The activation of oncogenes is essential to the development of lymphoid malignancies. Frequently, these oncogenes are involved in relaying extracellular messages to the nucleus by means of signaling pathways, causing changes in the cell transcriptional patterns. The altered function of those molecules associated with lymphomagenesis presents unique opportunities to specifically disrupt those signaling pathways critical to maintaining the malignant phenotype. Distinct oncogenic signaling cascades have been specifically associated with the different subtypes of non-Hodgkin lymphoma. Many of the effectors involved in these pathways may be essential for signaling function, thus offering an expansive array of candidate targets. Targeted therapeutic approaches are more likely to be specific and associated with fewer deleterious side effects. They are also expected to be highly active. Diverse and novel strategies have been used to target the products of these oncogenes in non-Hodgkin lymphoma; several have already reached the clinic with promising preliminary results.
The non-Hodgkin lymphomas (NHLs) are clonal malignant expansion of B or T cells that are at various stages of maturation. Multiple classification schemes have been employed for these diseases. Initially, these were based on the morphologic appearance of the neoplastic cell. However, as our understanding of the immunology, cytogenetics, and molecular biology of the lymphomas broadened, these classification systems changed in order to refine diagnoses and to provide a clinical perspective. Similarly, treatment of the NHLs evolved to include targeted therapies directed against specific signaling pathways critical to lymphomagenesis.
Human cells possess genes that are homologous to retroviral oncogenes. These normal cellular genes, referred to as proto-oncogenes, are involved in the regulation of proliferation, cell cycle progression, and apoptosis. These proto-oncogenes have the potential for becoming activated (oncogenes) by a variety of genetic mechanisms including amplification, point mutations, and chromosomal translocations. Frequently, these oncogenes are involved in relaying extracellular messages to the nucleus by means of signaling pathways, causing changes in the cell transcriptional patterns. Any of the effectors involved in these pathways may become oncogenic if overexpressed or inappropriately activated, leading to deregulation of cell growth. Therefore, intercepting involved signaling cascades at discrete points holds therapeutic promise. Distinct oncogenes have been specifically associated with the different subtypes of NHL. This review highlights promising targeted approaches to interfering with oncogenic signaling pathways in NHL.
Bcl-2 family
Role of Bcl-2 in lymphomagenesis
The Bcl-2 (B-cell CLL/lymphoma 2) protein is a member of a large family of proteins involved in the regulation of programmed cell death, primarily the intrinsic pathway. These proteins determine the cellular decision to live or die by their ability to modulate mitochondrial function. While some members, properly called antiapoptotic, preserve mitochondrial integrity, other proapoptotic members promote the release of cytochrome c from the intermembrane space of mitochondria (IMS). Following its release in the cytosol, cytochrome c interacts with Apaf-1 and caspase-9, forming an apoptosome, which activates caspase-9, which then activates caspase-3, the effector protease leading to the cleavage of target proteins and DNA fragmentation.1,2 All members in the Bcl-2 family contain one or more conserved Bcl-2 homology (BH) domains but have considerable variation in overall structure and function. The antiapoptotic members, which include Bcl-2 and Bcl-XL, share sequence homology in 4 BH domains designated BH1, BH2, BH3, and BH4. The proapoptotic members are divided into 2 groups, the multidomain Bax and Bak, which contain BH1 to BH3 domains, and the BH3-only proteins, including Bad, Bim, Bik, and Bid, which only have the BH3 domain. While Bcl-2 and Bcl-XL are integral membrane proteins that are predominantly located in the outer mitochondrial membrane (OMM), the multidomain proapoptotic and the BH3-only proteins are primarily localized in the cytosol and are translocated to the mitochondria following the activation of a death signal. The proposed model through which these proteins interact to determine the cell fate is intriguing and suggests that the BH3 segment of the entire BH3-only protein acts as a death ligand and hence can be exploited to develop synthetic peptide capable of inducing tumor cell death.2-4
Under normal conditions, Bax predominantly exists as a monomeric protein within the cytosol or is loosely attached to membranes. In response to an apoptotic stimulus, Bax translocates to the mitochondria where it deeply inserts into the OMM, undergoing a conformational change that induces its homooligomerization into large complexes, and facilitates release of cytochrome c, a process that is mediated by the BH3-only members and antagonized by Bcl-2 and Bcl-XL. The best understood example is that of Bid, where the generation of a signal through the death receptor (surface Fas and tumor necrosis factor [TNF] receptor) results in the cleavage of BH3-only protein Bid by caspase-8. The truncated version, tBid, then interacts with Bax or Bak and facilitates its translocation to OMM and oligomerization. The antiapoptotic property of Bcl-2 and Bcl-XL is mainly derived from their ability to sequester the BH3-only molecules and to interact with different components of the mitochondrial membrane promoting its stability. However, the recent work by Letai and colleagues2,3 established that there are 2 classes of BH3-only proteins involved in the execution of the apoptotic signal. By using BH3 peptidic sequences derived from representative BH3-only molecules, the authors described BH3 domains from Bid and Bim capable of activating mutidomain proapoptotic Bak and Bax and described BH3 domains from Bad and Bik that are incapable of inducing Bak oligomerization but effectively bind to antiapoptotic Bcl-2, displacing sequestered BID and BIM and making them available to activate Bak or Bax. There are several proposed mechanisms through which Bax, by means of its ability to interact with membrane lipids and protein channels, promotes the release of cytochrome c from the IMS. One mechanism, denoted as the mitochondrial permeability transition (MPT), involves an increase in the permeability of inner mitochondrial membrane (IMM) with secondary depolarization of the IMM and swelling of the matrix causing the rupture of the OMM. MPT is believed to be a consequence of the opening of a complex pore system designated as the permeability transition pore (PTP), which includes the voltage-dependent anion channel (VDAC) in the OMM and the adenine nucleotide translocator (ANT) in the IMM. Other mechanisms include the formation of de novo protein channels in the OMM from Bax oligomers, Bax regulation of VDAC activity without disruption of the ultrastructure or volume homeostasis of mitochondria, and the formation of lipidic channels by Bax induction of membrane instability without rupture.4
Enhanced Bcl-2 expression results most commonly from t(14; 18)(q32;q21) translocation that juxtaposes the Bcl-2 locus with the immunoglobulin heavy chain (IgH) enhancer.1,5-8 t(14;18)(q32; q21) is present in 70% to 90% of follicular lymphoma (FL) cases.1,8 Bcl-2 can be overexpressed in several B-cell malignancies through mechanisms other than t(14;18) and may confer a worse prognosis.9-12 The overexpression of Bcl-2 and/or Bcl-XL is believed to contribute to both progression of cancer cells and their resistance to chemotherapeutic drugs and radiation therapy.13-16 While Bcl-2 is important for the development of a B-cell malignancy, it is probably as important for the continuation of the malignant clone. The different aberrations accrued during tumorigenesis, like cell cycle dysregulation, angiogenesis, and invasion, are very likely to produce apoptotic signals. This can be viewed as an intrinsic checkpoint mechanism that is triggered by these breaches to terminate the abnormal cell. An apoptotic defect, like overexpression of Bcl-2, will be then necessary to oppose cell death signals. From a therapeutic point of view, eliminating Bcl-2 will remove the constraints on these inherent death signals and lead to cell demise. This was explored by Letai and colleagues, who generated mice expressing a conditional Bcl-2 gene and a constitutive c-Myc that develop lymphoblastic leukemia They demonstrated that Bcl-2 was required for the B-cell malignancy maintenance, because elimination of Bcl-2 resulted in rapid loss of the leukemic cells and prolonged survival of the mice. They also showed that while BAD BH3 did not affect mitochondria from nonmalignant pro-B lymphocytes, it resulted in release of cytochrome c from the lymphoblastic leukemia cells, which is consistent with the theory that Bcl-2 must be sequestering a large amount of continuously generated and activated BIM.2
Targeting Bcl-2 with ASOs
Disruption of these antiapoptotic signals became feasible with the advent of antisense oligonucleotides (ASOs). ASOs are short sequences of single-stranded DNA that are complementary to the mRNA of interest. The binding of ASO to mRNA on a base-pairing basis inhibits translation and also leads to cleavage of the mRNA by RNase H.8,17-20 The result is a reduction in the encoded protein pool. Additionally, there is evidence to support that ASOs have nonantisense effects including immune stimulation and enhancement of natural killer (NK) cell activity.8,21,22 Substituting the phosphodiester linkage of the bases with phosphorothioates conferred ASOs nuclease resistance. G3139, a prototype ASO targeting Bcl-2, down-regulated both mRNA and protein expression in Bcl-2–overexpressing lymphoma cell lines. After demonstrating therapeutic activity in tumor xenograft models, G3139 entered phase 1 clinical trials, both alone and in combination with chemotherapeutic agents.8,20,23,24 As a single agent in heavily pretreated patients with relapsed NHL expressing Bcl-2, G3139 resulted in responses and disease stability that correlated in most of these patients with down-regulation of Bcl-2.25,26 Combining G3139 with chemotherapeutic drugs is based on both in vitro as well as vivo experiments showing that targeting Bcl-2 with antisense therapy can promote apoptosis following cellular damage induced by chemotherapy.27-32 A phase 1 trial in relapsed FL using combination cyclophosphamide and G3139, in addition to other preclinical and clinical evidence, suggests that G3139 synergizes with many cytotoxic and biologic agents against a variety of hematologic malignancies including NHLs.8,33 Similar synergism may be achieved by the simultaneous disruption of the antiapoptotic (Bcl-2) and proliferation (mitogen-activated protein kinase kinase/mitogen-activated protein kinase [MEK/MAPK]) pathways to promote apoptosis and improve the cytotoxicity of conventional chemotherapeutic agents.34
Targeting Bcl-XL
There has also been interest in targeting another antiapoptotic molecule, Bcl-XL, with ASOs. Bcl-XL and proapoptotic Bcl-XS are products of different splicing of the same Bcl-X pre-mRNA. As a result, earlier ASOs that targeted Bcl-XL also possessed activity against the proapoptotic molecule Bcl-XS. Interestingly, these oligonucleotides were still able to induce apoptosis in several cancer cells.20,35-37 Subsequently, investigators looked for newer generations of ASOs that shift the splicing of the mRNA into producing Bcl-XS. Several were described and tested in cancer cells with promising results.20,38,39 Following the evidence that Bcl-2 and Bcl-XL have different roles, despite being closely related,13,40-43 a Bcl-2/Bcl-XL–bispecific ASO was designed with ability to induce in vitro apoptosis in tumor cells with diverse tissue origins.44-47
Low-molecular-weight compounds
The use of low-molecular-weight compounds to inhibit Bcl-2 and Bcl-XL function is a novel method with several potential therapeutic advantages. Small molecules inhibitors have the benefit of being more stable with better bioavailability as compared with other biologic and peptide inhibitors. They also have the ability to penetrate the central nervous system blood brain barrier.13 The search for small-molecule inhibitors of Bcl-2 and Bcl-XL became possible with the understanding of the detailed atomic structure of both the proapoptotic and antiapoptotic members. Both Bcl-2 and Bcl-XL bind, through their BH3 binding pockets, to the BH3 domains on proapoptotic members, an essential step in the execution of the antiapoptotic signal. The BH3 domains of these proapoptotic members are also required for implementing the apoptotic signal.13,48 Based on these findings, nonpeptide small molecules can be designed to compete with the proapoptotic members for binding to the BH3 binding pocket and subsequently disrupting the function of the antiapoptotic molecules. The first of these small molecules, HA14-1, was described by Wang et al.49 HA14-1 was shown to bind to the BH3 binding site on Bcl-2 protein and inhibit its interaction with Bak. At an inhibitory concentration of 50% (IC50) of 9 μM, it was able to induce apoptosis in human myeloid leukemia cells overexpressing Bcl-2.13,49 Afterward, several more small-molecule inhibitors of Bcl-XL and Bcl-2 were described and shown to bind to the BH3 binding site. An excellent review by Wang et al describes several of these small-molecule inhibitors of Bcl-2 and Bcl-XL and illustrates the various approaches entailed in discovering them.13 The work by Letai and colleagues describing 2 functionally different BH3-only proteins opens the door on several therapeutic potentials. Bad-like peptidomimetics or small molecules can be used to bind Bcl-2 and displace Bid. Because an apoptotic signal is needed to activate the BH3-only proteins, such agents may be harmless to normal tissues but lethal to transformed cells where inherent apoptotic signals are generated but blocked, for example, by excess Bcl-2 that sequesters BID.2,3 Walensky and colleagues designed a hydrocarbon-stapled BH3 helix that mimics Bid BH3 that activated apoptosis in vitro and inhibited leukemia cells in vivo. The use of α, α-disubstituted nonnatural amino acids containing olefinic side chains to tie amino acids within the BH3 peptide improved the stability and cell permeability of the mimics while preserving the helical structure, critical for biologic activity, and specificity.50 Moreover, a novel approach to inhibit Bcl-2 was recently reported by Cohen-Saidon and colleagues. Using a semisynthetic human phage-display library, they generated a single-chain Fv antibody (scFv) against Bcl-2. Attaching a basic domain of Tat to the antibody allowed the single-chain antibody complex to penetrate into several types of living cells. The anti–Bcl-2–scFv–Tat was specific and was directed to the BH1 domain of Bcl-2. The single-chain antibody was also shown to decrease the level of Bcl-2, depolarize the mitochondrial membrane, and induce cell apoptosis in 2 types of mast cells and in a human breast cancer cell line. This is a potentially promising approach that allows the construction of highly specific single-chain antibodies that are smaller than regular antibodies and thus easier to introduce into cells.51
Cyclin D1
Bcl-1 proto-oncogene, also called CCND1 and PRAD1, has been implicated in the pathogenesis of NHLs. Its gene product, cyclin D1, plays a critical role in cell transition from the G to S phase in response to mitogens. Cyclin D1 is 1 of at least 11 different mammalian cyclins that are involved in the regulation of cell cycle.52-54 Overexpression of cyclin D1 has been shown to shorten the G1 phase and reduce the dependency of the cell on mitogens.55,56 The prototypical NHL with deregulation of the Bcl-1 oncogene is mantle cell lymphoma (MCL).
The development of MCL involves the acquisition of specific cytogenetic abnormalities at the different phases of the disease. t(11;14)(q13;q32) juxtaposes the cyclin D1 (Bcl-1, CCND1, PRAD1) locus on chromosome 11 and the immunoglobulin heavy chain IGH locus on chromosome 14. The translocation results in overexpression of cyclin D1 with secondary cell cycle deregulation.52,57,58 Depending on which method is used, t(11;14)(q13;q32) is identified in 50% to 70% of cases of MCL. However, cyclin D1 RNA and/or protein are detected in 90% to 100% of cases, suggesting that alternative mechanisms including point mutations may also result in increased expression.59-64
Cell-cycle control
Cyclin D1, in association with cyclin-dependent kinase-4 (CDK4) and CDK6, will induce the cell to enter the S (DNA synthesis) phase by phosphorylating the retinoblastoma tumor-suppressing protein (pRB), which binds to transcription factors, including E2F.52,58 The process begins with phosphorylation of cyclin D1–bound CDK4 by CDK-activating kinase (CAK).58,65,66 The resultant active cyclin D–CDK4 complex phosphorylates pRB, releasing E2F and other DNA binding proteins that are necessary for transcriptional activation of genes required for cell transition into the S phase.52,58,67,68 Several CDK inhibitors have entered clinical trials, with others still undergoing preclinical testing. These agents fall into 2 categories depending on their mechanism of CDK inhibition. Flavopiridol, R-roscovitine (CYC202), and BMS-387032 directly bind to the CDK catalytic subunit, while 7-hydroxystaurosporine (UCN-01) modulates the upstream regulatory pathways needed for CDK activation.69
CDK inhibitors
Flavopiridol, a semisynthetic flavone derivative, is a protein kinase inhibitor with demonstrated activity against a number of protein kinases including cyclin D1 and CDK4. The drug exhibits in vitro activity against a variety of cycling as well as noncycling tumor cells including lymphomas.8 The antitumor effects of flavopiridol include cell cycle arrest, induction of apoptosis, activation of caspase-3, decreased expression of p53 protein, and down-regulation of MCL-1.8 Flavopiridol is the first CDK inhibitor to be tested in patients with MCL. Its efficacy was limited in both the Dana-Farber Cancer Institute and National Cancer Institute (NCI)–Canada trials but was also schedule dependent. In the NCI-Canada trial, flavopiridol was administered at a dose of 50 mg/m2/d by intravenous bolus for 3 consecutive days every 21 days. Although no complete responses (CRs) were seen, 3 patients had a partial response (11%), and 20 patients had stable disease (71%). At the Dana-Farber Cancer Institute, patients received flavopiridol 50 mg/m2/d given as a 72-hour continuous intravenous infusion every 14 days. There were no clinical responses, and only 3 patients maintained stable disease through treatment. This variability may be related to the pharmacokinetics of flavopiridol and its increased binding to human plasma proteins. This incited a phase 1 trial in which patients with refractory chronic lymphocytic leukemia received flavopiridol at total dose of either 60 mg/m2 or 80 mg/m2 weekly for 4 weeks on a 6-week schedule. Half the dose was administered as a 30-minute intravenous bolus followed by the other half given by 4-hour continuous infusion. The responses were promising, but life-threatening tumor lysis occurred that led to the interruption of trial.70-72
When tested against a number of cell lines, cytotoxic synergy between flavopiridol and various chemotherapeutic agents could be demonstrated based on a specific sequence of drug administration. Thus, flavopiridol has possible utility for lymphoid malignancies, likely in combination with antineoplastic drugs.8,70 The preclinical and clinical studies with most of the other CDK inhibitors similarly demonstrated nonselectivity of these agents, with activity against multiple kinase proteins and CDK members and modest antitumor activity as monotherapy.69,73 Additionally, although the 2 novel agents CYC202 and BMS-387032 are more selective CDK inhibitors, none is a potent inhibitor of CDK4, thereby limiting their use.69,74
c-Myc
Role of c-Myc in lymphomagenesis
Another attractive candidate for targeted therapy is the c-Myc protein that is highly expressed in Burkitt lymphoma (BL). The up-regulation of the c-Myc protein is usually the result of chromosomal translocations that place the c-myc gene located on 8q24 under the control of immunoglobulin (Ig) enhancers. The most frequent translocation partner, detected in 80% of BL, is the Ig heavy chain region on chromosome 14: t(8;14)(q24;q32). The other 2 translocation partners include the kappa light chain locus on chromosome 2, t(2;8)(p12;q24), and the lambda light chain locus on chromosome 22, t(8;22)(q24;q11), occurring in 15% and 5% of cases, respectively.1,75-79 The Ig enhancers are responsible for the inappropriately high transcription rate of the translocated c-myc allele.75,78-81
The detailed function of c-Myc is not yet completely understood, but it is believed to involve the promotion of both cell proliferation and apoptosis.10,78,79 The activity of c-Myc depends on its association with the Max protein. The formation of the Myc-Max heterodimer promotes its binding to hexameric DNA sequence termed the E-box, with subsequent interaction with Trrap and recruitment of histone acetylases to activate transcription. Other proteins including Mad and Mxi-1 are involved in down-regulating the c-Myc protein function, both by binding to Max and making it unavailable for c-Myc and through forming heterodimers (Max-Mad, and Mad-Mad) that compete with Myc-Max complexes for binding to DNA target sites. The oncogenic properties of c-Myc are probably the result of its constitutive expression leading to increased levels of Myc-Max heterodimers.10,75,78 Multiple cellular processes are perturbed by c-Myc up-regulation, some involved in promoting malignant cellular transformation and others implicated in antagonizing it. c-Myc is an attractive therapeutic target for several reasons. It is highly expressed only in cycling cells, and thus the toxicities expected from its inhibition should be limited to highly dividing tissues like the gastrointestinal tract and bone marrow. Also, c-Myc is the convergence point of several upstream signaling pathways that can be affected by genetic mutations in various tumors. Finally, several in vitro studies have shown that while c-Myc overexpression can sensitize cells to the cytotoxic effects of some chemotherapeutic agents, inhibiting c-Myc can enhance the sensitivity of tumor cells to several other antineoplastic agents.82-87
Targeting c-Myc at the level of RNA and DNA
Down-regulation of c-Myc by ASOs has been tested in a large array of cancer cells including hematologic and solid tumors.20 Reduction in cell proliferation occurred when ASOs were used either alone or in combination with antineoplastic agents.20 Using in vivo models of solid tumors, a better antitumor activity of ASOs was observed when they were administered after the chemotherapeutic agent.20,88,89
Newer generations of ASOs with greater resistance to endogenous nucleases, improved cellular uptake, and increased stability with their target sequence are being developed.82 Most promising are the phosphorodiamidate morpholino ASOs (PMOs) with documented activity against c-Myc expression.89-92
Several other related approaches have been developed to interfere with c-Myc function. Peptide nucleic acids (PNAs) and locked nucleic acids (LNAs) are capable of complexing with both DNA and RNA and subsequently inhibiting transcription and translation.82,93-95 Triple helix–forming oligonucleotides (TFOs) are purine-rich or mixed purine/pyrimidine-rich oligonucleotides that target c-Myc gene directly. TFOs stably bind to duplex DNA within the major groove and prevent the binding of transcription factors.82,87 Several lymphoma cell lines were treated with these TFOs in vitro, with secondary cell arrest followed by apoptosis.96,97 Some researchers exploited the DNA secondary structure to use quadruplex-forming oligonucleotides to inhibit transcription.98,99 Others identified a cationic porphyrin molecule that can specifically interact with the P1 promoter and stabilize DNA in a quadruplex structure that represses transcription.100 Targeting c-Myc by ribozymes, which are RNA molecules capable of degrading specific target RNA, and short interfering RNAs (siRNAs) are promising techniques, yet the optimal delivery system still needs to be determined.82,87 Introducing ribozymes into tumor cell lines by means of retroviruses down-regulated c-Myc gene.101,102 c-Myc inhibition by siRNAs has not yet been reported.82 Another untested strategy is the use of decoy oligonucleotides (DOs) that contain c-Myc DNA binding sites. DOs are short, double-stranded oligonucleotides with DNA binding sites that compete with the real genomic sites for binding to transcription factors.82 Recently, low-molecular-weight compounds were effectively used both in vitro and in vivo to disrupt the formation of Myc-Max heterodimers.82,87,103,104 However, high concentrations of these compounds were needed to be effective, which carries the potential for significant toxicities.82 An alternative approach to target the Myc-Max interaction is to shift the heterodimerization balance by ectopically inducing the overexpression of Mad family members.82,105,106 Interference with c-Myc function may also be potentially achieved at the level of the TRAAP–histone acetylase complex.82,87
NF-kB family
The nuclear factor–kB (NF-kB) pathway is active in several of the non-Hodgkin lymphomas and confers cancer cells with a survival advantage by inhibiting apoptosis. The term “NF-kB” is used to describe several dimeric transcription factors that belong to the c-Rel family.107,108 Some factors are retained in the cytoplasm as mature products by a specific inhibitor called the inhibitor of kB (IkB), while others are derived by proteolytic cleavage of larger intracytoplasmic precursors. After activation, NF-kB translocates from the cell cytoplasm to the nucleus, where it participates in gene transcription.107-110 Based on murine studies, NF-kB is believed to play a central role in the regulation of innate and adaptive immunity, inflammatory response, lymphoid organ development, as well as apoptotic inhibition.108,111-113 NF-kB activity is tightly controlled during normal B-cell development, where different NF-kB heterodimers are differentially expressed in the B-cell lineage, emphasizing their role as stage-specific regulators of B-cell development, survival, division, and immunoglobulin expression.114 Additionally, the NF-kB pathway is one of the key transcription factors activated by B-cell receptor (BCR) signaling. Engagement of BCR with secondary activation of NF-kB is critical for the survival of quiescent B cells in the periphery, allowing them to evade apoptosis; it is equally important for the survival and expansion of mitogen-activated B cells by increasing cell division and decreasing apoptosis.114-116 The NF-kB function of inhibiting programmed cell death is derived from its ability to induce the expression of antiapoptotic factors including cellular inhibitor of apoptosis (c-IAP), Fas-associating protein with death domain–like interleukin-1–converting enzyme (FLICE) inhibitory protein (c-FLIP), TNF receptor (TNFR)–associated factor 1 (TRAF1) and TRAF2, as well as members of the Bcl-2 family. NF-kB is a potent IAP that is triggered by either death receptors or the mitochondrial-dependent pathway, making it an attractive target for antitumor therapy (Figure 1).108
Role of NF-kB in lymphomagenesis
The deregulation of NF-kB pathway plays a role in the development of several NHLs. Approximately half of extranodal marginal zone B-cell lymphomas of mucosal-associated lymphoid tissue (MALT) type harbor 1 of 3 specific chromosomal translocations that result in a dramatic increase in NF-kB activity.117 Constitutive activation of NF-kB is also a common molecular feature for other B-cell malignancies such as Hodgkin lymphomas and mediastinal B-cell lymphomas where amplifications of c-REL are frequently seen.118,119 About 23% of diffuse large B-cell lymphomas (DLBcls) show amplification of the c-REL proto-oncogene (2p12-16).120 However, based on recent work by Houldsworth and colleagues in DLBcl, the amplification of the c-REL locus did not necessarily correlate with activation of NF-kB pathway. No significant association was found between the Rel copy number, REL nuclear accumulation, and the differential expression of NF-kB target genes, suggesting that Rel may not be the functional target of 2p amplification.121 Nonetheless, constitutive activation of NF-kB is critical for one subtype of DLBcl. DLBcl encompasses 2 distinct diseases that differ in the expression of hundreds of genes, based on gene expression profiling, and also have markedly different clinical outcome. One subtype, germinal center B-cell–like (GCB) DLBcl that resembles normal germinal center B cells, has a good prognosis, whereas activated B-cell–like (ABC) DLBcl that resembles BCR-stimulated blood B cells has a poor outcome. Constitutive activation of the NF-kB pathway is required for survival of ABC DLBcl as illustrated in the work by Davis and colleagues. The ABC subtype expressed known NF-kB target genes and showed high levels of NF-kB DNA binding activity, with constitutive IkB kinase (IKK) activity and IkB degradation, validating the constitutive activation of NF-kB pathway. On the other hand, this molecular profile was not observed in the GCB subtype. Inhibition of the NF-kB through the introduction of a super-repressor IkB that cannot be phosphorylated by IKK was selectively toxic to ABC but not GCB DLBcl, causing apoptosis and G1-phase cell cycle arrest.122
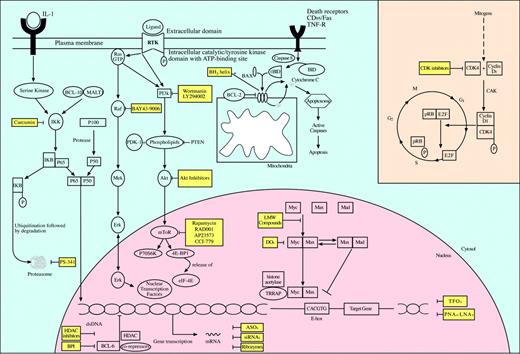
Inhibition of molecular targets for the treatment of non-Hogkin lymphomas. Activation of oncogenes is a critical event in the development of non-Hodgkin lymphomas. Oncogenic proteins are key effectors in signaling pathways that regulate cell proliferation, cell cycle progression, and apoptosis. Their inhibition via specific small molecules and pharmacologic agents offers a novel approach in the targeted treatment of non-Hodgkin lymphomas. While several have already entered clinical trials with promising preliminary results, other agents are still awaiting preclinical validation. LMW indicates low molecular weight; CAK, CDK-activating kinase.
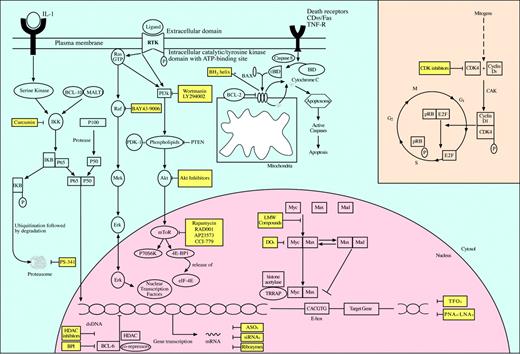
Inhibition of molecular targets for the treatment of non-Hogkin lymphomas. Activation of oncogenes is a critical event in the development of non-Hodgkin lymphomas. Oncogenic proteins are key effectors in signaling pathways that regulate cell proliferation, cell cycle progression, and apoptosis. Their inhibition via specific small molecules and pharmacologic agents offers a novel approach in the targeted treatment of non-Hodgkin lymphomas. While several have already entered clinical trials with promising preliminary results, other agents are still awaiting preclinical validation. LMW indicates low molecular weight; CAK, CDK-activating kinase.
The translocation t(11;18)(q21;q21) is the most common translocation, occurring in 18% to 35% of MALT lymphomas cases. It results in the juxtaposition of the apoptosis inhibitor-2 (API2) gene on chromosome 11 and MLT (MALT lymphoma–associated translocation) gene on chromosome 18. API2 encodes for an IAP protein, and MLT encodes for the MALT1 protein. IAP family proteins inhibit activated caspases, resulting in arrest of programmed cell death, while MALT1 contains a caspaselike domain with a death domain that recruits the protein to the cell death signaling pathway.117,123,124 The AIP2-MALT1 fusion protein is also capable of directly activating IKK, a function that requires the presence of both proteins of the fusion product, with resulting increased translocation of NF-kB into the nucleus.117,124 The other translocations reported in MALT lymphomas are t(1;14)(p22;q32), which brings the entire coding region of the Bcl-10 gene under the control of enhancer elements of the IGH locus on chromosome 14, and t(14;18)(q32;q21), with resultant IGH-MLT rearrangement. Bcl-10 normally activates the NF-kB pathway through binding to MALT1 and subsequent activation of IKK complex. The upregulation of Bcl-10 in t(1;14) or MALT1 in t(14;18) will increase the amount of the protein required to activate IKK with secondary increase in translocation of NF-kB to the nucleus.117,124,125
Proteasome inhibitors
Several molecular therapeutic approaches have been designed to inhibit NF-kB. Among these, the proteasome inhibitors have shown promising results. The proteasome is a large protease complex that possesses a central role in cellular metabolism through the elimination of intracellular proteins that have been tagged for degradation. The process requires the initial modification of the proteins by conjugation to ubiquitin prior to proteolytic degradation within the core of the proteasome. Among the targets for ubiquitination and proteolysis are proteins involved in cell cycle regulation, transcription factor activation, apoptosis, and control of chemotaxis, angiogenesis, and cell adhesion.8,126 Inhibition of NF-kB is one of several mechanisms through which proteasome inhibitors derive their antitumor effect. These mechanisms have been best studied in the context of multiple myeloma cells lines treated with PS-341 or bortezomib, a boronic acid peptide that is a specific inhibitor of the 26S proteasome. Specific inhibition of NF-kB resulted in apoptosis via mitochondrial cytochrome c release and caspase-9 activation, decreased expression of angiogenic cytokines and adhesion molecules, and growth factors such as interleukin-6 (IL-6). Several other signaling molecules were also affected by PS-341 independent of NF-kB inhibition, including the induction of apoptosis via activation of c-jun NH2-terminal kinase (JNK) and a Fas/caspase-8–dependent apoptotic pathway, increasing p53 activity by p53 protein phosphorylation and degrading Mdm2, cleaving the DNA protein kinase catalytic subunit and ataxia telangiestasia mutated protein (ATM) involved in DNA repair, activation of BAX proteins, and accumulation of CDK inhibitors p21 and p27. Additionally, PS-341 down-regulates several effectors of the protective cellular response to genotoxic stress, like topoisomerase II, restoring the sensitivity of myeloma cells to DNA-damaging chemotherapeutic agents.127-129
As a single agent, bortezomib induced apoptosis in many types of human cancer cell lines, including those derived from hematologic malignancies as well as in a murine model of BL.8,130 Moreover, the drug demonstrated marked synergy with chemotherapeutic agents like irinotecan, gemcitabine, and doxorubicin, and ionizing radiation.8,126 Lactacystin, a bacterial metabolite with a natural ability to inhibit proteasome, has been tested in DLBcl cells with proven ability to prevent IkB degradation.131 Constitutive activation of NF-kB in MCL has been observed by Lan Pham et al.132 Treatment of the MCL cells with PS-341 or the specific IkBa inhibitor BAY 11-7082 resulted in decreased NF-kB DNA binding activity and stabilization of IkB. Cell growth inhibition occurred through both cell cycle arrest and induction of apoptosis.132 Proteasome inhibition in MCL may carry therapeutic potentials through other pathways unrelated to NF-kB inhibition. There are data to suggest that the low levels of the CDK inhibitor p27 may be a consequence of increased proteasome-mediated degradation.133 This is supported by experiments in cell models where proteasome inhibition resulted in up-regulation of p27.126,132 In human phase 1 and 2 trials, bortezomib was well tolerated, with toxic effects being limited to diarrhea, thrombocytopenia, neutropenia and peripheral neuropathy.134 Currently, bortezomib has a definite role in the treatment of myeloma. In a phase 2 trial in patients with relapsed and refractory myeloma, bortezomib was administered at a dose of 1.3 mg/m2 intravenously twice weekly for 2 weeks every 21 days, with addition of dexamethasone of 20 mg on the day of and day after each dose of bortezomib for patients with progressive disease after 2 cycles. The rate of response was 35% and included a CR of 4%. The most common adverse events were thrombocytopenia, fatigue, peripheral neuropathy, and gastrointestinal side effects.135 According to preliminary data from phase 1 trials, several patients with NHLs are experiencing responses to bortezomib.126,131,136 Similar encouraging results were seen in a preliminary report from a phase 2 trial by O'Connor et al37 in patients with indolent lymphomas, particularly FL and MCL, and in a phase 2 study in relapsed or refractory B-cell NHL showing overall response rate of 41% among patients with MCL.126,137,138 A trial combining bortezomib with EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) in patients with NHLs is also being studied.134
Other inhibitors of the NF-kB pathway
Inhibiting the NF-kB pathway has been also achieved through other routes. One group identified curcumin (diferuloylmethane) as an effective agent that blocks constitutive NF-kB in myeloma cells.139 Curcumin, which has been shown to possess chemopreventive activity against various tumors in animal models, is a safe agent in humans as demonstrated in phase 1 clinical trials.140 Curcumin inhibited IKK activity with secondary down-regulation of NF-kB and activation of caspases leading to apoptosis of myeloma cells.139 G06976, protein kinase C inhibitor, has been shown to inhibit IKK activity in estrogen receptor–negative (ER-) human and mouse mammary adenocarcinoma cell line with demonstrable antitumor activity in vivo (Table 1).141
PI3K/Akt/mTOR pathway
Protein kinases are generally classified as either tyrosine or serine/threonine specific, although some are capable of phosphorylating both tyrosine and serine/threonine residues. The protein-tyrosine kinases (PTKs) include several families of transmembrane-spanning receptors (receptor protein tyrosine kinases [RPTKs]) as well as cytoplasmic, nonreceptor PTKs. The catalytic activity of PTKs is tightly controlled by multiple autoinhibitory mechanisms, and the oncogenic transformation of PTKs can be viewed as a consequence of the relief of these normal autoinhibitory constraints. The loss of these regulatory restraints can result from several mechanisms, including chromosomal translocations, gain of function (GOF) mutations or small deletions, and gene amplification.142 However, malignant transformation of cells can also arise from chromosomal perturbations that involve the downstream effectors of PTKs, including the Ras–Raf–extracellular signal-regulated kinase (Ras-Raf-ERK) and the lipid kinase phosphoinositide 3-OH kinase (PI3K) signaling pathways. PI3K is a family of proteins that phosphorylate the 3′-OH group of the inositol ring in inositol phospholipids, and hence they are lipid kinases. This protein family can be subdivided in 3 classes, but class I is the most studied of PI3Ks and the most important in hematopoietic cells. Class I PI3Ks are heterodimers consisting of a catalytic subunit (p100) and a regulatory subunit, and their preferred substrate is phosphoinositide (4,5) diphosphate (PtdIns(4,5)P2). The activation of class I PI3Ks starts with their recruitment to the plasma membrane, where they associate with an activated RPTK or its substrates. These interactions lead to allosteric activation of the catalytic subunit with secondary production of PtIns(3,4,5)P3 and PtIns(3,4)P2. The phospholipid products of PI3K activate downstream targets, including 3′-phosphoinositide-dependent kinase (PDK-1), Akt/protein kinase B (PKB), and PKC. The activation of Akt also requires its interaction with PDK with subsequent phosphorylation of threonine 308 and serine 473 in Akt. Activated Akt is then translocated to the nucleus where several of its substrates reside, including regulators of glucose transport/metabolism, apoptosis, and cell cycle transit. In this manner, the PI3K/PDK-1/Akt pathway is viewed as the controlling mechanism allowing the cell to undergo cell cycle transit provided that there are sufficient nutrients. Several phosphatases negatively regulate the PI3K/PDK-1/Akt pathway, including the tumor-suppressor gene PTEN. High levels of Akt activation have been described in multiple cancers including NHLs.142-147 The transforming effects of PI3K/Akt are dependent on the regulation of another downstream target, the mammalian target of rapamycin (mTOR), also called FK506-binding protein (FKBP)–rapamycin-associated protein (FRAP). Activation of mTOR results in phosphorylation of p70S6 kinase (p70S6K), required for ribosome biogenesis, and 4E-BP1 translational repressor with secondary release of eIF-4E that is critical for assembly of a translational complex. The end result is cap-dependent translation of proteins required for cell cycle transit, specifically cyclin D1 and c-Myc.142,147,148 However, there is evidence that cells are capable of expressing these proteins through cap-independent mechanisms, providing a salvage pathway that allows the cells to survive during starvation conditions.147 This is important because it implies that inhibiting mTOR may not lead to sufficient decreased levels of cell cycle proteins leading to G1 arrest. Gera et al147 demonstrated in cell cultures and mouse xenografts with high Akt activity that mTOR inhibitors, rapamycin and CCI-779, resulted in cell cycle arrest through down-regulation of cyclin D1 and c-Myc. These data suggested simultaneous inhibition of both the cap-dependent and salvage pathways. When tested in cells with a low Akt activity, mTOR inhibitors resulted in up-regulation of cyclin D1 and c-Myc expression through activation of the salvage or cap-independent translation pathway.147
Role of PI3K/PDK-1/Akt/mTOR pathway in lymphomagenesis
The PI3K/PDK-1/Akt/mTOR pathway plays an important role in lymphomagenesis. Three Akt members are associated with different types of malignancies, but Akt1 specifically has been mapped to chromosome 14q32, a region frequently involved in translocations in leukemia and lymphoma.143,149 The role of PI3K in cell survival and proliferation of immortalized B lymphocytes was examined in Burkitt lymphoma (BL) cells and Epstein-Barr virus (EBV)–immortalized B cells, lymphoblastoid cell lines (LCLs), which are a model for the cells in posttransplantation lymphoproliferative disease (PTLD). Treatment of these cells with the PI3K inhibitor, LY294002, induced apoptosis in BL cells and cell cycle arrest at G1 phase in LCL cells.150 Some investigators suggested that induction of apoptosis in B-cell lymphoma cell lines by anti-IgM and anti-IgD is mediated through inhibitory effects on the PI3K pathway.151 Others investigated apoptosis in DLBcl as mediated by inhibition of the PI3K/Akt pathway.152 There are also data showing interplay between eIF-4E and c-Myc in promoting lymphomagenesis153 as well as data to suggest that the T-cell leukemia 1 (TCL1) proto-oncogene, which is highly expressed in a variety of NHLs, induces B-cell malignancies by potentiating the activity of Akt.154 Recently, there was a report that inhibition of apoptosis by activation of the AKT signaling pathway is an important mechanism involved in a subset of NHLs associated with MCT-1 overexpression.155 Several agents have been investigated to block this important pathway in search of new dugs for cancer therapy. Although some showed disappointing results, other agents proved to be promising.
Inhibitors of mTOR
Inhibition of PI3K catalytic subunit with wortmannin and LY294002 carries several limitations. These include the very short half-life of wortmannin, nonspecificity of LY294002, and the potential for disrupting glucose transport and metabolism in normal cells.156,157 Several Akt inhibitors have been developed and shown to inhibit the growth of various cancer cell lines.158-161 There are 3 mTOR inhibitors in clinical development, RAD001, AP23573, and CCI-779. All 3 are rapamycin analogs that act through binding to the intracytoplasmic receptor FK506-binding protein-12 (FKBP12) with subsequent inhibition of the kinase activity of mTOR. In phase 1 and 2 trials, the mTOR inhibitors demonstrated clinical activity against a variety of solid tumors.162,163 Preclinical studies in human multiple myeloma cells using a xenograft model confirmed the activity of CCI-997 in myeloma cells through inhibition of proliferation, angiogenesis, and induction of apoptosis. The sensitivity of the myeloma cells to the growth inhibitory effects of CCI-997 was also shown to correlate with high Akt activity.164 Wendel and colleagues used a mouse model of B-cell lymphoma to demonstrate that activated Akt promoted lymphomagenesis by disabling apoptosis. The phenotype was resistant to conventional chemotherapy agents when used alone. However, the combination with rapamycin restored the cells sensitivity to these drugs. Similar activity of rapamycin was not demonstrated in chemoresistant lymphoma cells that depended on Bcl-2 for survival, again underlying the selective activity of mTOR inhibitors.165
The MAPK pathway
The Ras/Raf/Mek/Erk or MAPK pathway is another potential target for the development of new therapeutic agents in NHLs. The MAPK pathway possesses dual roles in promoting cellular proliferation. It regulates the production of various cytokines and growth factors, and then it relays the message generated by these cytokines and growth factors by mediating primarily mitogenic and antiapoptotic signals.166 The Ras proteins are located at the inner surface of the plasma membrane and are inactive in their guanosine diphosphate (GDP)–bound state but are active in the guanosine triphosphate (GTP)–bound state. By means of cell surface receptor tyrosine kinases (RTKs) and other membrane receptors, diverse extracellular ligand–mediated stimuli transiently promote the formation of the active GTP-bound state as regulated by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). The interaction between activated Ras and Raf leads to localization of Raf to the plasma membrane. Activated Raf subsequently phosphorylates and activates dual-specificity kinase MEK (MAPK kinase) that phosphorylates and activates the ERK1/2 family of MAPK. The activated ERKs then translocate to the nucleus where they phosphorylate transcriptional regulatory proteins.167 Constitutive activation of the MAPK pathway with subsequent deregulation of the cell proliferation program can occur via 3 main routes. These include activating mutations in Ras, activating mutations in Raf, as well as up-regulation of RTK. The MAPK pathway is involved in the regulation of normal hematopoietic cell proliferation and differentiation.166 Its role in the pathogenesis of lymphomas is suggested by studies of lymphoma-derived cell lines.168 One study suggested that rituximab sensitizes NHL B-cell lines to chemotherapeutic agents by down-regulaing the Erk1/2 signaling pathway.169 Another MAPK implicated in lymphomagenesis, specifically transformed FL and EBV-related lymphomas, is p38 MAPK.170-172 Targeting the MAPK pathway has already been accomplished at 2 important levels, Ras and Raf. The arsenal against Raf currently includes ASO of Raf-1, ISIS 5132, that has been tested in phase 1 trials and the Raf-1 kinase inhibitor BAY 43-9006 (Bayer, West Haven, CT).173,174 BAY 43-9006 was shown to have in vitro activity against a multitude of kinases including wild-type and mutant (V599E) B-Raf and Raf-1 kinase isoforms as well as vascular endothelial growth factor receptor-2 (VEGFR-2). The antitumor activity of BAY 43-9006 in NHLs is awaiting the results of phase 2 trials.174,175
Bcl-6
Role of Bcl-6 in lymphomagenesis
The Bcl-6 protein is a potent sequence-specific transcriptional repressor that is involved in the regulation of lymphoid development. Bcl-6 is normally expressed in B cells within the germinal centers (GCs) but is absent in pre-GC and differentiated progenies. This finding highlights the function of Bcl-6; it is needed to license the B cells for the GC reaction (class switch recombination and somatic hypermutation) but is subsequently down-regulated to permit B-cell exit from GCs and terminal differentiation.1,10,176 Consistent with its role in modulating B-cell responses in GCs, Bcl-6 has been shown to repress signal transducer and activator of transcription-2 (STAT-6)–dependent IL-4–induced induction of Ig epsilon transcripts and expression of the p27kip1, cyclin D2, and Blimp-1 target genes, with the later being involved in B-cell differentiation. On the other hand, Bcl-6 protein, in response to activation of BCR, is down-regulated by MAPK-induced phosphorylation, which marks Bcl-6 for degradation by the ubiquitin-proteasome pathway. Also, Bcl-6 RNA is down-regulated by CD40 receptor signaling.1,176 The continued expression of Bcl-6, by means of chromosomal translocations of 3q27, will result in repression of genes involved in B-cell activation, differentiation, cell cycle arrest, and apoptosis, contributing to the development of NHL. The deregulation of Bcl-6 expression is the result of promoter substitution, wherein the 5′ noncoding sequence in the Bcl-6 gene is truncated and promoters from immunoglobulin (Ig) genes or a non-Ig gene are placed adjacent to the intact coding exons of Bcl-6, preventing normal down-regulation of Bcl-6 transcription that occurs during B-cell terminal differentiation. Such translocations are reported in 20% to 40% of DLBcl and 6% to 14% of FL (and have also been observed in cases of MCL, MALT lymphoma, Hodgkin lymphoma, and chronic lymphocytic leukemia/small lymphocytic lymphoma).1,10,176 Yet, somatic mutations in the 5′ noncoding region of the gene may also cause deregulated expression of Bcl-6. Generally, mutations in this region can be found in one third of normal GC and memory B cells and up to 75% of DLBcl cases, but specific nucleotide substitutions capable of disrupting a negative autoregulatory circuit have been associated with DLBcl.176
Targeting Bcl-6
Interfering with Bcl-6 function for potential therapeutic benefits in NHL has been explored at several levels. The regulation of gene expression by Bcl-6 involves the recruitment of corepressor complexes by means of its broad complex/tramtrack/bric-a-brac (BTB) domain. A lateral groove motif specific to Bcl-6 BTB domain binds to a conserved sequence called the BTB binding domain (BBD) found in silencing mediator of retinoid and thyroid receptors (SMRTs), nuclear receptor corepressor (N-CoR), and Bcl-6 corepressor (BCoR). Bcl-6 also binds through its middle region to metastases-associated genes (MTA3) protein, a subunit of nucleosome remodeling and deacetylase (NuRD) complex that contains a histone deacetylase (HDAC). These repressor programs may have different sets of target genes with different functions, where the former permits the B cells to undergo rapid proliferation and survive the DNA damage occurring as part of the GC reaction and the latter blocks differentiation.177 Using crystallographic data, peptides were designed to disrupt the interaction between BTB lateral groove and BBD motif. The Bcl-6 peptide inhibitors (BPIs) efficiently penetrated cells, localized to the nucleus, and competed with corepressors for binding to Bcl-6 lateral groove, with no binding to other BTB proteins. They were also shown to disrupt Bcl-6–mediated transcriptional expression with secondary reactivation of target genes and induce apoptosis and cell cycle arrest in Bcl-6–positive DLBcl cells. Similar results were also obtained by using siRNA. Targeting the NuRD repression mechanism may also possess therapeutic potentials.177 Recent observations have found that the acetylation of Bcl-6 inhibits its function and that 2 deacetylase pathways including HDAC-2 and silent information regulator SIR-2 control the level of Bcl-6 acetylation. Using HDAC inhibitor trichostatin A and SIR-2 inhibitor nicotinamide can induce Bcl-6 acetylation and therefore inhibit its function.176
Conclusions and future directions
The recent interest in developing new antineoplastic treatments, based on the molecular features of particular tumors, has been driven by several recent accomplishments in understanding the pathogenesis of cancer. Among those was the discovery of the role played by activation of oncogenes and inactivation or loss of tumor suppressor genes in tumorgenesis. The proteins encoded by these genes are mostly involved in regulation of cell proliferation, cell cycle control, and apoptosis, which make them ideal targets for the development of cancer therapy. Targeted therapeutic agents are therefore more likely to be specific and associated with fewer deleterious side effects. They are also expected to be highly active. Because tumor cells accumulate additional genetic changes, multiple factors are likely to contribute toward the abnormal proliferation of cancer cells. This implies that disruption of multiple cellular pathways may be needed to achieve therapeutic efficacy. While no single pathway can solely determine whether a cell will survive or undergo programmed death, the ultimate fate of the cell will depend on the complex balance of multiple antiapoptotic to proapoptotic factors As a consequence, targeted therapy aiming at shifting the balance toward the proapoptotic pathway may require the interruption of multiple antiapoptotic signals to be effective. The new paradigm may involve combining molecular targeted therapy with conventional cytotoxic therapy. Currently, there is a large arsenal of targeted agents of potential use in the treatment of NHLs. Some are still in preclinical testing, but others have already entered the clinical arena.
Oligonucleotide-based therapy, including ASOs, DOs, and other oligonucleotide-mediated therapy, has several unresolved problems. They all share a short half-life and undergo extensive degradation by endocytosis and nucleases. DOs additionally are required to translocate from the cytoplasm into the cell nucleus to exert their effects. The design of a suitable vector to ensure efficient delivery of the oligonucleotides to target cells and enhancing their efficacy and specificity are also critical to minimize nonspecific effects of high doses of oligonucleotides. Several steps have already been undertaken to circumvent these barriers, including the chemical modification of oligonuleotides.
Proteasome inhibition holds promise as a novel effective therapy for several subtypes of NHLs with minimal toxicities. Intuitively, it would seem that proteasome inhibition should be associated with significant toxicities to normal tissues, because the ubiquitin-proteasome pathway is essential to the survival of all cells. However; several reports have documented the greater susceptibility of transformed cells to proteasome inhibition as compared with nonmalignant cells.178,179 Several factors probably contribute to such selectivity, but the exact mechanisms are not fully understood. The proteasome inhibitor is also very effective in inducing cell cycle arrest and apoptosis in different types of cancer cells. This is derived from its ability to affect many pathways involved in promotion of tumorigenesis and apoptosis, resulting in decreased NF-kB–dependent gene transcription, increased level of the tumor suppressor p53, accumulation of p21 and p27 with secondary induction of cell cycle arrest, accumulation of the proapoptotic protein BAX, and down-regulation of signaling through p44/42 MAPK.134
Small-molecule inhibitors or low-molecular-weight compounds will probably prove to be a useful class of therapeutic agents in NHLs. The nonpeptidic compounds are attractive because they share excellent molecular properties in terms of stability, bioavailability, cell permeability, and central nervous system penetration.13 Using structure-based methods, several of these small molecules can be designed or screened for in chemical directories and subsequently tested for inhibitory effects on oncogenes or their products and for in vivo antitumor activity.
Targeting oncogenes represents an important step forward in the treatment of NHLs. The strategies employed, albeit imperfect, continue to evolve. The careful selection of patients based on their molecular profile holds great potential for increased efficacy and diminished toxicities in the future therapy of lymphoid malignancies.
Supported in part through funding from the Veterans Administration Merit Review Award (R.B.G.).
Prepublished online as Blood First Edition Paper, March 31, 2005; DOI 10.1182/blood-2004-12-4621.