Comment on Secchiero et al, page 4122
In this issue, Secchiero and colleagues show that most CLL B cells are sensitive to the orally bioavailable drug nutlin-3, with synergy for killing leukemic B cells when combined with traditional chemotherapeutic agents.
p53 plays a protective role in normal somatic tissues by removing and/or preventing propagation of damaged cells.1 Its growth-suppressive and proapoptotic activity is a powerful weapon against cancer cells that have retained the functionality of the p53 pathway. In contrast, p53 is a potent growth-suppressive and proapoptotic molecule that could harm normal proliferating cells if left uncontrolled. Therefore, the cellular level and activity of p53 are subjected to a rigid control system both under normal physiologic conditions and during stress. p53 is a short-lived protein, and its cellular level is regulated primarily by degradation via the ubiquitin-proteasome pathway. A growing number of cellular proteins have been implicated in the regulation of p53 stability, including the so-called murine double minute-2 gene (MDM2). MDM2 appears to function as a “master regulator” of p53.2 Its essential role in controlling p53 stability and activity is supported by the fact that genetic disruption of the MDM2 gene in mice is embryonic lethal but can be rescued by concomitant disruption of the p53 gene.3 Could restoration of p53 function by antagonizing MDM2 be considered a feasible approach in the treatment of this leukemia?
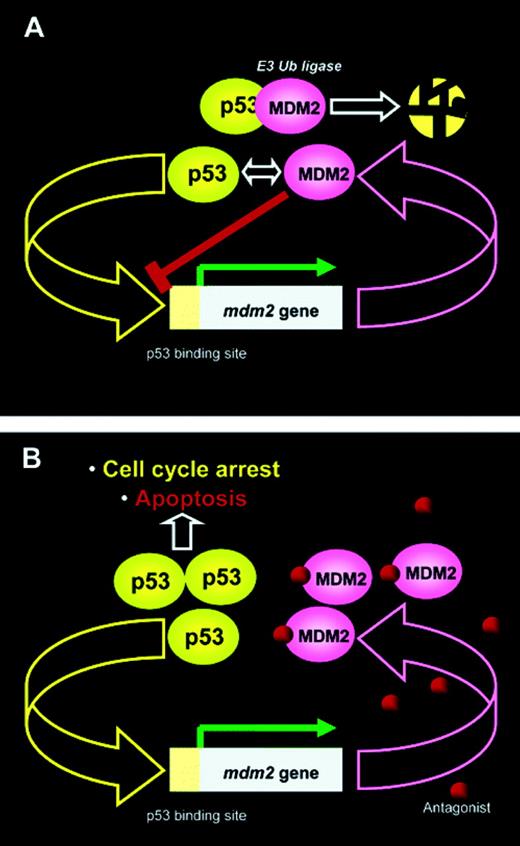
MDM2 regulates p53 via an autoregulatory feedback loop in which both proteins mutually control their cellular level. MDM2 protein has multiple options for abrogating p53 function, including blocking access of p53 to basal transcription molecules, preventing its C-terminal acetylation, and inducing p53 ubiquitination (see figure for more detail). Several classes of low-molecular-weight inhibitors of the p53-MDM2 interaction have been reported that can disrupt the binding between the 2 proteins. Recently, the first potent and selective small-molecule antagonists of MDM2 have been developed.4 These molecules, termed nutlins, have shown the ability to activate the p53 pathway in vitro and in vivo. Nutlins represent a class of cis-imidazoline analogues that bind to the p53 pocket on the surface of MDM2 in an enantiomer-specific manner. MDM2 interacts through its 100-residue N-terminal domain with the N-terminal transactivation domain of p53 (residues 1-75). This protein-protein dialogue inhibits the p53-MDM2 interface by mimicking the interaction of the 3 critical p53 amino acid residues within the hydrophobic cavity of MDM2. Of importance, this interaction, while blocking MDM2-mediated inhibition of p53, does not interfere with p53 function and has little toxicity in animal models. Thus, it would be anticipated that nutlin can activate the p53 pathway with resultant antitumor effects. This has been shown now for a wide variety of human cancer cell lines; however, the antitumor activity is greater in overexpressing MDM2 cell lines (reviewed in Vassilev4 ).FIG1
p53-MDM2 autoregulatory circuit. (A) MDM2 and p53 mutually regulate their levels in unstressed proliferating cells. MDM2 expression is controlled by a p53-dependent promoter and increases when the level of p53 rises. In turn, MDM2 binds p53 and inhibits its transcriptional activity by blocking the transcriptional activation domain of the transcription factor. MDM2 also serves as the E3 ubiquitin ligase for p53 and facilitates its ubiquitin-dependent degradation in the proteasome. As a result, both p53 and MDM2 are kept at very low levels in proliferating cells. (B) Small-molecule antagonists of MDM2 that can bind to the p53 pocket on the surface of the molecule will inhibit the p53-MDM2 interaction and release p53 from negative control. p53 will stabilize, accumulate in cell nuclei, and activate the p53 pathway. Reproduced with permission from J Med Chem. 2005,48,4491-4499. Copyright 2005 Am. Chem. Soc.
p53-MDM2 autoregulatory circuit. (A) MDM2 and p53 mutually regulate their levels in unstressed proliferating cells. MDM2 expression is controlled by a p53-dependent promoter and increases when the level of p53 rises. In turn, MDM2 binds p53 and inhibits its transcriptional activity by blocking the transcriptional activation domain of the transcription factor. MDM2 also serves as the E3 ubiquitin ligase for p53 and facilitates its ubiquitin-dependent degradation in the proteasome. As a result, both p53 and MDM2 are kept at very low levels in proliferating cells. (B) Small-molecule antagonists of MDM2 that can bind to the p53 pocket on the surface of the molecule will inhibit the p53-MDM2 interaction and release p53 from negative control. p53 will stabilize, accumulate in cell nuclei, and activate the p53 pathway. Reproduced with permission from J Med Chem. 2005,48,4491-4499. Copyright 2005 Am. Chem. Soc.
More recently, nutlin-3 has been shown to induce apoptosis in hematologic malignancies including acute myeloid leukemia and myeloma cell lines.5,6 In the latter case, nutlin-3 was shown to be cytotoxic even when myeloma cells were being sustained by the presence of stromal cells. This advantage was also noted in the context of little apparent damage to stromal cells by exposure to nutlin-3. A recent study has also found that chronic lymphocytic leukemia (CLL) B cells exposed to nutlin-3 generated p53 pathway activation and concomitantly induction of apoptosis in cells with wild-type p53, but not mutant p53.7 This study also found that nutlin-3 was synergistic with several commonly used drugs in CLL: chlorambucil and fludarabine.
Secchiero and colleagues add to this work in CLL by also finding that nutlin-3 can induce apoptosis in almost 100% of CLL B-cell clones tested. Intriguing aspects of this study were that not only were p53 protein levels increased but also that gene profiling after nutlin exposure revealed the up-regulation of several p53-responsive genes. In addition, the authors found, as did an earlier report,7 that this drug synergizes with both chlorambucil and fludarabine. This is particularly relevant, as our current paradigm in treating CLL patients is that combination therapies are required for the most effective clinical responses.
What kind of toxicity profiles are to be expected with the use of nutlinlike drugs in CLL? In the earlier CLL study,7 it was found that blood T cells are not as susceptible to killing by nutlin-3 as B-CLL cells, suggesting relatively low toxicity toward the immune system, a very welcome attribute in CLL therapy. Further encouraging findings in the study by Secchiero et al are that despite induction of p53 in normal lymphocytes, there was less cytotoxicity for other cells including bone marrow and CD34+ cells. Thus, nutlin-3 has a growing array of positive attributes; it can be used orally, penetrates cell membranes, is effective preclinically at very low doses (100-300 nM), does not have a high level of toxicity, and synergizes with traditional chemotherapies.
It is important to remember that nutlin-3 should be effective only in leukemic cells that possess a functional p53 pathway. In CLL, the structural changes in p53 required to inactivate p52 are usually rare and more often late in the disease. An additional point is that induction of apoptosis by p53 is complex, and therefore, is a likely target for inactivation in tumor cells, which could obviate any significant clinical impact. Despite these caveats, the recent work on nutlin-3 seems almost too good to be true, and we await the results of clinical trials with this agent in CLL as well as other hematologic malignancies. ▪