Comment on Barosi et al, page 2849
“The illness which he'd been smitten should have been analyzed when caught something like spleen that scourge of Britain or Russia's chondria for short”
—Alexander Pushkin
Eugene Onegin (1823), stanza 38
For nearly 150 years, the disease myelofibrosis with myeloid metaplasia, or equivalently agnogenic myeloid metaplasia, has been known by at least 45 different names, each reflecting the anatomic or hematologic findings of the observer. Like Pushkin, nearly all the writers made reference to the spleen. And as the blind man palpating an elephant (heaven forbid!), the terms were descriptive but didn't make hematologic sense. Then William Dameshek described this illness as 1 of 4 myeloproliferative diseases which include polycythemia vera, essential thrombocythemia, chronic myeloid leukemia, and agnogenic myeloid metaplasia.1 The phenotypic characteristics of the diseases of those patients are striking and similar—they have a leukocythroblastic peripheral blood smear, splenomegaly, and varying degrees of fibrosis in the marrow, terminating with variable frequency in acute leukemia. The latter is perhaps abetted by the unintentional consequences of well-intentioned nonspecific therapy, which almost always included chemotherapy of one type or another.FIG1
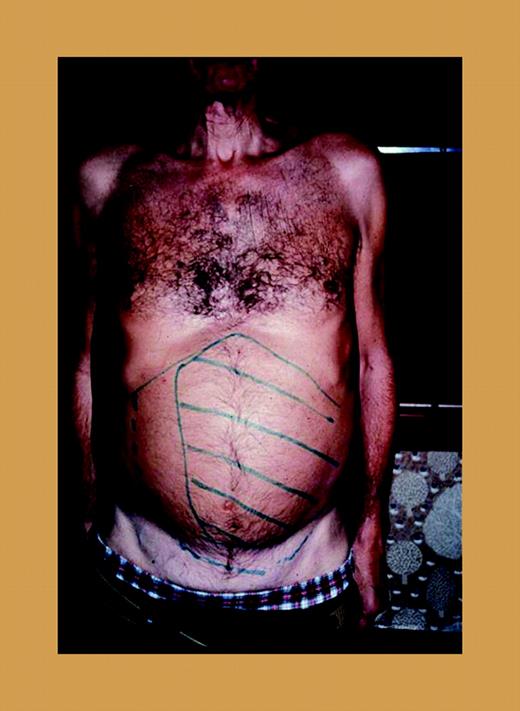
A patient with marked splenomegaly, agnogenic myeloid metaplasia (myelofibrosis with myeloid metaplasia).
A patient with marked splenomegaly, agnogenic myeloid metaplasia (myelofibrosis with myeloid metaplasia).
Within the past 5 years, significant advances have been made in treatment of these illnesses as the diagnostic characteristics have become better defined. So far, the zenith has been reached, of course, in chronic myeloid leukemia, where not only is the disease specifically defined by cytogenetic and molecular abnormalities, but targeted chemotherapy based upon these abnormalities has resulted in spectacular therapeutic advances. A lesser degree of success has been achieved in the remaining 3 disorders, but even in these, significant advances have been made in their clinical and laboratory definition. This is especially true with the discovery of the Janus kinase 2 (JAK2) mutation, which pervades these illnesses, especially polycythemia vera, but not chronic myeloid leukemia.2 Similarly, these heretofore therapeutically resistant illnesses have likewise been responsive to new innovative therapies including, for example, interferon, imatinib,3 thalidomide,4 and bone marrow transplantation.5
Because of the lack of precision in defining response, any codified effort evaluating therapeutic effect is to be applauded. The present effort of the advisory board of the consensus conference (ABCCs) by Barosi and colleagues is a laudable beginning. It is noted that from a standpoint of writing response guidelines, they are based upon “physicians' consensus judgement,” which is an expert opinion, not evidence based. The authors state that defining response is a complex issue, with which I agree. Their proposal basically offers a number of parameters that can be evaluated so that those interested in the treatment of this disease may readily communicate with each other, much like the tumor-node-metastasis (TNM) staging used in cancer. Yet, except for the changes in the bone marrow, many drugs will affect the white blood cell count (WBC), platelet count, hemoglobin, and spleen size without affecting marrow histologically or overall life-span. Thus, relatively minor changes will be considered “drug response” by these criteria. They correctly point out that monitoring changes in bone histology is not routine in either clinical practice or in clinical trials. This is true even in recent publications. Simplified and reproducible scoring systems for fibrosis are much needed. Whatever restores normal marrow histology is the sina qua non of therapy. I am impressed by the basic requirement of a “representative biopsy” defined as artifact free and at least 1.5 cm in length. This will require not only improvement in our hematopathology laboratories, but will also require tempered steel biopsy needles and a hematologist with a mighty arm. Likewise, many aspirations, even in early-stage disease, yield dry taps, making marrow cytogenetic analysis impossible. Perhaps one or another peripheral blood fluorescent in-situ hybridization (FISH) test for more common cytogenetic abnormalities can be considered.FIG2
Unlike Pushkin, who reserved his splenic remarks to 2 countries, Barosi and colleagues come from 8. This is not only a numerical achievement, but a splendid example of a multinational scientific accomplishment. They are to be congratulated. ▪