression plasmid. Activity of the wild-type protein was set to 100%. The results represent the mean plus or minus standard deviation of at least 3 different experiments. (F) Jurkat cells were cotransfected with Tax or Tax mutants. NF-κB DNA-binding activity was assessed by electrophoretic mobility shift assay using a consensus oligonucleotide for NF-κB. Specificity of the NF-κB complex was determined by the addition of an excess of an unlabeled consensus (cold) or mutated (mutant) oligonucleotides.
Tax C-terminal lysines are critical for NF-κB, but not CREB, activation. (A) Schematic representation of the lysine-to-arginine Tax mutants. (B) 293T cells were transfected with the various Tax-6His plasmids and proteins purified by denaturing Ni-NTA pull-down were blotted with mabs to poly-ubiquitin chains (top panel) or to Tax (middle and bottom panels). (C) Representation of Tax mutants in which lysines 7 and/or 8 were reintroduced to the lysineless protein K1-10R-6His. (D) 293T cells were transfected with Tax-6His, the lysineless Tax K1-10R-6His, or the indicated reverse mutants in the presence of an HA-Ub plasmid to favor Tax ubiquitylation. Proteins purified by denaturing Ni-NTA pull-down were blotted with mabs to HA (top panel) or to Tax (middle and bottom panels). (E) Jurkat cells were cotransfected with the NF-κB-Luc (top panel) or the HTLV-LTR-Luc (bottom panel) reporter plasmids, and the plasmids encoding for Tax or Tax mutants. For normalization, cells were cotransfected with renilla luciferase expression plasmid. Activity of the wild-type protein was set to 100%. The results represent the mean plus or minus standard deviation of at least 3 different experiments. (F) Jurkat cells were cotransfected with Tax or Tax mutants. NF-κB DNA-binding activity was assessed by electrophoretic mobility shift assay using a consensus oligonucleotide for NF-κB. Specificity of the NF-κB complex was determined by the addition of an excess of an unlabeled consensus (cold) or mutated (mutant) oligonucleotides.
We then selectively reintroduced lysines 7 and/or 8 in the lysineless protein Tax K1-10R-His (Figure 1C). Mutants were cotransfected with HA-Ub to favor detection of ubiquitylated products. Reintroduction of K7 partially restored Tax ubiquitylation whereas reintroduction of K8 had no effect (Figure 1D). Reintroduction of both lysines (Tax R7-8K) further restored Tax ubiquitylation. Hence, lysines 7 and 8, closely positioned at positions 280 and 284, constitute major functional Tax ubiquitylation sites. However, other C-terminal lysines also contribute to Tax ubiquitylation since combined mutation of lysines 4 to 8 was required to abolish mono- and oligo-ubiquitylated Tax (Figure 1B; WB Tax).
C-terminal Tax lysines are critical for NF-κB, but not CREB, activation
We next investigated the ability of lysine-deficient Tax mutants to activate the NF-κB or the CREB pathways (Figure 1E). Jurkat cells were cotransfected with either pNF-κB-Luc or HIV-LTR-Luc (NF-κB; Figure 1E, top), or HTLV-LTR-Luc (CREB; Figure 1E, bottom) reporters together with the indicated Tax plasmids. As expected, Tax expression strongly induced NF-κB and CREB reporters (100% of activity, 49- and 300-fold induction, respectively) and the previously described M47 (CREB-defective) and M22 (NF-κB–defective) mutants gave the expected opposite phenotypes.12 The lysineless Tax K1-10R and the Tax K4-10R mutants did not activate NF-κB– or CREB-dependent reporters, presumably because of a structural or folding defect. Tax K1-3R, lacking the N-terminal lysines, retained a full activity for NF-κB, but was reduced for CREB induction. Such defect in CREB activation likely relates to the reported role of amino acids 81 to 95 of Tax in CBP/p300 binding.17 Tax K4-8R, K6-8R, and K7-8R were significantly impaired for NF-κB activation, but could still activate the CREB pathway, although with a reduced efficiency for K4-8R (63-fold or 21% of Tax), demonstrating that their defect in NF-κB activation was not due to a mutation-induced impaired conformation (Figure 1E). Mutation of lysine 6 did not affect Tax transactivation, whereas mutation of K7 or K8 specifically reduced induction of the NF-κB pathway. Interestingly, reintroduction of both lysines 7 and 8 in the lysineless Tax K1-10R protein (R7-8K), but not of either lysine alone, resulted in a partial but significant recovery of NF-κB induction (14-fold), whereas no restoration was found for CREB (Figure 1E). These results indicate that the integrity of lysines 7 and 8 is required for a complete NF-κB, but not CREB, induction in Jurkat cells. Similar results were observed in HeLa cells. However, mutation of lysines 6, 7, and/or 8 attenuated, rather than abrogated, NF-κB induction; but again, the sole restoration of lysines 7 and 8 in the lysineless mutant was sufficient to recover NF-κB induction (not shown).
Specific lysine residues are required for NF-κB complex formation
We examined the NF-κB DNA-binding activity by electrophoretic mobility shift assay in Tax-expressing cells (Figure 1F). Tax expression in Jurkat cells yielded a strong retarded complex, specifically competed by an excess of unlabeled, but not mutated, consensus NF-κB oligonucleotide. As expected, this NF-κB complex was observed with M47 but not with M22. Results obtained with the lysine mutants essentially mirrored those of the luciferase assays, as a strong induction of DNA/protein complex was observed with Tax K1-3R, whereas only a faint band was observed with Tax K1-10R, K4-10R, K4-8R, K6-8R, and K7-8R proteins. NF-κB subunit specificity was demonstrated using RelA- and p50-specific antibodies. RelA-containing heterodimers were only detected in cells transfected with Tax or NF-κB active Tax mutants, whereas inactive p50 homodimers were found in both Tax-positive and -negative cells (not shown). Comparable results were observed in HeLa cells (not shown), except that surprisingly, Tax K6-8R and K7-8R mutants retained some activity in these cells. However, in both Jurkat and HeLa cells, a strong induction was observed with the R7-8K mutant, whereas R7K and R8K mutants remained inactive (Figure 1F and not shown). Hence, the suppression of K7 and K8 in Tax affects the formation of NF-κB/DNA complexes and their dual presence is sufficient to restore this function, underlying their critical role for the induction of NF-κB DNA-binding activity.
Tax C-terminal lysines are critical for IKK binding, IKK activation, and nuclear translocation of RelA. (A) HeLa cells were transfected with Tax or Tax mutants. Lysates were immunoprecipitated with anti-Tax mab and recovered proteins were blotted using anti-Tax, anti-IKKα, anti-IKKβ, or anti-IKKγ antibodies, as indicated. (B) HeLa cells were transfected as in panel A. Lysates were immunoprecipitated using anti-IKKγ antibodies and recovered proteins were blotted using anti-Tax, anti-IKKα, anti-IKKβ,or anti-IKKγ antibodies, as indicated. (C) Jurkat cells were transfected with Tax or Tax mutants. Activity of immunoprecipitated IKK was assessed by kinase assay using GST-IκB-α as substrate (top panel). Immunoprecipitates were probed with anti-IKKα antibody to determine the amounts of precipitated kinase and with anti–GST-IκB-α to determine the amount of substrate. Equal amounts of lysates were determined by blotting with anti-GAPDH antibody. (D) HeLa cells were transfected with either Tax plasmid and stained with anti-Tax and anti-RelA antibodies. The percentage of nuclear translocation of RelA in Tax-positive cells is indicated.
Tax C-terminal lysines are critical for IKK binding, IKK activation, and nuclear translocation of RelA. (A) HeLa cells were transfected with Tax or Tax mutants. Lysates were immunoprecipitated with anti-Tax mab and recovered proteins were blotted using anti-Tax, anti-IKKα, anti-IKKβ, or anti-IKKγ antibodies, as indicated. (B) HeLa cells were transfected as in panel A. Lysates were immunoprecipitated using anti-IKKγ antibodies and recovered proteins were blotted using anti-Tax, anti-IKKα, anti-IKKβ,or anti-IKKγ antibodies, as indicated. (C) Jurkat cells were transfected with Tax or Tax mutants. Activity of immunoprecipitated IKK was assessed by kinase assay using GST-IκB-α as substrate (top panel). Immunoprecipitates were probed with anti-IKKα antibody to determine the amounts of precipitated kinase and with anti–GST-IκB-α to determine the amount of substrate. Equal amounts of lysates were determined by blotting with anti-GAPDH antibody. (D) HeLa cells were transfected with either Tax plasmid and stained with anti-Tax and anti-RelA antibodies. The percentage of nuclear translocation of RelA in Tax-positive cells is indicated.
Specific lysines are critical for IKK binding and nuclear translocation of RelA
Since Tax C-terminal lysines are critical for Tax-induced NF-κB activation and DNA/protein complex formation, we tested their role in IKK binding and RelA nuclear translocation. HeLa cells were transfected with various Tax plasmids and immunoprecipitated with anti-Tax mab. Blotting with anti-IKKα, -IKKβ, or -IKKγ antibodies showed that all 3 IKK subunits were present in Tax/IKK immunoprecipitates (Figure 2A). The Tax K1-3R, K6-8R, K7-8R, K7R, and K8R proteins were also able to bind IKK proteins, whereas no coprecipitation was found for the lysineless mutant K1-10R and the Tax K4-10R and K4-8R proteins. Importantly, reintroduction of K7 and K8 (R7-8K), but not of either lysine alone, fully restored Tax ability to bind IKKγ and coprecipitate IKKα and IKKβ (Figure 2A).
When the same extracts were immunoprecipitated with anti-IKKγ, blotting with anti-Tax antibodies showed that the wild-type Tax or M47 proteins were present in assembled IKK immunoprecipitates (Figure 2B), whereas M22 protein could not be recovered, as reported.18 Again, Tax K1-10R, K4-10R, and K4-8R were not coprecipitated with anti-IKKγ. By contrast, Tax K1-3R, K6-8R, K7-8R, and importantly, R7-8K, were all present in Tax/IKK immunoprecipitates.
Kinase assays performed in Jurkat T cells using IκB-α as a substrate confirmed that IKK kinase activity was massively induced by wild-type Tax or M47, whereas, as expected, M22 had no effect (Figure 2C). IKK kinase activity was not induced by Tax K1-10R, K4-10R, and K4-8R, whereas a massive induction was detected in the presence of Tax K7-8R and importantly R7-8K, in agreement with the immunoprecipitation results. These results indicate that Tax-mediated activation of IKK is modulated by C-terminal lysines.
Tax expression also induced RelA nuclear translocation, as detected by immunofluorescence (Figure 2D), indirectly reflecting Tax-induced IKK activation. Consistent with the immunoprecipitation results, M22 and K4-8R mutants failed to induce RelA nuclear translocation (1% and 2% of cells, respectively), whereas the M47, K6-8R, K7-8R, and R7-8K Tax mutants were able to induce efficient RelA translocation (100%, 81%, 89%, and 54% of cells, respectively; Figure 2D and not shown). Altogether, these results indicate that Tax C-terminal lysines, particularly K4 to K8, mediate the recruitment of Tax to the IKK complex, which in turn triggers IKK activation and nuclear translocation of RelA. Interestingly, K7-8R mutation, which disrupts NF-κB activation but not CREB activation in reporter assays, did not disrupt IKK binding, IKK kinase activity, or RelA nuclear translocation.
Tax ubiquitylation mediates IKK binding and nuclear translocation of RelA
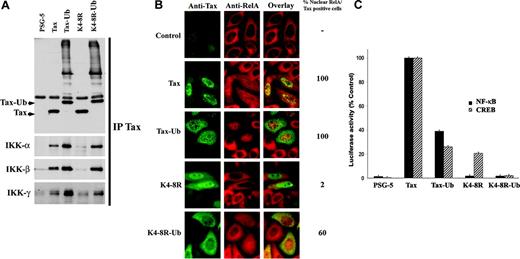
Since mutations of lysines 4 to 8 abolish Tax ubiquitylation, Tax binding to the IKK complex, and nuclear translocation of RelA, we directly assessed the role of Tax ubiquitylation in NF-κB activation. We used Tax-Ub constructs in which an ubiquitin moiety was fused to the carboxy-terminal end of Tax or Tax K4-8R. Blotting of anti-Tax–precipitated proteins with anti-Tax mab revealed mono- and poly-ubiquitylated proteins in HeLa cells transfected with Tax-Ub or K4-8R-Ub (Figure 3A). Importantly, while K4-8R failed to immunoprecipitate IKKs, K4-8R-Ub completely recovered this activity (Figure 3A), directly demonstrating that Tax binding to IKK is mediated by C-terminal Tax ubiquitylation. Consistently, immunofluorescence showed that K4-8R-Ub (60% of cells), but not K4-8R (2% of cells), induced RelA nuclear translocation (Figure 3B), reflecting Tax-induced IKK activation. These results directly demonstrate that ubiquitylation of the C-terminal part of Tax is essential for the formation of an active IKK complex and for RelA nuclear translocation.
However, in agreement with Peloponese et al,11 C-terminal ubiquitin fusion to Tax reduced both NF-κB and CREB activities in reporter assays. Consistently, Tax K4-8R-Ub fusion failed to activate both NF-κB and CREB reporters (Figure 3C). As CREB activation is insensitive to Tax ubiquitylation (Chiari et al10 and Figure 1), the reduced transcriptional activity of Tax-Ub might be due to altered protein conformation or reduced expression. Alternatively, additional Tax modifications, such as sumoylation, might be required for complete NF-κB activation, beyond RelA nuclear translocation.
Tax ubiquitylation mediates IKK binding and nuclear translocation of RelA. (A) HeLa cells were transfected with wild-type Tax, Tax-Ub, Tax K4-8R, or Tax K4-8R-Ub. After immunoprecipitation with anti-Tax mab, recovered proteins were blotted with anti-Tax, anti-IKKα, anti-IKKβ, or anti-IKKγ antibodies, as indicated. (B) HeLa cells were transfected as in panel A and stained by dual immunofluorescence with anti-Tax (green) and anti-RelA (red) antibodies. The percentage of nuclear translocation of RelA in Tax-positive cells is indicated. (C) Jurkat cells were cotransfected with the NF-κB-Luc (▪) or the HTLV-LTR-Luc (▨) reporters, renilla luciferase, and either Tax plasmid. Activity of the wild-type protein was set to 100%.
Tax ubiquitylation mediates IKK binding and nuclear translocation of RelA. (A) HeLa cells were transfected with wild-type Tax, Tax-Ub, Tax K4-8R, or Tax K4-8R-Ub. After immunoprecipitation with anti-Tax mab, recovered proteins were blotted with anti-Tax, anti-IKKα, anti-IKKβ, or anti-IKKγ antibodies, as indicated. (B) HeLa cells were transfected as in panel A and stained by dual immunofluorescence with anti-Tax (green) and anti-RelA (red) antibodies. The percentage of nuclear translocation of RelA in Tax-positive cells is indicated. (C) Jurkat cells were cotransfected with the NF-κB-Luc (▪) or the HTLV-LTR-Luc (▨) reporters, renilla luciferase, and either Tax plasmid. Activity of the wild-type protein was set to 100%.
Tax is sumoylated on lysines 7 and 8
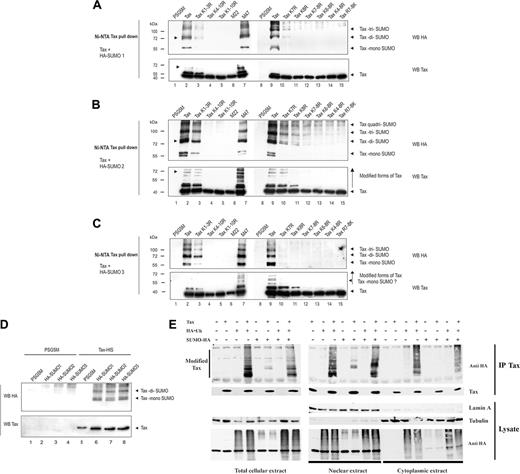
We then investigated whether Tax is sumoylated using 293T cells cotransfected with either Tax-His plasmids together with HA-tagged SUMO1, -2, or -3. Tax proteins were recovered following denaturing Ni-NTA pull-down as shown in Figure 1B. Tax blot indicates that Tax proteins were produced at comparable levels and shows various amounts of high-molecular-weight Tax species (Figure 4A-C, lower panels). No HA-reactive species were found in the absence of Tax expression (Figure 4A-C, upper panels), reflecting purification efficiency. HA-reactive products were readily detected in cells expressing wild-type Tax and either HA–SUMO-1, -2, or -3, formally demonstrating that Tax is modified by all 3 SUMOs. Molecular weights of conjugated Tax products were consistent with attachment of 1 to 3 SUMO monomers. Jurkat T cells were also cotransfected with Tax and either HA-SUMO plasmids. HA-reactive Tax products were found in Jurkat cells expressing either HA–SUMO-1, -2, or -3 (Figure 4D), demonstrating that Tax is also efficiently sumoylated in T lymphocytes.
In 293T cells, sumoylated species were detected for M47 but not M22, suggesting that sumoylation might regulate Tax-induced NF-κB activation. Mutation of C-terminal lysines (K4-10R) abolished Tax sumoylation, whereas mutation of N-terminal lysines (K1-3R) had a moderate effect. Combined mutation of K4-8R or K6-8R massively reduced Tax sumoylation. Importantly, combined mutation of K7-8R was sufficient to strongly reduce sumoylation (Figure 4A-C), but not to abrogate mono- and oligo-ubiquitylation (Figure 1B). However, reintroduction of K7 or K8 (R7-8K) was not sufficient to restore Tax sumoylation. These results indicate that, as for ubiquitylation, the C-terminal part of Tax is the major target for conjugation to SUMO and that lysines 7 and 8 are preferential sites, although other determinants in Tax are also required.
Tax is SUMO-conjugated in the nucleus. (A-D) 293T (A-C) or Jurkat (D) cells were transfected with the plasmids encoding for Tax-6His or Tax mutants in the presence of HA-SUMO1 (A,D), HA-SUMO2 (B,D), or HA-SUMO3 (C,D) plasmids. Proteins purified by denaturing Ni-NTA pull-down were blotted with anti-HA (top panel) or anti-Tax (bottom panel) mabs. (E) HeLa cells were cotransfected with Tax and either Ub-HA and/or HA-SUMO3 plasmids. After immunoprecipitation with anti-Tax mab, recovered proteins were blotted with anti-Tax or anti-HA antibodies. Cell fractionation was performed as described in “Materials and methods.” Total lysates (left panels), nuclear extracts (lamin positive, tubulin negative; middle panels), and cytoplasmic extracts (lamin negative, tubulin positive; right panels) are displayed. Lysates were blotted with anti-HA and antitubulin antibodies to ensure equal loading.
Tax is SUMO-conjugated in the nucleus. (A-D) 293T (A-C) or Jurkat (D) cells were transfected with the plasmids encoding for Tax-6His or Tax mutants in the presence of HA-SUMO1 (A,D), HA-SUMO2 (B,D), or HA-SUMO3 (C,D) plasmids. Proteins purified by denaturing Ni-NTA pull-down were blotted with anti-HA (top panel) or anti-Tax (bottom panel) mabs. (E) HeLa cells were cotransfected with Tax and either Ub-HA and/or HA-SUMO3 plasmids. After immunoprecipitation with anti-Tax mab, recovered proteins were blotted with anti-Tax or anti-HA antibodies. Cell fractionation was performed as described in “Materials and methods.” Total lysates (left panels), nuclear extracts (lamin positive, tubulin negative; middle panels), and cytoplasmic extracts (lamin negative, tubulin positive; right panels) are displayed. Lysates were blotted with anti-HA and antitubulin antibodies to ensure equal loading.
Tax sumoylation is involved in the formation of Tax nuclear bodies and transcriptional activation
Since Tax is modified by both ubiquitin and SUMO on the same target lysines, we investigated the relationship between these 2 modifications. HeLa cells were cotransfected with plasmids encoding for Tax together with HA-tagged Ub and/or HA-tagged SUMO3, and immunoprecipitated with the anti-Tax mab. In cells expressing Tax and either HA-Ub or HA-SUMO3, blotting with anti-HA antibodies confirmed the presence of either polyubiquitylated or sumoylated Tax products (Figure 4E). Nuclear fractions (lamin positive, tubulin negative) and cytoplasmic fractions (tubulin positive, lamin negative) were then prepared. Anti-HA immunoblots demonstrated the presence of both ubiquitylated and sumoylated Tax in the nuclear extracts, whereas the cytoplasmic extracts contained only ubiquitylated Tax. Cotransfection of Tax with both Ub and SUMO3 plasmids resulted in a marked reduction of ubiquitylated Tax in both total and cytoplasmic fractions, whereas sumoylated Tax increased in the nuclear fraction. These results suggest that sumoylation favors Tax targeting to the nucleus or stabilizes the nuclear form of Tax.
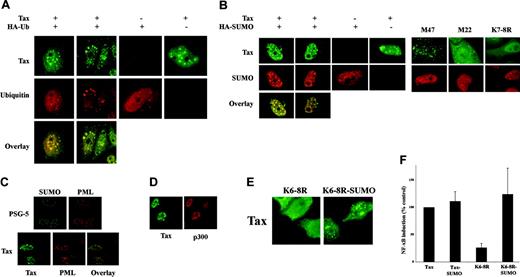
Immunofluorescence showed that cotransfection of Tax with HA-Ub significantly increased cytoplasmic Tax localization (from 30% to 54% of cells) without affecting the percentage of cells containing Tax nuclear bodies (> 90%). Tax strongly localized to nuclear bodies and perinuclear aggregates, together with a diffuse staining in the nucleoplasm and cytoplasm (Figure 5A). However, anti-HA antibodies decorated mostly perinuclear aggregates with an appreciable diffuse staining of the nucleoplasm and cytoplasm. Overlay demonstrated complete colocalization of Tax and ubiquitin within the perinuclear aggregates (31% of cells) and a diffuse overlap in the nucleoplasm, with distinct ubiquitin-free Tax nuclear bodies (94% of cells). In contrast, cotransfection of Tax with HA-SUMO3 (Figure 5B) revealed that Tax is present within nuclear bodies (96% of cells), together with a diffuse staining in the nucleoplasm and a very weak cytoplasmic staining. SUMO3 localized to the nucleus preferentially in nuclear bodies. Overlay revealed colocalization of SUMO3 in Tax nuclear bodies (63% of cells). In addition, Tax-free SUMO nuclear bodies were observed in both Tax and mock-transfected cells and likely represent promyelocytic leukemia (PML) nuclear bodies (Figure 5C). These data strongly argue for the specific targeting of sumoylated Tax to nuclear bodies, whereas ubiquitylated Tax can be encountered in a diffuse nuclear staining, as well as in the cytoplasm.
Tax colocalizes with RelA and p300 (Figure 2C and Figure 5D) in nuclear foci that have been proposed to play an important role in transcriptional activation.6 To investigate the respective role of ubiquitylation and sumoylation in RelA nuclear translocation and foci formation, we analyzed the localization of various mutants by immunofluorescence. As shown in Figure 2D, the NF-κB defective mutants M22 and K4-8R failed to induce RelA nuclear translocation, whereas the M47 mutant triggered both RelA nuclear translocation and nuclear body formation (Figure 2C). Importantly, the sumoylation-defective Tax K6-8R, K7-8R, and R7-8K mutants were able to induce RelA translocation (Figure 2C and Figure 5E), but were defective in recruiting SUMO or in forming nuclear bodies (< 1% of cells; Figure 2C and Figure 5B). We then examined directly the contribution of SUMO to Tax nuclear body formation. We found that SUMO fusion to K6-8R (K6-8R-SUMO) partially rescued nuclear body formation (Figure 5E) and significantly restored NF-κB transcriptional activation (Figure 5F). Thus, Tax sumoylation mediates both formation of Tax nuclear bodies and full NF-κB transcriptional activation.
Tax sumoylation is involved in the formation of Tax nuclear bodies and transcriptional activation. (A-B) HeLa cells were transfected with either Tax plasmid together with HA-Ub (A) or HA-SUMO3 (B), and stained by dual immunofluorescence with anti-Tax (green) or anti-HA (red) mabs. (C-D) HeLa cells were transfected with a control plasmid or the Tax plasmid and stained with anti-SUMO, anti-Tax (green), anti-PML, or anti-p300 (red) antibodies. (E) HeLa cells were transfected with Tax K6-8R or Tax K6-8R-SUMO1 plasmids and stained with anti-Tax antibodies. (F) Jurkat cells were cotransfected with the NF-κB-Luc reporter, renilla luciferase, and the plasmid encoding wild-type Tax, Tax-SUMO1, Tax K6-8R, or Tax K6-8R-SUMO1. Activity of the wild-type protein was set to 100%.
Tax sumoylation is involved in the formation of Tax nuclear bodies and transcriptional activation. (A-B) HeLa cells were transfected with either Tax plasmid together with HA-Ub (A) or HA-SUMO3 (B), and stained by dual immunofluorescence with anti-Tax (green) or anti-HA (red) mabs. (C-D) HeLa cells were transfected with a control plasmid or the Tax plasmid and stained with anti-SUMO, anti-Tax (green), anti-PML, or anti-p300 (red) antibodies. (E) HeLa cells were transfected with Tax K6-8R or Tax K6-8R-SUMO1 plasmids and stained with anti-Tax antibodies. (F) Jurkat cells were cotransfected with the NF-κB-Luc reporter, renilla luciferase, and the plasmid encoding wild-type Tax, Tax-SUMO1, Tax K6-8R, or Tax K6-8R-SUMO1. Activity of the wild-type protein was set to 100%.
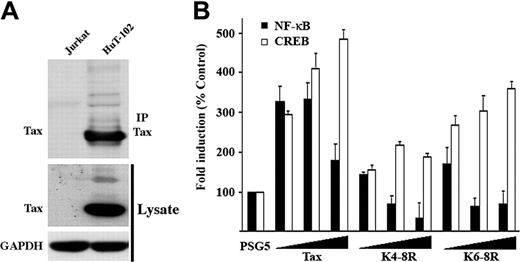
Endogenous Tax is ubiquitylated. (A) HuT-102 or Jurkat cells were either directly lysed in Laemli buffer or processed for immunoprecipitation with anti-Tax mab and blotting with anti-Tax sera. Lysates were also blotted with anti-GAPDH antibody. (B) C8166 cells were cotransfected with the NF-κB-LacZ (▪) or the HTLV-LTR-LacZ (□) reporter plasmids, and increasing doses of either Tax plasmid. The amount of transfected DNA was maintained at 3 μg using the PSG5 plasmid. Results correspond to β-gal activity after subtraction of the signal obtained in absence of LacZ plasmid and normalization to the amount of total proteins. Activity of the wild-type Tax protein was set to 100%.
Endogenous Tax is ubiquitylated. (A) HuT-102 or Jurkat cells were either directly lysed in Laemli buffer or processed for immunoprecipitation with anti-Tax mab and blotting with anti-Tax sera. Lysates were also blotted with anti-GAPDH antibody. (B) C8166 cells were cotransfected with the NF-κB-LacZ (▪) or the HTLV-LTR-LacZ (□) reporter plasmids, and increasing doses of either Tax plasmid. The amount of transfected DNA was maintained at 3 μg using the PSG5 plasmid. Results correspond to β-gal activity after subtraction of the signal obtained in absence of LacZ plasmid and normalization to the amount of total proteins. Activity of the wild-type Tax protein was set to 100%.
Endogenous Tax is ubiquitylated
Ubiquitylated Tax products can be easily detected in transfected 293T cells in the absence of proteasome inhibition, suggesting that they do not mediate a proteolytic function. Similarly, endogenously expressed reduced-mobility Tax forms were detected in HTLV-1–infected HuT-102 cells, in both total cell lysates and anti-Tax immunoprecipitates blotted with anti-Tax sera (Figure 6A). The protein species have the same electrophoretic mobility as the Tax-ubiquitin adducts detected in cotransfected cells, which suggests that endogenous Tax proteins undergo stable ubiquitylation that likely mediates a nonproteolytic function.
To confirm the regulatory role of lysine ubiquitylation or sumoylation in the functions of endogenous Tax, we investigated the ability of lysine-deficient Tax mutants to exert a dominant-negative effect on endogenous Tax produced by the HTLV-I–transformed C8166 T cells (Figure 6B). C8166 cells were cotransfected with either HIV-LTR-LacZ (NF-κB), or HTLV-LTR-LacZ (CREB) reporter plasmids together with increasing doses of Tax plasmids. NF-κB and CREB reporter activities of endogenous Tax (control transfection) was set as 100%. Tax transfection increased both NF-κB or CREB activation. In contrast, NF-κB activation was dose-dependently reduced in cells expressing the NF-κB defective TaxK4-8R or TaxK6-8R mutants, whereas no reduction was found for CREB. Such a selective dominant-negative effect of these 2 ubiquitin/SUMO-deficient Tax mutants strongly suggests that NF-κB activation by endogenous Tax is dependent on C-terminal lysines, even in the natural context of infected T lymphocytes.
Discussion
We provide direct evidence that Tax ubiquitylation on C-terminal lysines, particularly lysines 4 to 8, is mandatory for Tax binding to the IKK complex and nuclear translocation of RelA. Of note, Tax proteins mutated on K6, K7, and K8 were fully active on CREB-dependent promoters, ruling out a nonspecific role of lysine mutations linked to incorrect Tax conformation or localization. Furthermore, Tax K6-8R and K4-8R mutants exert a dominant-negative effect on NF-κB activation by endogenous Tax in HTLV-1–infected cells, strongly arguing for a critical role of C-terminal lysines in the ability of both transiently expressed and endogenous Tax to activate the NF-κB pathway.
Protein ubiquitylation controls NF-κB activation through both proteolytic and nonproteolytic mechanisms. NF-κB activation is mediated by the poly-ubiquitylation of phosphorylated IκB proteins followed by their proteasomal degradation,19 while IKK activation is mediated by phosphorylation-dependent monoubiquitylation of IKKβ.20 In addition, ATM-dependent ubiquitylation of IKKγ mediates NF-κB activation by genotoxic stress.21 However, our study is the first to show that ubiquitylation of an essential viral transactivator of the NF-κB pathway is a prerequisite for NF-κB activation, through facilitation of the formation of the IKK complex. In contrast with our observations, Peloponese et al11 reported that mono-ubiquitylation of Tax down-regulates its transcriptional function on both NF-κB and CREB reporters. As CREB activation is insensitive to Tax ubiquitylation10 (Figure 1), we believe that the reduced activity of Tax-Ub in reporter assays reflects an altered protein conformation due to ubiquitin fusion or a reduced expression.
We also demonstrate that Tax sumoylation mediates the formation of RelA-enriched Tax nuclear bodies and full NF-κB transcriptional activation. These nuclear bodies play an important transcriptional role in Tax-induced NF-κB activation through the recruitment of p300 to RelA.6 Similarly, sumoylation of PML or TCF-1 is required for the recruitment of partner proteins within nuclear bodies and for optimal transcriptional activation.22 In that respect, nonsumoylated Tax mutants (K6-8R, K7-8R, R7-8K) may promote RelA nuclear translocation, but not nuclear body formation, and are not fully active for NF-κB activation. Importantly, SUMO fusion to K6-8R mutant restores nuclear body formation and transcriptional activation, suggesting that sumoylation is implicated in the genesis of nuclear bodies.
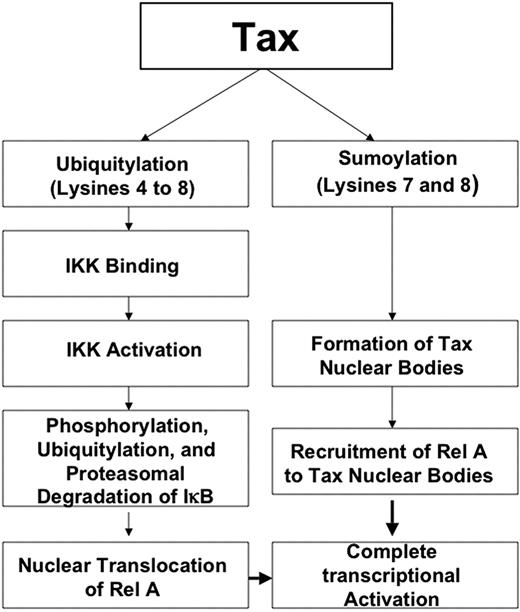
Proposed model for the role of Tax ubiquitylation and sumoylation in NF-κB activation.
Proposed model for the role of Tax ubiquitylation and sumoylation in NF-κB activation.
We demonstrated that ubiquitin and SUMO compete for the same target lysines on Tax. While competition of SUMO and ubiquitin for the same lysines accounts for inhibition of IκB-α degradation23 and plays a critical role in RAD6-dependent DNA repair,24 they appear in the case of Tax to take place in distinct subcellular compartments. Note that IKKγ, a key partner of Tax, is also present in a sumoylated form in the nucleus and in an ubiquitylated form in both the nucleus and the cytoplasm.21 IKKγ sumoylation results in its nuclear targeting, which allows its subsequent ATM-dependent ubiquitylation to ultimately activate IKK. These findings are strikingly similar to those identified here for Tax, suggesting that modifications by ubiquitin and SUMO might represent a general mode of regulation of the NF-κB pathway.
Taken together, our results converge toward a new model in which Tax is posttranslationally modified by ubiquitylation and sumoylation. Tax ubiquitylation mediates IKK complex binding, IKK activation, and RelA nuclear translocation, whereas Tax sumoylation mediates Tax nuclear body formation and final transcriptional activation (Figure 7). It remains to be demonstrated whether (1) the same molecule carries both modifications, (2) ubiquitylation is required for subsequent deubiquitination coupled with sumoylation, (3) ubiquitylation results in Tax localization to a subcellular region where sumoylation then occurs, or (4) molecules carry independent and competing modifications. In any case, our identification of a critical posttranslational control of Tax activation of the NF-κB pathway through a dual ubiquitin/SUMO targeting should allow further mechanistic dissection of Tax-dependent transformation, and raises the prospect of pharmacologic uncoupling of the NF-κB and the CREB pathways.
Prepublished online as Blood First Edition Paper, January 19, 2006; DOI 10.1182/blood-2005-09-3572.
Supported by the American University of Beirut University Research Board (URB) and Medical Practice Plan (MPP), the Lebanese Council for Scientific Research, and the Diana Tamari Sabbagh (DTS) Foundation, Beirut, Lebanon; the CNRS, Alliance des Recherches sur le Cancer (ARECA), HTLV European Research Network (HERN), and the Association pour la Recherche contre le Cancer (ARC) (no. 4781), Paris, France; the Fondation de France; and the Eli Lilly International Foundation, Hamburg, Germany. R.N. is the recipient of a grant from the Lady Tata Memorial Trust, London, United Kingdom. E.C. is the recipient of a grant from ARC.
R.N. and E.C. contributed equally to this work; M.E.-S. and R.M. contributed equally to this work; and C.P. and A.B. are both last authors of this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Françoise Bex and Isabelle Lamsoul (Brussels, Belgium) for the generous gift of Tax-Ub and Tax K4-8R-Ub plasmids and to Valérie Lallemand (Paris, France) for the help in the analysis of Tax sumoylation.