Comment on Krauss et al, page 2200
In this issue of Blood, Krauss and colleagues provide evidence that erythropoiesis is indeed a unique process of terminal differentiation that results in nuclear condensation and extrusion without caspase activation of apoptotic machinery.
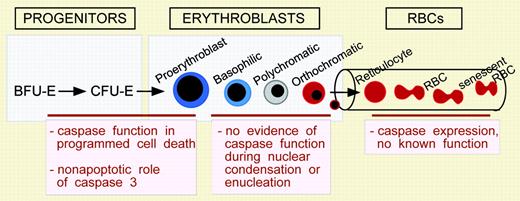
Most cells in the body are nucleated and rely on apoptotic signals to facilitate their demise. Programmed cell death is characterized by the activation of a set of intracellular cysteine proteases, termed caspases, that cleave target proteins to cause characteristic morphologic changes associated with cell death. These changes include nuclear condensation, which is a prominent feature of erythroblast maturation prior to their enucleation (see figure). In fact, red cells are not the only enucleated terminally differentiated cells in mammals. Keratinocytes in the skin and lens epithelial cells in the eye also lose their nuclei during maturation. Limited studies in these 2 cell types indicate that the process of nuclear loss is associated with the activation of caspases, raising the question of whether caspase activation plays a role in terminal maturation of erythroid cells.
In this issue of Blood, Krauss and colleagues examine the process of nuclear condensation in murine erythropoiesis using primarily the in vitro differentiation of Friend virus–infected erythroid cells. No evidence was found for caspase-induced cleavage of lamin B, nuclear pore proteins, or components of the spliceosome as the erythroid nucleus condensed. These findings are consistent with the lack of DNA laddering even after erythroid nuclei are extruded.1 Furthermore, immunohistochemical studies revealed that nuclear pores remain intact but redistribute during late stages of erythroblast maturation, supporting the concept that RNA transcripts continue to traffic to the cytoplasm prior to nuclear extrusion.
Caspases have potential roles at early and possibly late stages of erythroid differentiation, but not as morphologically distinguishable erythroblasts undergo progressive nuclear condensation and enucleation.
Caspases have potential roles at early and possibly late stages of erythroid differentiation, but not as morphologically distinguishable erythroblasts undergo progressive nuclear condensation and enucleation.
Is there a role for programmed cell death in erythropoiesis? Immature erythroid cells, particularly at the erythroid colony-forming unit (CFU-E) and proerythroblast stages, are highly dependent on the hormone erythropoietin for survival and undergo apoptosis upon cytokine withdrawal (see figure). This is thought to be an important mechanism for the regulation of steady-state levels of red cells and in the “stress” response following acute blood loss, and it likely involves caspase cleavage of target proteins, including GATA-1.2 However, caspases may also have nonapoptotic roles in erythropoiesis, since knock-down of caspase-3 transcript levels in erythroid burst-forming unit (BFU-E) leads to a block in erythroid maturation.3 Little is known about the potential nonapoptotic functions of the cell death pathway. Caspases are also expressed in aged erythrocytes, but they do not appear to play a role in the “death” of senescent red cells.4 Thus, paradoxically, caspases appear to function at an early stage of erythroid differentiation but not when erythroblasts undergo the changes in nuclear morphology associated with programmed cell death. It is becoming ever more apparent that the processes of nuclear condensation and enucleation that lead to the mature mammalian erythrocyte are unique in the animal kingdom. ▪