In aplastic anemia, immune destruction of hematopoietic cells results in bone marrow failure. Type 1 cytokines, especially IFN-γ, have been implicated in the pathophysiology of T-cell–mediated, Fas-mediated stem cell apoptosis of hematopoietic cells. Here, we show that the transcription factor T-bet (T-box expressed in T cells) is increased in T cells from patients with aplastic anemia. Patients' T-bet bound directly to the proximal site of the IFN-γ promoter without any prior stimulation, in contrast to healthy controls. Increased levels of Itk kinase participated in T-bet up-regulation and active transcription of the IFN-γ gene observed in these patients. Blocking PKC-θ, a kinase that lies downstream of Itk kinase, decreased T-bet protein and IFN-γ intracellular levels. These data suggest that the increased IFN-γ levels observed in aplastic anemia patients are the result of active transcription of the IFN-γ gene by T-bet. Blocking the transcription of the IFN-γ gene with kinase inhibitors might lead to the development of novel therapeutic agents for patients with aplastic anemia and other autoimmune diseases.
Introduction
Aplastic anemia, the paradigm of bone marrow failure syndromes, is characterized by peripheral blood pancytopenia and an empty bone marrow.1 In most cases, aplastic anemia is an immune-mediated disease with active destruction of hematopoietic cells by T lymphocytes.2 The aberrant immune response may be triggered by drugs, virus, or chemical exposure, but in the majority of cases there is no obvious etiologic factor.3,4 The clinical observations that most patients respond to immunosuppressive treatment with cyclosporine and antithymocyte globulin–based regimens5,6 is the most powerful evidence for the pivotal role of the immune system in the pathophysiology of aplastic anemia. Excessive production of interferon-γ (IFN-γ), tumor necrosis factor (TNF), and interleukin-2 (IL-2) from patients' T cells suggests that the hematopoietic cells are destroyed through a Th1 T-cell response.7-11 This Th1 “shift” in aplastic anemia results in both Fas-mediated cell death and inhibition of hematopoietic stem cell proliferation.2,12 Oligoclonal expansion of cytotoxic T lymphocytes (CTLs) correlates with disease activity.13,14 In an animal model of aplastic anemia, injection of alloreactive lymphocytes results in bone marrow failure, but pancytopenia can be prevented with anti–IFN-γ and anti-TNF monoclonal antibody.15
IFN-γ, the hallmark cytokine of the Th1 immune response, is produced primarily by T cells and natural killer (NK) cells. Following activation, naive T cells differentiate into Th1 CD4+ and cytotoxic CD8+ cells that secrete IFN-γ and other cytokines, and Th2 CD4+ cells that produce IL-4 and other cytokines. Two transcription factors are responsible for the shift of CD4+ T cells into the Th1 or Th2 phenotype: T-bet for Th1 and GATA-3 for Th2.16,17 IFN-γ is also produced when T cells are stimulated with IL-12 or IL-18 secreted by antigen-presenting cells (APCs). Regulation of IFN-γ production occurs primarily at the level of transcription.18 The proximal site of the IFN-γ gene (–75 to –45 bp of the IFN-γ promoter) is a binding site for nuclear factor for activated T cells (NFATs), AP-1, ATF, and CREB transcription factors.19,20 In the proximal IFN-γ promoter site, a half T-box sequence allows T-bet binding, resulting in increased IFN-γ production.21
T-bet is a member of the T-box family of transcription factors.22 This family contains a highly conserved DNA binding domain, the T-box. T-box binds to a specific sequence in the promoter of different genes. T-bet is found in Th1 but not in Th2 cells and is the key regulator of Th1 development and function.16,23 Mice lacking T-bet fail to develop Th1 cells and are driven toward Th2-mediated disease.24 Overexpression of T-bet in Th2 cells results in loss of the Th2 phenotype and increased production of IFN-γ.16 Activated T cells result in increased T-bet expression, which induces IL-12Rβ2 expression.25 T-bet also positively regulates its own expression through an autoregulator loop involving Hlx, a homeobox gene.26
T-cell engagement through the T-cell receptor (TCR) activates various protein kinases27 ; early in this cascade, the Itk of the Tec kinase family is stimulated and with other kinases is responsible for the downstream activation of other kinase members, including members of the PKC family. From this group, PKC-θ has a prominent role in T-cell activation, proliferation, and cytokine production.27,28 PKC-θ activates multiple transcription factors, including NF-κB and AP-1, which in turn bind to the promoter of different genes and promote their transcription. The selective expression of PKC-θ for T-cell activation and survival has suggested that blocking of PKC-θ could be a potential target for activated T cells in autoimmune diseases.29
Here, we show that T cells from patients with aplastic anemia display increased protein levels of T-bet compared with healthy controls; the increased IFN-γ production in aplastic anemia is the result of active IFN-γ gene transcription mediated by the binding of increased T-bet protein levels to the proximal site of the IFN-γ promoter. These effects are related to the activation of T-bet in part from Itk and PKC-θ; if PKC-θ is blocked, T-bet protein levels and intracellular IFN-γ levels return to normal.
Patients, materials, and methods
Patients and controls
After informed consent was obtained in accordance with the Declaration of Helsinki and using protocols approved by the institutional review board of the National Heart, Lung, and Blood Institute, heparinized peripheral blood samples were obtained from 28 patients with acquired aplastic anemia (age range, 10-79 years) who were treated at the Hematology Branch (Table 1). The diagnosis of aplastic anemia was based on the criteria of the International Agranulocytosis and Aplastic Anemia Study. Ten healthy volunteers served as controls (age range, 18-55 years). Marrow cytogenetics were normal in all cases and Fanconi anemia was excluded in children and younger adults. No patient was transfused immediately before blood sampling; the transfusion statusof all patients is shown in Table 1. TERC mutations were excluded in patient nos. 3, 4, 14, 16, 19, and 21, but a mutation was found in patient no. 9. Three patients with sickle cell disease (transfusion dependent), a patient with myelodysplastic syndrome (refractory anemia, transfusion dependent), 3 patients with refractory anemia with excess of blasts (RAEBs), and 2 patients with autoimmune hemolytic anemia (AIHA, transfusion dependent) served as secondary controls.
Lymphocyte isolation and stimulation conditions
Peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation with lymphocyte separation medium (Organon, Durham, NC), and T cells were separated subsequently by magnetic depletion of non-T cells, as recommended by the manufacturer (MACS Pan T cells isolation kit II; Miltenyi Biotec, Auburn, CA). Briefly, non-T cells (B cells, monocytes, NK cells, dendritic cells, erythroid cells, and platelets) from PBMCs were indirectly magnetically labeled using a biotin-conjugated cocktail of CD14, CD16, CD19, CD36, CD56, CD123, and glycophorin A and magnetic-activated cell sorter (MACS) microbeads coupled to antibiotin mAb. The magnetically labeled cells were depleted by retaining them on a MACS column in the magnetic field of miniMACS. The purified T cells were used immediately for the preparation of the extracts. Stimulation of T cells was performed using 10 ng/mL PMA and 0.5 μg/mL ionomycin.
Antibodies
Anti–T-bet monoclonal antibody (mAb), antiactin Ab, and the HRP-conjugated secondary Abs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Itk mAb and anti-STAT1 mAb were from Upstate Biotechnology (Lake Placid, NY).
Inhibitors
Calphostin C (0.05 μM) and rottlerin (30 μM) were used for the inhibition of PKC and PKC-θ, respectively.30,31 SB203580 (10 μM) and PD98059 (50 μM) were used for the inhibition of p38 pathway and the MEK/Erk (mitogen-activated protein/extracellular signal regulated kinase kinase) pathway, respectively.32,33 All inhibitors were from Calbiochem (La Jolla, CA). Where mentioned, freshly isolated T cells were incubated with the inhibitors for 30 minutes at 37°C (95% O2, 5% CO2) followed by stimulation with PMA and ionomycin for 24 hours or collected without any prior stimulation for preparation of the extracts.
Cytoplasmic and nuclear extracts
Freshly isolated T cells were used for the preparation of the extracts as previously described.30 Briefly, 3 to 5 million T cells were resuspended in buffer A (10 mM Hepes-KOH, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA) with a mixture of protease inhibitors (Santa Cruz Biotechnology) at 4°C. After adding 20 μL 10% nonidet-NP40, cells were centrifuged and the supernatant cytoplasmic extract was collected in a new tube. The nuclear pellet was resuspended in buffer B (20 mM Hepes-KOH, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA) with protease inhibitors and incubated for 10 minutes at 4°C. Following centrifugation, the supernatant nuclear extract was kept at –80°C until assayed. The protein content of the extracts was determined using the Micro BCA Protein Assay kit (Pierce, Rockford, IL).
Immunoblot and electrophoretic mobility shift assays (EMSAs)
Proteins (10 μg/lane) were resolved in 12% Tris-glycine SDS gels (Invitrogen, Carlsbad, CA); electrophoresis was performed at 125 V. Resolved proteins from the gel were transferred to polyvinylidene difluoride (PVDF) membranes (Invitrogen). The membrane was blocked for 2 hours with 5% BSA in PBS and 0.05% Tween-20 followed by incubation in primary Ab. Subsequently, membranes were incubated in HRP-conjugated secondary Ab, and detection of the bands of interest was performed using the enhanced chemiluminescence (ECL) plus system (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Membranes were stripped after the first blotting in ImmunoPure IgG elution buffer (Pierce), reblocked, and reblotted with another Ab. To evaluate equal loading of the lanes, membranes were reblotted with antiactin polyclonal Ab. Densitometry analysis of the bands of interest was performed using the ImageQuant analysis software from Amersham Biosciences (Piscataway, NJ).
The dsDNA probe of the proximal site of the IFN-γ promoter used was 5′-aaaacttgtgaaaatacgtaatcctcag-3′ and synthesized by Integrated DNA Technologies (Coralville, IA). EMSAs were performed as previously described.34 The protein-DNA complexes were visualized using phosphorimager Storm 860 and the ImageQuant software (Amersham Biosciences).
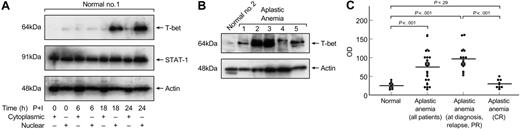
Increased T-bet levels in T cells from patients with aplastic anemia. (A) Cytoplasmic and nuclear extracts from healthy volunteers were analyzed in immunoblots for T-bet expression. T-bet could be detected only in the nucleus of normal T cells after at least 18 hours of stimulation with PMA and ionomycin. STAT1 served as a control to show differences in cytoplasmic-nuclear localization. Actin was used to show equal loading of the samples. (B) Unstimulated nuclear extracts from healthy volunteers and aplastic anemia patients were analyzed for T-bet expression. All patients' samples were run side-by-side with healthy donors' samples. Patients had significantly increased levels of T-bet compared with controls. (C) Densitometric intensity of immunoblots results from all the subjects studied. The results obtained from unstimulated T-cell T-bet expression between healthy individuals and aplastic anemia patients were highly significant (P < .001). Horizontal lines indicate mean values.
Increased T-bet levels in T cells from patients with aplastic anemia. (A) Cytoplasmic and nuclear extracts from healthy volunteers were analyzed in immunoblots for T-bet expression. T-bet could be detected only in the nucleus of normal T cells after at least 18 hours of stimulation with PMA and ionomycin. STAT1 served as a control to show differences in cytoplasmic-nuclear localization. Actin was used to show equal loading of the samples. (B) Unstimulated nuclear extracts from healthy volunteers and aplastic anemia patients were analyzed for T-bet expression. All patients' samples were run side-by-side with healthy donors' samples. Patients had significantly increased levels of T-bet compared with controls. (C) Densitometric intensity of immunoblots results from all the subjects studied. The results obtained from unstimulated T-cell T-bet expression between healthy individuals and aplastic anemia patients were highly significant (P < .001). Horizontal lines indicate mean values.
Flow cytometry
The expression of cell surface markers and IFN-γ was performed as previously described10 using the Fix and Perm cell permeabilization kit based on manufacturer's instructions (Caltag Laboratories, Burlingame, CA). Freshly isolated PBMCs from 10 patients and 5 healthy volunteers were examined using CD4-ECD–, CD8-PC5–(Beckman Coulter, Hialeah, FL), IL-12Rβ1-PE–, IL-12Rβ2-PE–, and IFN-γ-FITC–conjugated Abs (BD Biosciences, San Jose, CA). Isotype IgG1 PE–, FITC–, PC5–, and ECD–conjugated controls were from BD Biosciences. Analysis was performed with the FC500 Flow Cytometer (Beckman Coulter).
ELISA
PBMCs from 8 patients with aplastic anemia and 4 healthy volunteers were incubated for 24 hours in culture media without any stimulation. In vitro IFN-γ production was measured in culture supernatants by ELISA based on manufacturer's instructions (R&D Systems, Minneapolis, MN).
Statistical and densitometry analysis
Densitometry analysis of the bands of interest was performed using the ImageQuant analysis software from Amersham Biosciences. Statistical analysis was performed using the Mann-Whitney test.
Results
Increased T-bet protein in T cells from patients with aplastic anemia
T cells from patients with aplastic anemia overexpress IFN-γ.8,10 Regulation of IFN-γ production occurs primarily at the level of transcription, and T-bet has a central role in the transcriptional regulation of the IFN-γ gene.17,35 We first examined the expression of T-bet in normal T cells. Cytoplasmic and nuclear protein lysates were obtained from unstimulated cells and after 6, 18, 24, and 48 hours of stimulation with PMA and ionomycin from healthy volunteers, as described in “Patients, materials, and methods.” As shown in Figure 1A, T-bet was located in the nucleus of normal T cells and was not present in the cytoplasm. In healthy donor cells, T-bet expression was induced after 18 hours of stimulation, and maximal T-bet protein levels could be detected after 24 to 48 hours with PMA and ionomycin. STAT1 served as a control for differences in cytoplasmic-nuclear localization (Figure 1A); a polyclonal antibody against actin was used to show equal loading of the samples. Based on these data, we subsequently used only nuclear extracts for the immunoblot experiments for T-bet expression.
We next examined the expression of T-bet in nuclear protein lysates from aplastic anemia patients' T cells. From each nuclear protein lysate, 10 μg was separated electrophoretically on 12% Tris-glycine SDS gels and analyzed for T-bet expression. Nineteen (68%) of 28 patients had increased T-bet protein levels in unstimulated T cells compared with the absence of T-bet in all unstimulated healthy controls (Figure 1B). The densitometric intensities of immunoblot results from all the subjects studied are collectively presented in Figure 1C; the results obtained from unstimulated T-cell T-bet expression between healthy individuals and aplastic anemia patients were highly significantly different (P < .001); significant differences were detected in all patients when examined at diagnosis, partial remission, and relapse (P < .001). There was no difference in T-bet protein levels between controls and patients in complete remission (CR) (P = .29, Figure 1C).
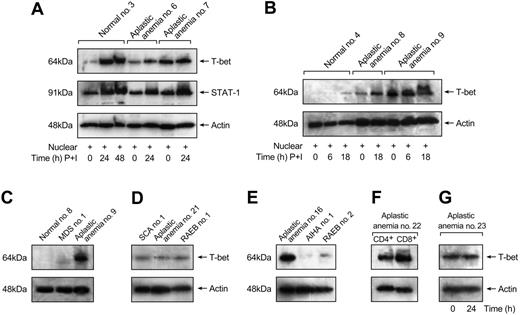
The kinetics of T-bet expression by T cells from patients with aplastic anemia and healthy individuals were also examined. As shown in Figure 2A-B, in patients with high and aberrant expression of T-bet in unstimulated T cells, stimulation did not affect T-bet protein levels; in patients with lower T-bet protein levels, stimulation induced further T-bet expression. The same blots were reblocked and reblotted with STAT-1 polyclonal Ab as a control (STAT-1 is a transcription factor that also induces IFN-γ transcription, and STAT1 expression is induced after stimulation). No differences in STAT-1 expression were detected between patients and controls (Figure 2A). Actin was used to show equal loading of the gel lanes.
Differences in T-bet expression from patients with aplastic anemia were compared with patients with other hematologic diseases who were transfusion dependent to exclude the possibility that our results were due to T-cell activation by transfusions. Patients with sickle cell anemia (n = 3, Figure 2D), a transfusion-dependent patient with myelodysplastic syndrome (Figure 2C), and patients with AIHA (n = 2, Figure 2E) showed undetectable amounts of T-bet protein in unstimulated T cells. Patients with RAEBs were also examined as secondary controls (n = 3). We did not detect any T-bet expression in unstimulated T cells from these patients (Figure 2D-E).
T-bet expression by separated CD4+ and CD8+ T cells from patients with aplastic anemia was studied. In patients with increased expression of T-bet (n = 3), CD8+ cells expressed more T-bet compared with CD4+ cells (Figure 2F). Aplastic anemia patients in complete remission (n = 2) had undetectable levels of T-bet in both CD4+ and CD8+ T cells (data not shown).
Stimulation induces T-bet expression in healthy controls but not in aplastic anemia patients. (A) Stimulation conditions with PMA and ionomycin did not have any effect on T-bet levels in patients with aberrant expression of T-bet (patient no. 7). In patients with lower T-bet levels, stimulation induced further T-bet expression (patient no. 6). No significant differences in STAT1 protein levels were detected between aplastic anemia patients and controls. (B) Healthy controls did not express T-bet in unstimulated T cells. T-bet expression was induced after stimulation with PMA and ionomycin. Stimulation conditions induced T-bet expression in patients with lower T-bet protein levels (aplastic anemia no. 8). (C-E) Patients with myelodysplastic syndrome (MDS), refractory anemia with excess of blasts (RAEBs), sickle cell anemia (SCA), and autoimmune hemolytic anemia (AIHA) showed undetectable T-bet levels in immunoblot experiments comparable with healthy controls and patients in remission (patient no. 21). (F) CD8+ T cells express increased T-bet levels compared with CD4+ T cells. (G) T-bet protein levels are not affected by in vitro maintenance after 24 hours without T-cell stimulation.
Stimulation induces T-bet expression in healthy controls but not in aplastic anemia patients. (A) Stimulation conditions with PMA and ionomycin did not have any effect on T-bet levels in patients with aberrant expression of T-bet (patient no. 7). In patients with lower T-bet levels, stimulation induced further T-bet expression (patient no. 6). No significant differences in STAT1 protein levels were detected between aplastic anemia patients and controls. (B) Healthy controls did not express T-bet in unstimulated T cells. T-bet expression was induced after stimulation with PMA and ionomycin. Stimulation conditions induced T-bet expression in patients with lower T-bet protein levels (aplastic anemia no. 8). (C-E) Patients with myelodysplastic syndrome (MDS), refractory anemia with excess of blasts (RAEBs), sickle cell anemia (SCA), and autoimmune hemolytic anemia (AIHA) showed undetectable T-bet levels in immunoblot experiments comparable with healthy controls and patients in remission (patient no. 21). (F) CD8+ T cells express increased T-bet levels compared with CD4+ T cells. (G) T-bet protein levels are not affected by in vitro maintenance after 24 hours without T-cell stimulation.
T-bet protein levels in aplastic anemia patients' cells were not affected by in vitro maintenance without T-cell stimulation; no difference in T-bet protein levels was observed after 24 hours (Figure 2G) or after 48 hours (data not shown).
These data indicated that T cells from patients with aplastic anemia had increased T-bet protein levels in the absence of stimulation compared with controls.
T-bet binds to the proximal site of the IFN-γ promoter in aplastic anemia T cells
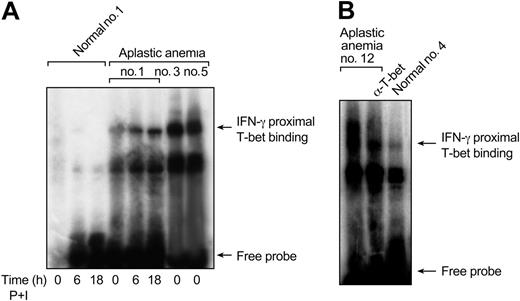
To determine whether increased protein levels of T-bet in aplastic anemia patients were functional, we performed EMSA experiments using an oligonucleotide that spans from –75 to –45 bp (proximal IFN-γ promoter site) on the human IFN-γ promoter. We examined nuclear extracts from unstimulated T cells from healthy individuals (n = 4) and patients (n = 8). There was significantly increased binding in nuclear extracts from unstimulated T cells from all patients examined; in contrast, binding from healthy individuals was undetectable in unstimulated nuclear extracts (Figure 3A-B). The binding observed in unstimulated protein lysates from patients was diminished in supershift experiments using an Ab against T-bet, indicating that binding was specifically due to T-bet (Figure 3B). Binding in nuclear extracts from normal T cells could be detected only after 24 to 48 hours of stimulation with PMA and ionomycin; binding could be completely inhibited when using excess unlabeled oligonucleotide, indicating specificity (data not shown). The binding of T-bet to the IFN-γ promoter in aplastic anemia patients correlated directly to T-bet protein levels (Figure 1A and Figure 3A [patient nos. 1, 3, and 5]).
Together, these experiments demonstrated that increased T-bet in aplastic anemia patients bound to the proximal site of the IFN-γ promoter without stimulation and could potentially drive the transcription of the IFN-γ gene, leading to increased IFN-γ production.
Increased T-bet protein levels correlate with increased intracellular IFN-γ levels and IL-12R in aplastic anemia patients
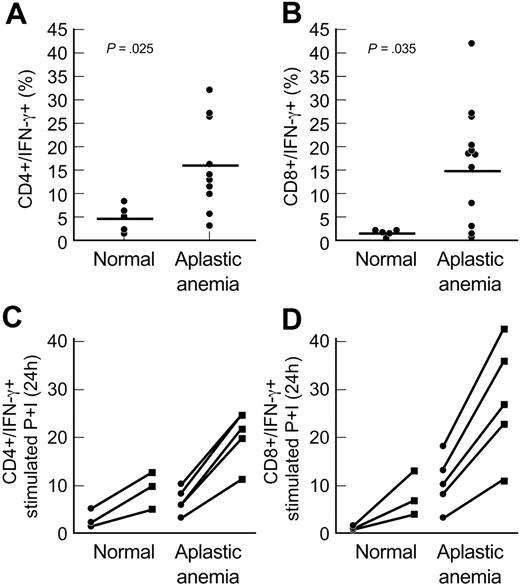
To further determine whether increased T-bet protein levels observed in aplastic anemia patients correlated directly with IFN-γ levels, we determined the intracellular levels of IFN-γ by flow cytometry. PBMCs from patients with aplastic anemia (n = 10) and healthy volunteers (n = 5) were permeabilized and stained with FITC-conjugated IFN-γ mAb and the cell surface fluorochrome-conjugated Abs, as described in “Patients, materials, and methods.” Unstimulated PBMCs from 8 of 10 aplastic anemia patients expressed increased levels of IFN-γ in CD4+ and CD8+ T cells, compared with healthy controls (Figure 4A-B). Increased expression of IFN-γ in aplastic anemia patients correlated with the increased T-bet protein levels observed in the same patients. PBMCs and separated CD4+ and CD8+ T cells from 5 patients with aplastic anemia and 3 healthy controls were examined for IFN-γ with PMA and ionomycin stimulation; all patients' samples showed increased levels of IFN-γ after stimulation compared with controls (P = .022 for CD4+/IFN-γ+, Figure 4C; P = .03 for CD8+/IFN-γ+, Figure 4D).
T-bet binds to the proximal site of the IFN-γ promoter in patients with aplastic anemia. (A) Nuclear extracts from healthy controls and patients were examined in EMSA experiments using an oligonucleotide that spans from –75 to –45 bp on the human IFN-γ promoter site (proximal IFN-γ promoter site). Unstimulated extracts from aplastic anemia patients revealed increased T-bet binding. In patients with lower T-bet protein levels (patient no. 1, Figure 1B) this binding was induced after stimulation. No binding could be detected in unstimulated nuclear extracts from healthy controls. (B) The binding observed in unstimulated extracts from aplastic anemia patients was decreased in supershift experiments using a T-bet mAb, indicating binding specificity.
T-bet binds to the proximal site of the IFN-γ promoter in patients with aplastic anemia. (A) Nuclear extracts from healthy controls and patients were examined in EMSA experiments using an oligonucleotide that spans from –75 to –45 bp on the human IFN-γ promoter site (proximal IFN-γ promoter site). Unstimulated extracts from aplastic anemia patients revealed increased T-bet binding. In patients with lower T-bet protein levels (patient no. 1, Figure 1B) this binding was induced after stimulation. No binding could be detected in unstimulated nuclear extracts from healthy controls. (B) The binding observed in unstimulated extracts from aplastic anemia patients was decreased in supershift experiments using a T-bet mAb, indicating binding specificity.
To further determine that increased IFN-γ levels in patients with aplastic anemia correlated with increased T-bet levels observed in the same cases, PBMCs from patients with aplastic anemia (n = 8) and healthy controls (n = 4) were examined for IFN-γ secretion in culture supernatants with ELISA; all patients' samples showed increased levels of IFN-γ compared with controls (healthy controls' mean IFN-γ levels ± SEM: 11.9 ± 1.334 pg/mL; aplastic anemia patients' mean IFN-γ levels ± SEM: 20.31 ± 1.049 pg/mL, P = .004; data not shown).
T-bet up-regulates IL-12Rβ2.23 IL-12Rβ2 participates in IFN-γ production through the transcription factor STAT4.25,36 We examined whether increased T-bet protein levels observed in aplastic anemia patients affected IL-12Rβ2 levels. Unstimulated PBMCs from 8 patients with aplastic anemia and 4 healthy controls were examined for IL-12Rβ1 and IL-12Rβ2 levels using flow cytometry. PBMCs from aplastic anemia patients did not show significant differences in IL-12Rβ1 or IL-12Rβ2 levels compared with controls, despite the increased IFN-γ levels observed in the same patients (data not shown). Next, we examined IL-12Rβ1 and IL-12Rβ2 levels in stimulated PBMCs (PMA and ionomycin) from patients with aplastic anemia (n = 5) and healthy controls (n = 3). There were no significant differences despite increased IFN-γ levels observed in the same patients after stimulation (P = .41 for CD4+/IL-12Rβ2+; P = .37 for CD8+/IL-12Rβ2+; data not shown).
Itk protein levels correlate with T-bet expression in T cells from patients with aplastic anemia
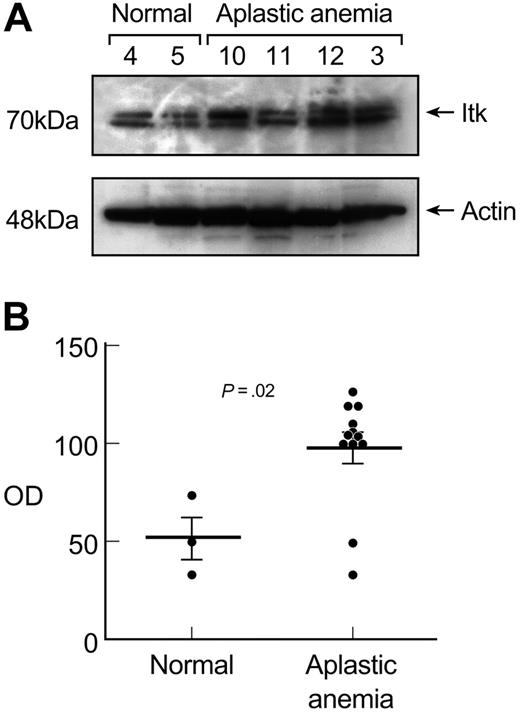
Itk is a Tec family kinase that can migrate from the cytoplasm to the nucleus; Itk participates in the inducible expression and activation of T-bet.35 Cytoplasmic extracts from unstimulated T cells from patients with aplastic anemia (n = 12) and healthy volunteers (n = 3) were examined by immunoblot for Itk expression. T cells from patients expressed increased Itk protein levels compared with healthy controls (P = .02; Figure 5A). The elevated Itk protein levels observed in patients correlated with the increased T-bet protein levels in the nuclear lysates from the same cases. Two patients who did not express T-bet protein in unstimulated T cells showed Itk protein levels at healthy control levels. The difference of the densitometric readings obtained from Itk protein expression in immunoblots between healthy individuals and aplastic anemia patients was significant (P = .02; Figure 5B).
Rottlerin inhibits T-bet and IFN-γ in aplastic anemia T cells
Downstream from Itk, PLC is activated and hydrolyzes inositol phospholipids into the second messengers IP3 and DAG.37 Subsequently, various kinases are activated, leading to the phosphorylation and activation of transcription factors, including T-bet, and therefore the transcription of multiple genes.38 PKC-θ, downstream of Itk in the signal transduction activation cascade in T cells, plays an important role in the activation of T cells and is the predominant PKC isoform in T cells.39,40 Since there is no commercially available inhibitor for Itk, we examined the effect of rottlerin, a PKC-θ inhibitor, on T-bet protein levels and compared rottlerin to other inhibitors: calphostin C (a PKC inhibitor), SB203580 (a p38 MAPK pathway inhibitor), and PD98059 (an Erk pathway inhibitor).
T-bet levels correlate with intracellular IFN-γ levels in aplastic anemia patients. (A-B) We examined the intracellular IFN-γ levels using flow cytometric analysis in PBMCs from aplastic anemia patients (n = 10) and healthy controls (n = 5). CD4+ and CD8+ T cells from aplastic anemia patients showed increased intracellular levels of IFN-γ compared with controls. The increased expression of intracellular IFN-γ in aplastic anemia patients correlated with increased T-bet protein levels observed in the same patients. The differences in the percentages of double-positive CD4+/IFN-γ+ and CD8+/IFN-γ+ cells between healthy controls and patients examined were significant (P = .025 and P = .035, respectively). (C-D) CD4+ and CD8+ T cells from aplastic anemia patients showed increased intracellular IFN-γ levels after stimulation for 24 hours with PMA and ionomycin (P+I). The graphs show the percent of CD4+/IFN-γ and CD8+/IFN-γ cells before (•) and after (▪) stimulation. The differences in stimulated cells were also significant. Horizontal lines represent mean values.
T-bet levels correlate with intracellular IFN-γ levels in aplastic anemia patients. (A-B) We examined the intracellular IFN-γ levels using flow cytometric analysis in PBMCs from aplastic anemia patients (n = 10) and healthy controls (n = 5). CD4+ and CD8+ T cells from aplastic anemia patients showed increased intracellular levels of IFN-γ compared with controls. The increased expression of intracellular IFN-γ in aplastic anemia patients correlated with increased T-bet protein levels observed in the same patients. The differences in the percentages of double-positive CD4+/IFN-γ+ and CD8+/IFN-γ+ cells between healthy controls and patients examined were significant (P = .025 and P = .035, respectively). (C-D) CD4+ and CD8+ T cells from aplastic anemia patients showed increased intracellular IFN-γ levels after stimulation for 24 hours with PMA and ionomycin (P+I). The graphs show the percent of CD4+/IFN-γ and CD8+/IFN-γ cells before (•) and after (▪) stimulation. The differences in stimulated cells were also significant. Horizontal lines represent mean values.
Itk protein levels correlate with T-bet expression in aplastic anemia patients. (A) Cytoplasmic extracts from unstimulated T cells from patients with aplastic anemia (n = 12) and healthy controls (n = 3) were examined in immunoblot experiments for Itk expression. T cells from patients revealed increased Itk protein levels compared with healthy controls. The elevated Itk protein levels correlated with the increased T-bet protein levels observed in the same patients. Patient no. 11, a patient in remission, showed Itk protein levels comparable with healthy controls and did not express any T-bet in unstimulated T cells (data not shown). (B) Densitometric intensity of all the subjects studied is shown. The differences in Itk protein levels expressed in aplastic anemia patients and controls in unstimulated T cells were significant (P = .02). Horizontal lines represent mean values.
Itk protein levels correlate with T-bet expression in aplastic anemia patients. (A) Cytoplasmic extracts from unstimulated T cells from patients with aplastic anemia (n = 12) and healthy controls (n = 3) were examined in immunoblot experiments for Itk expression. T cells from patients revealed increased Itk protein levels compared with healthy controls. The elevated Itk protein levels correlated with the increased T-bet protein levels observed in the same patients. Patient no. 11, a patient in remission, showed Itk protein levels comparable with healthy controls and did not express any T-bet in unstimulated T cells (data not shown). (B) Densitometric intensity of all the subjects studied is shown. The differences in Itk protein levels expressed in aplastic anemia patients and controls in unstimulated T cells were significant (P = .02). Horizontal lines represent mean values.
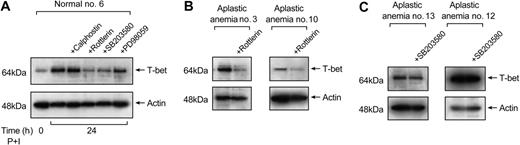
Normal T cells were treated with the inhibitors (as described in “Patients, materials, and methods”) prior to stimulation with PMA and ionomycin. Nuclear lysates (5 μg) prepared from T cells treated with inhibitors were examined for T-bet expression. Figure 6A is representative of 5 different experiments performed. Rottlerin decreased T-bet protein levels by about 50%, suggesting PKC-θ's role in T-bet activation in normal T cells. SB203580 and PD98059 decreased T-bet protein levels by 35% and 10%, respectively; calphostin did not affect T-bet protein levels significantly (Figure 6A).
Next, we examined whether rottlerin affected T-bet expression in T cells from patients with aplastic anemia. Rottlerin treatment decreased T-bet protein levels by 50%, compared with T-bet levels observed in untreated aplastic anemia T cells (n = 3) (Figure 6B). SB203580 resulted in a 15% (mean) decrease in T-bet protein levels in unstimulated T cells from patients with aplastic anemia (n = 3) (Figure 6C).
PKC-θ participates in the inducible expression of T-bet. (A) Calphostin C (0.05 μM) and rottlerin (30 μM) were used for the inhibition of PKC and PKC-θ, respectively. SB203580 (10 μM) and PD98059 (50 μM) were used for the inhibition of the p38 and the MEK/Erk kinase pathway, respectively. Normal T cells were treated with these inhibitors prior to stimulation with PMA and ionomycin followed by preparation of nuclear extracts. The nuclear extracts were used in immunoblot experiments for T-bet expression. In normal T cells, calphostin did not have any effect on T-bet expression, while rottlerin inhibited T-bet protein levels by 50%. SB203580 and PD98059 decreased T-bet levels by 35% and 10%, respectively. The results shown here are representative of 5 different experiments performed. (B) When T cells from aplastic anemia patients were treated with rottlerin, T-bet expression was decreased by 50%. (C) SB203580 showed a mean 15% decrease in T-bet protein levels in aplastic anemia patients.
PKC-θ participates in the inducible expression of T-bet. (A) Calphostin C (0.05 μM) and rottlerin (30 μM) were used for the inhibition of PKC and PKC-θ, respectively. SB203580 (10 μM) and PD98059 (50 μM) were used for the inhibition of the p38 and the MEK/Erk kinase pathway, respectively. Normal T cells were treated with these inhibitors prior to stimulation with PMA and ionomycin followed by preparation of nuclear extracts. The nuclear extracts were used in immunoblot experiments for T-bet expression. In normal T cells, calphostin did not have any effect on T-bet expression, while rottlerin inhibited T-bet protein levels by 50%. SB203580 and PD98059 decreased T-bet levels by 35% and 10%, respectively. The results shown here are representative of 5 different experiments performed. (B) When T cells from aplastic anemia patients were treated with rottlerin, T-bet expression was decreased by 50%. (C) SB203580 showed a mean 15% decrease in T-bet protein levels in aplastic anemia patients.
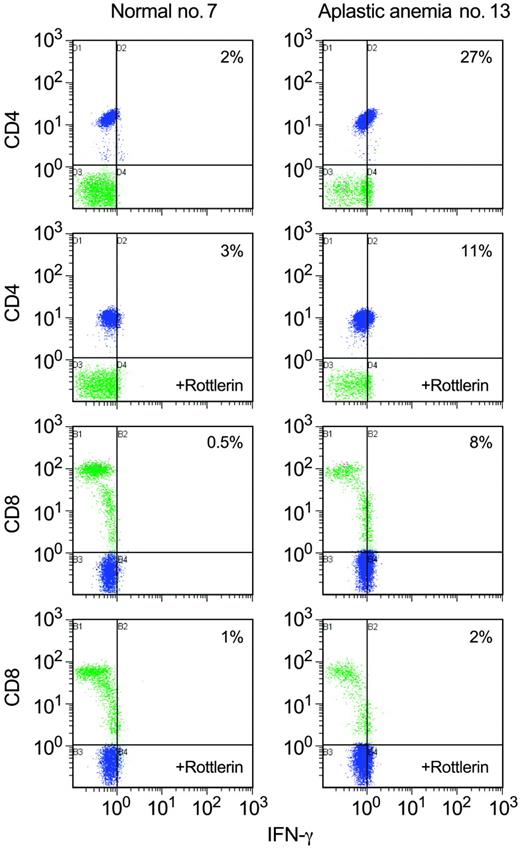
Rottlerin decreases IFN-γ levels in aplastic anemia T cells. We examined the effect of rottlerin on intracellular IFN-γ levels using flow cytometric analysis in PBMCs from aplastic anemia patients (n = 4). In patients with increased IFN-γ levels (3 of 4 patients examined), rottlerin diminished IFN-γ by approximately 50%. A representative experiment of all patients and controls examined is shown. Blue indicates CD4+; green, CD8+.
Rottlerin decreases IFN-γ levels in aplastic anemia T cells. We examined the effect of rottlerin on intracellular IFN-γ levels using flow cytometric analysis in PBMCs from aplastic anemia patients (n = 4). In patients with increased IFN-γ levels (3 of 4 patients examined), rottlerin diminished IFN-γ by approximately 50%. A representative experiment of all patients and controls examined is shown. Blue indicates CD4+; green, CD8+.
We also determined the effect of rottlerin on IFN-γ levels. PBMCs from patients (n = 4) were treated with rottlerin and examined for intracellular IFN-γ levels by flow cytometry. In patients with increased IFN-γ levels (3 of 4 patients studied), rottlerin diminished IFN-γ by approximately 50% (Figure 7); in the patient with low intracellular IFN-γ levels, rottlerin had no effect on IFN-γ levels (data not shown).
T-bet protein levels correlate with disease activity
We analyzed the relationship among disease status and treatment modalities in aplastic anemia patients and T-bet protein levels as determined by immunoblots (Table 1). Patients in complete remission (n = 9) did not express T-bet in their unstimulated T cells. All other patients examined (n = 19) were either sampled at first diagnosis, had been previously treated and subsequently relapsed, or were in partial remission; they all expressed increased levels of T-bet (Figure 1C).
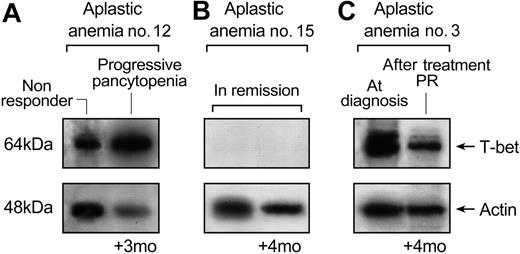
In 6 cases, T-bet protein levels were measured 3 to 4 months later, after the first sampling. In patients whose disease status was unchanged, there were no differences in T-bet expression. In patients who had responded to treatment, T-bet levels were lower (Figure 8). Patient no. 12, who relapsed when first studied, by 3 months had progressive pancytopenia, and T-bet levels were elevated at both time points (Figure 8A). Patient no. 15 was in remission and continued in remission 4 months later; no T-bet could be detected at either occasion (Figure 8B). Patient no. 3 was at diagnosis when there was high expression of T-bet; 4 months later, response to treatment was associated with lower levels of T-bet (Figure 8C).
Discussion
Laboratory studies and clinical observations suggest that aplastic anemia is an immune-mediated disease.2,4 The development of an autoimmune disease is a multistep process; genetic, environmental, and immunoregulatory factors affect different cell types and contribute to the clinical expression of a complex disease. Alterations in T-cell function and cytokines have a central role in these diseases, including aplastic anemia. The increased T-bet and IFN-γ levels observed in circulating aplastic anemia lymphocytes, without the requirement for in vitro stimulation, might represent endogenous stimulation in aplastic anemia T cells and support the Th1/Tc1 autoimmune basis of this rare and complex disease.10,41 In this study, we provide evidence of the molecular mechanism for the Th1/Tc1 immune response; increased T-bet protein levels in T cells likely are responsible for the increased IFN-γ levels and the Th1 shift in aplastic anemia patients.
T-bet protein levels correlate with disease activity. Patients were re-examined for T-bet levels 3 to 4 months after they were first examined for T-bet expression. In patients whose disease status was unchanged, there were no differences in T-bet expression. In patients who had responded to treatment, T-bet levels were lower. (A) Patient no. 12, who relapsed when first studied, by 3 months had progressive pancytopenia, and T-bet levels were elevated at both time points. (B) Patient no. 15 was in remission and continued in remission 4 months later; no T-bet could be detected at either occasion. (C) Patient no. 3 was at diagnosis when there was high expression of T-bet; 4 months later, response to treatment was associated with lower levels of T-bet.
T-bet protein levels correlate with disease activity. Patients were re-examined for T-bet levels 3 to 4 months after they were first examined for T-bet expression. In patients whose disease status was unchanged, there were no differences in T-bet expression. In patients who had responded to treatment, T-bet levels were lower. (A) Patient no. 12, who relapsed when first studied, by 3 months had progressive pancytopenia, and T-bet levels were elevated at both time points. (B) Patient no. 15 was in remission and continued in remission 4 months later; no T-bet could be detected at either occasion. (C) Patient no. 3 was at diagnosis when there was high expression of T-bet; 4 months later, response to treatment was associated with lower levels of T-bet.
In aplastic anemia, the pathogenetic Vb T-cell clones and increased IFN-γ levels correlate with disease activity.10,13 In the current work, T-bet levels also were related to disease activity. Patients with active disease expressed significantly increased T-bet protein levels, whereas patients in remission showed undetectable amounts of T-bet protein levels in unstimulated T cells, as did healthy controls. Whether the increased T-bet levels in patients correlate with the CDR3 skewing is to be established.
Our results are consistent with findings in other autoimmune diseases.42 In patients with inflammatory bowel disease,43 T-bet is up-regulated and appears to control the mucosal cytokine balance and be associated with clinical disease, suggesting a pivotal role of T-bet in the pathogenesis of T-cell–mediated colitis. In active Behçet disease,44 T-bet is also up-regulated. In a systemic lupus erythematosus murine model, the absence of T-bet led to decreased autoantibody production and decreased immune-mediated renal disease.45 Similarly, T-bet–deficient mice are characterized by overproduction of Th2 cytokines and an asthma phenotype.22 Patients with type 1 diabetes, a Th1-mediated disease, have polymorphisms in the T-bet gene.46 These findings suggest that T-bet has a central role in the development of autoimmune diseases.
In normal T cells, upon TCR engagement, there is a rapid activation of Lck and Zap-70, which in turn phosphorylate LAT and SLP-76. The latter serve as a platform for the recruitment of molecules into a signaling complex, including Tec kinases.47 From the Tec family of kinases, Itk appears to have the most important role for the activation of PLC-γ.48 This leads to the activation of protein kinase C and the Ras/MAPK pathway kinases, which in turn activate multiple transcription factors. Itk-deficient T cells show defective transcriptional regulation of the IL-2 gene through NFAT and AP-1, leading to decreased IL-2 production.49 Itk participates in the activation of T-bet.35 Itk, along with Vav1 kinase, is required for cell polarization and recruitment of PKC-θ upon TCR stimulation.50,51 Based on these observations, and because T-bet up-regulation in autoimmune diseases is unexplained, we examined the Itk levels in aplastic anemia patients. We speculated that the increased T-bet level observed in patients is active since it bound to the IFN-γ promoter without prior stimulation. In an attempt to identify kinases that activate T-bet, we showed that Itk is increased only in patients with increased T-bet levels. We also show for the first time that PKC-θ, a kinase involved in the interferon responses in T cells,52 participates also in the inducible expression of T-bet.
Alterations in other transcription factors in aplastic anemia cannot be excluded and may also contribute to the increased IFN-γ levels. The oligonucleotide used in our EMSA experiments includes the binding sites of other transcription factors. In our supershift experiments, the binding observed was significantly decreased by a T-bet mAb, but residual binding was still detected (Figure 3B); kinases that are responsible for T-bet activation could potentially activate other transcription factors, and these transcription factors could contribute to the increased transcription of the IFN-γ gene. Additionally, alterations in the methylation or acetylation status of the IFN-γ promoter or differential expression of corepressors (such as mSin3a) could also play role in increased T-bet binding.21
Although we could not detect any significant differences on IL-12Rβ2 expression between aplastic anemia patients and healthy controls, despite significant differences in intracellular IFN-γ and T-bet protein levels, alterations in other members of the IL-12 family (especially IL-23 and IL-27) cannot be excluded.53 The small number of patients examined might not allow determination of significant difference, or immunosuppressive treatment received by some patients may have compensated for the effects of T-bet on IL-12Rβ2. The small differences observed of IL-12Rβ1 and IL-12Rβ2 levels may be of biologic significance but further analysis is needed.
In summary, these data show that T-bet, the key regulator of Th1 development and function, is aberrantly expressed in aplastic anemia T cells, correlated with increased IFN-γ production. One possible explanation for our results is an intrinsic defect in the TCR, which could be the first step in altered signal transduction, increased IFN-γ production, and subsequent inhibition of hematopoietic stem cell proliferation as observed in aplastic anemia patients. Blocking PKC-θ and subsequently down-regulation of T-bet, and probably down-regulation of other transcription factors regulated by PKC-θ, may be important in the control of altered T-cell expression in an immune-mediated disease. The paradigm of imatinib, the ABL kinase inhibitor first introduced in the treatment of chronic myeloid leukemia, was rapidly extended to the development of a variety of new kinase inhibitors for human malignancies with promising results.29,54,55 Whether PKC-θ inhibitors may also have a role as therapeutic agents remains to be established. Since T-bet is related directly to the disease activity, T-bet levels can be used as indicators of active disease. Additional studies are needed to understand the pathways responsible for the aberrant T-bet/IFN-γ expression that could possibly lead to the development of novel therapeutic agents for the treatment of aplastic anemia and other autoimmune diseases.
Prepublished online as Blood First Edition Paper, January 24, 2006; DOI 10.1182/blood-2005-10-4201.
Supported by the Intramural Research Program of the NIH and a bursary award from the Aplastic Anemia and Myelodysplastic Syndrome International Foundation awarded to E.E.S.
E.E.S. and N.S.Y. conceived the study, designed the experimental approach, interpreted the data, and wrote the report. Under the supervision of N.S.Y., E.E.S. was responsible for conduct of the study, execution of experiments, and data collection and analysis. K.K. provided technical support in flow cytometry experiments. All investigators contributed to critical revision of the report.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all patients and healthy controls for donating blood samples. We thank our research nurses Olga Nunez and Barbara Weinstein for collection of the samples, Faith Williams for help in preparing the figures, and our lab manager Spencer Green.