Abstract
Multiple myeloma is an incurable form of lymphoid cancer characterized by accumulation of neoplastic plasma cells in the bone marrow cavity. Little is known about the mechanisms regulating myeloma cell movement within the bone marrow and metastasis to secondary sites. Herein, we identify multiple members of the wingless/int (Wnt) family as promoters of myeloma cell migration/invasion. Wnt-mediated migration was associated with the Wnt/RhoA pathway and did not necessitate signaling through β-catenin. Activation of both RhoA and members of the protein kinase C (PKC) family, including PKCα, PKCβ, and PKCμ, were required for induction of migration. Activated RhoA and PKCα, PKCβ, and PKCμ appear to assemble in macromolecular signaling complexes that are associated with the cell membrane. These results suggest that Wnt responsiveness of myeloma plasma cells may be a significant factor in disease progression. (Blood. 2005;106: 1786-1793)
Introduction
Multiple myeloma (MM) is a malignancy of end-stage B-lineage cells characterized by accumulation of neoplastic plasma cells in the bone marrow (BM) cavity. Although myeloma accounts for only a small percentage of human cancers, this disease is responsible for approximately 20% of all deaths from lymphoid neoplasias as a result of its inherently fatal nature. Myeloma cells are assumed to first enter blood vessels in the periphery from which they migrate through vascular endothelium to the BM microenvironment. Here, they interact with BM stromal cells and subsequently migrate to secondary sites in the BM where they eventually proliferate.
Migration is one of the important processes fundamental to myeloma cell invasion and dissemination. However, little is known about the mechanisms regulating this phenomenon. Clearly, an understanding of this regulation is important, not only in terms of the basic biology of the disease, but also in the development of new clinical strategies to affect progression. Several proteins such as insulin-like growth factor-I (IGF-I),1,2 stromal cell-derived factor-1α,3 macrophage inflammatory protein 1-α,4 monocyte chemoattractant protein-1,5 and vascular endothelial growth factor6 have been suggested to participate in myeloma cell migration, but the complexity of this process makes it likely that other, as yet unidentified molecules, are likely to play significant roles. Previous studies7 have indicated that wingless/ints (Wnts) induce striking morphologic changes in myeloma cells, suggesting altered motility and making Wnts likely candidates as participants in the migratory process.
Wnts comprise a family of glycoproteins that have been shown to be critical for normal development8,9 and have also been implicated in a number of cancers.10,11 Wnt binding to receptor complexes containing a 7 transmembrane protein, Frizzled (Fz), and the low-density lipoprotein receptor-related protein (LRP) 5/612 leads to activation of downstream elements known as Dishevelleds (Dvls)13 and subsequently a number of intracellular signaling cascades. The most well studied of these is referred to as the “canonical” (Wnt/β-catenin) pathway wherein a degradation complex of adenomatous polyposis coli/axin/glycogen synthase kinase-3β is disrupted, leading to stabilization and accumulation of β-catenin. β-Catenin then translocates to the nucleus where, in combination with members of the T-cell transcription factor/lymphoid enhancer factor family, it functions as a transcriptional activator.14 In addition to the canonical pathway, Wnts initiate a second cascade (Wnt/RhoA) that does not require the LRP coreceptor leading to activation of RhoA and associated downstream kinases.7,15-18 RhoA is a member of a family of small guanosine triphosphatases (GTPases) that includes Rac and Cdc42.19 This pathway has been implicated in cell motility and adhesion,20 and several cell types, including melanoma21 and intestinal epithelial cells,22 have been shown to respond with changes in these properties in response to a variety of Wnts. Wnt-mediated migration appears to require activation of both RhoA and, in melanoma cells, members of the protein kinase C (PKC) family of isoenzymes,23 which have also been reported to be involved in other aspects of Wnt signaling.24,25 Activation of PKCs has been associated with a third Wnt signaling pathway characterized by calcium flux and likely involving G-protein-coupled receptors.26,27
Members of the PKC family have been implicated in multiple biologic responses, including cytoskeletal changes, cell adhesion, and motility.28,29 On the basis of their structures and cofactor requirements, the PKC family has been classified into 3 major groups: calcium-dependent classical PKCs (cPKCs) including α, β, and γ; calcium independent, novel PKCs (nPKCs), including δ, ϵ, η, and θ; and calcium and lipid-independent atypical PKCs (aPKCs) including ζ and ι/λ. A fourth subgroup contains PKCμ (also known as PKD), which has distinct structural and enzymatic properties, is ubiquitously expressed and is activated by lipids and platelet-derived growth factor.30
Myeloma cells have been shown to respond to Wnt-3a by activation of both the Wnt/β-catenin and Wnt/RhoA pathways but not the Wnt/Ca++ cascade.7 Wnt-induced morphology changes and cytoskeletal rearrangements, suggesting altered motility in these cells, were entirely associated with the Wnt/RhoA pathway. The present studies were, therefore, undertaken to investigate the question of whether Wnts might actually regulate migration and invasion of myeloma cells and to characterize the signaling elements required for such activity.
Materials and methods
Myeloma cells, cell lines, and conditioned medium
Primary myeloma samples were obtained from patients and CD138+ plasma cells prepared as previously described.31 The Institutional Review Board of the University of Arkansas for Medical Sciences approved the research studies, and all subjects provided written informed consent in accordance with the Declaration of Helsinki. Human MM cell lines ANBL6, Brown, Delta47, H929, MM144, OPM-2, RPMI8226, GX7 and a human T-cell line, Jurkat, were cultured in RPMI-1640 (Biofluids, Rockville, MD) as previously described.32 Human bone marrow stromal cell lines HS-27A and HS-533 were cultured in RPMI-1640 containing heat-inactivated 5% fetal bovine serum. Human umbilical vein endothelial cells (HUVECs)34 were cultured in M199 containing 5% heat-inactivated human serum and 20% fetal calf serum, heparin (25 μg/mL), 2 mM l-glutamine, penicillin (100 U/mL), streptomycin (100 μg/mL). Wnt-3a-conditioned medium (CM) or control medium (con-CM) was prepared in L cells as described.35
Antibodies and reagents
Antibodies used in the present studies with their indicated specificities were purchased from the following sources: p-Ser744/748-PKD/PKCμ, p-PKC (pan), p-Ser638/641-PKCα/β, p-Thr538-PKCθ, p-Thr410/403-PKCζλ, PKCμ, Cell Signaling Technology (Beverly, MA); PKCα, PKCβ, PKCγ, PKCδ, PKCϵ, PKCη, PKCθ, PKCζ, and PKCι/λ, RhoA, anti-Flag, Santa Cruz Biotechnology (Santa Cruz, CA); Rac and Cdc42, Transduction Laboratories (Lexington, KY); horseradish peroxidase-conjugated anti-mouse or antirabbit antibodies, Transduction Laboratories. Inhibitors used were as follows: LY294002, Cell Signaling Technology; Go6976, Go6983, Y27632, CalBiochem, (San Diego, CA). Inhibitors were dissolved in sterile dimethyl-sulfoxide (Sigma, St Louis, MO), divided into aliquots, and stored at -20°C. All compounds were diluted to final concentrations in RPMI-1640 medium immediately before usage.
Transmigration/invasion assays
Wnt-3a-induced MM transendothelial migration was performed as previously described.2 Briefly, HUVECs or bone marrow stromal cell lines HS-5 and HS-27A (seeded at 105/well) were grown on the insert of transwell plates (Corning Costar, Cambridge, MA) for 24 hours at which time the plated cells were determined to be confluent by microscopic observation. Wnt-3a CM diluted to varying concentrations was added to the lower chamber. MM cell suspensions (104/well) were loaded onto the insert and then incubated for 4 hours at 37°C. In experiments performed with specific inhibitors, cells not pretreated or pretreated with inhibitors for 1 hour were loaded onto inserts and incubated in the presence or absence of Wnt-3a. At the end of the incubation period medium from the lower chamber was collected and centrifuged, and the migrating cells were resuspended and enumerated following trypan blue staining in a standard microscope counting chamber. Matrigel invasion chambers (BD Bioscience, Bedford, MA) were seeded with 5 × 104 myeloma cells, and assays were performed as described by the manufacturer.
Constructs, transfectants, retrovirus production, and infection
Plasmids encoding Wnt-1, Wnt-3a, and Wnt-4 cDNAs in pUSEamp vector were purchased form Upstate Biotechnology (Lake Placid, NY). The constructs were transfected into H929 and OPM-2 cells using Lipofectamine (Invitrogen-Life Technologies, Carlsbad, CA) according to manufacturer's instructions. Clonal cell lines were generated by limited dilution in growth media containing 1 mg/mL G418. Positive clones were detected by anti-HA (hemagglutinin) antibody. Wild-type (pCEV-rhoA/WT) and mutant (pCEV-rhoA-N19) RhoA were kindly provided by Dr Toru Miki, Division of Basic Research, National Cancer Institute. The inserts from pCEV-rhoA/WT or the mutant derivative were released with BamHI and EcoRI and ligated into the pFB-neo retroviral vector (Stratagene, La Jolla, CA) containing a Flag tag (Five NH2-terminally deleted epitope-tagged) sequence. The recombinant retrovirus DNAs were transfected into 293T cells with Lipofectamine (Invitrogen-Life Technologies) according to manufacturer's instructions. After 48 hours, packaged virus was collected and used to infect H929 and OPM-2 cells in the presence of polybrene (8 μg/mL). Clonal cell lines were generated by limited dilution in growth media containing 1 mg/mL G418. Positive clones were detected by anti-Flag antibody.
RT-PCR analysis
First-strand cDNA synthesis was performed using ProSTAR Ultro HF reverse transcriptase-polymerase chain reaction (RT-PCR) Kit (Stratagene) primed with random hexamer in a 50-μL reaction mixture containing 1 μg total RNA. The first-strand cDNA mixture (1 μg) was subjected to PCR using PCR Kit (Applied Biosystem, Foster City, CA) in a 50-μL volume according to manufacturer's instructions. All PCR reactions were initiated with a first cycle at 94°C for 3 minutes and a final cycle at 72°C for 10 minutes. Reactions were carried out for 40 cycles under the following conditions: Wnt primers, 94°C for 30 seconds, 60°C for 45 seconds, 72°C for 1 minute. Primer sequences were as follows: Wnt-1, 5′-ATG AAC CTT CAC AAC AAC GAG-3′/5′-TTTCTC GAA GTA GAC GAG GTC-3′; Wnt-2B, 5′-CAC CTG CTG GCG TGC ACT CTC AGA-3′/5′-GGG CTT TGC AAG TAT GGA CGT CCA CAG TA-3′; Wnt-3, 5′-CGG CTG TGA CTC GCA TCA TAA G-3′/5′-CGG TGC TTC TCT ACT ACC ATC TCC-3′; Wnt-3A, 5′-GCC CCA CTC GGA TAC TTC TTA CTC-3′/5′-CTC CTG GAT GCC AAT CTT GAT G-3′; Wnt-4, 5′-AC GTG CGA GAA ACT CAA GGG-3′/5′-CA CAA ACG ACT GTG AGA AGG-3′; Wnt-5A, 5′-CAA GGT GGG TGA TGC CCT GAA GGA G-3′/5′-CGT CTG CAC GGT CTT GAA CTG GTC GTA-3′; Wnt-7A, 5′-GCC GTT CAC GTG GAG CCT GTG CGT GC-3′/5′-AGC ATC CTG CCA GGG AGC CCG CAG CT-3′; Wnt-10B, 5′-GGA GGG CGG CCC CAG AGT TCC-3′/5′-AAG CTG CCA CAG CCA TCC AAC AGG-3′; Wnt-11, 5′-CTG GAA ATG AGG TGT AGG TGC-3′/5′-TGT GTC CCG TGG GAG CCC ACC-3′; Wnt-13, 5′-AAG ATG GTG CCG ACT TCA CCG-3′/5′-CTG CTT TCT TGG GGG CTT TGC-3′; β-actin, 5′-CCACTGGCATC GTGATGGAC-3′/5′-GCGGATGTCC CACGTCACACT-3′.
Subcloning of PCR fragments and DNA sequence analysis
PCR fragments were separated on 1.2% agarose gels and purified using QIAEX Gel Extraction Kit (QIAGEN, Valencia, CA). The PCR fragments were subcloned using TOPO-TA cloning vector according to manufacturer's instructions (Invitrogen). Candidate clones from PCR fragments were subjected to DNA sequence analysis using vector M13 primers. Sequences were determined at the National Cancer Institute (NCI) DNA Sequencing Minicore facility. Data were analyzed using Sequencer 3.1 software and compared with Gene Bank using NCBI BLAST.36
Immunoblotting and immunoprecipitation (IP)
Cells (1 × 107) starved overnight were treated with Wnt-3a for indicated times, or not treated. Following treatment, cells were lysed, extracts were prepared, and Western blotting was performed as previously described.7 For IP, whole-cell lysates (500 μg protein) from cells treated with Wnt-3a-CM or con-CM for indicated times were prepared and precleared by incubation with protein G-Sepharose. Lysates were incubated with anti-PKC antibodies for 2 hours at 4°C. Immune complexes were then adsorbed to protein G-Sepharose beads and washed 3 times. Treated complexes were subjected to immunoblot analysis with anti-RhoA antibody.
Cell fractionation
Cytosolic and membrane proteins were prepared by fractionating cell lysates as described37 with minor modifications. Briefly, cells treated with Wnt-3a or not treated were harvested in hypotonic buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4, 1 mM MgCl2, 0.5 mM CaCl2, and 1 mM EDTA [ethylenediaminetetraacetic acid]) and then homogenized by 30 strokes of a Dounce homogenizer. After removing the nuclear pellet, lysates were separated into cytosolic and membrane fractions by ultracentrifugation at 100 000g (35 000 rpm) for 60 minutes. The membrane pellets were resuspended in hypotonic buffer containing 0.1% sodium dodecyl sulfate (SDS). Proteins from each fraction were subjected to immunoblot analysis.
Rho family GTPase activation assay
Statistical analysis
Student t test was performed to analyze the statistical significance of differences between experimental groups using the Microsoft Excel t test 2 sample assuming unequal variance statistical software package (Microsoft, Redman, WA). The P values less than .05 by the 2-tailed test were considered significant.
Results
Wnts stimulate transmigration by myeloma cell lines and patient plasma cells
Initial studies were performed to assess the effect of Wnt-3a on migration and invasion using a transwell migration assay. Wnt-3a CM or con-CM was added to the lower chamber of transwell plates containing micropore filters precoated with HUVECs after which MM cells were added to the upper chamber. As shown in Figure 1A-B, Wnt-3a significantly induced migration of MM cell lines H929 (Figure 1A) and OPM-2 (Figure 1B) through HUVECs in a dose-dependent manner. Maximal responses were seen at 100% of Wnt-3a CM for both lines (P < .01), compared with con-CM treatment. In similar experiments, using 2 BM-derived stromal cell lines HS-27A (epithelial-like) and HS-5 (fibroblast-like) to coat membranes, Wnt-3a induced transmigration of both myeloma lines (Figure 1C-D) in a manner comparable to that seen with HUVECs. Experiments using matrigel-coated invasion chambers similarly revealed Wnt-3a mediated invasion through extracellular matrix (not shown) in agreement with results of the transmigration assay. To extend this observation to patient material, CD138+ primary myeloma plasma cells isolated from clinical samples were similarly assayed. Wnt-3a significantly (P < .05) induced migration in 4 of 7 patient samples (Figure 1E). The magnitude of this effect was comparable in 3 of 4 patients to that seen with IGF-I (P < .01), which has previously been shown to be a strong chemoattractant for myeloma cells.2 Of the 3 samples that did not respond to Wnt-3a, 2 were also unresponsive to IGF-I. Enhancement of migration was not observed with a series of B-cell lymphomas or Epstein-Barr virus-derived “normal” B-cell lines (not shown). Additional biochemical analysis of lysates from plasma cells of 6 patients, including 5 used in the migration assay (D62, D70, D73, D81, and D95), revealed that Wnt-3a induced stabilization of β-catenin in all samples (data not shown), suggesting that functional Wnt signaling pathways are present in virtually all primary myeloma.
Wnt-3a induces migration and invasion by MM cell lines and CD138+primary plasma cells. MM cells (H929 [A,C] and OPM-2 [B,D]) were starved in serum-free medium for 3 hours and added to transwell chambers containing a polycarbonate pore membrane (5-μM pore size) on which HUVECs (A-B) or bone marrow stromal cell lines (C-D) were pregrown for 24 hours. Wnt-3a CM diluted in con-CM (vol/vol) (A-B) or undiluted (C-D,E) was added to the lower chamber and after 4 hours of incubation cells in lower chamber were harvested and counted. The results are shown as mean ± SE (n = 3). Results are representative of 3 independent experiments. Primary plasma cells purified from bone marrow of patients with myeloma (E) were assayed for transmigration through HS-5 bone marrow stromal cell as described for panel C using IGF-I as a positive control for migration. The results are shown as mean ± SE (n = 3). *P < .05, **P < .01, ***P < .001 versus control.
Wnt-3a induces migration and invasion by MM cell lines and CD138+primary plasma cells. MM cells (H929 [A,C] and OPM-2 [B,D]) were starved in serum-free medium for 3 hours and added to transwell chambers containing a polycarbonate pore membrane (5-μM pore size) on which HUVECs (A-B) or bone marrow stromal cell lines (C-D) were pregrown for 24 hours. Wnt-3a CM diluted in con-CM (vol/vol) (A-B) or undiluted (C-D,E) was added to the lower chamber and after 4 hours of incubation cells in lower chamber were harvested and counted. The results are shown as mean ± SE (n = 3). Results are representative of 3 independent experiments. Primary plasma cells purified from bone marrow of patients with myeloma (E) were assayed for transmigration through HS-5 bone marrow stromal cell as described for panel C using IGF-I as a positive control for migration. The results are shown as mean ± SE (n = 3). *P < .05, **P < .01, ***P < .001 versus control.
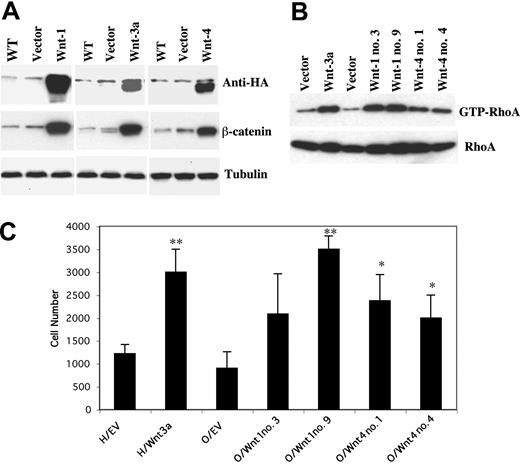
To investigate the possibility that other Wnt family members might also promote myeloma cell migration, we first examined endogenous Wnt mRNA expression by RT-PCR in a number of myeloma cell lines. As shown in Table 1, Wnt-1, -3, -3a, and -4 were the only family members not detected in any myeloma lines. Wnt-1 and -4 were therefore selected for further study with Wnt-3a included as a control. Since soluble forms of Wnt-1 and -4 are not available, myeloma lines were transfected with constructs encoding corresponding cDNAs. Expression of Wnts in isolated clones, as detected by anti-HA antibody, led to both stabilization of β-catenin (Figure 2A) and activation of RhoA (Figure 2B) but not other members of the RhoA family, including Rac and Cdc-42 (not shown). These clones were next evaluated for migration using a modification of the transwell assay in which minimal migration-inducing concentrations of IGF-I were placed in the lower chambers and the transfected myeloma cells in the upper chamber. Following incubation, chambers were scored for the ability of transfected Wnts to enhance the IGF-1 effect. As shown in Figure 2C, expression of Wnt-3a, Wnt-1 (1 of 2 clones), or Wnt-4 (2 of 2 clones) all significantly promoted IGF-I-mediated migration. These results indicate that multiple Wnts are capable of affecting this biologic process.
Expression of Wnt-1, -3a, and -4 enhances IGF-I-mediated migration of MM cells. (A) H929 and OPM-2 cells were transfected with empty vector or plasmid constructs containing Wnt-1, -3a, and -4 cDNAs with an HA-tag as described in “Material and methods.” Cell lysates were resolved on 12% SDS-polyacrylamide gels, proteins transferred to membranes and blotted with anti-HA antibody to confirm Wnt expression (top). Cytosolic fractions from cell lysates of positive clones were resolved on 8% SDS-polyacrylamide gels, transferred to membranes, and blotted with anti-β-catenin antibody (middle). The same fractions were also blotted with antitubulin as a control for protein loading (bottom). (B) Positive clones expressing Wnts (H929/Wnt-3a and OPM-2/Wnt-1, -4) or empty vector were starved in serum-free medium for 12 hours. Cell lysates were prepared and incubated with GST-Rho binding domain glutathione beads. Beads were collected by centrifugation, and the bound proteins were analyzed by Western blotting using RhoA-specific antibodies. The same lysates were subjected to 12% SDS-PAGE and blotted with anti-RhoA as a control for protein loading. (C) H929 cells expressing Wnt-3a (H/Wnt3a) or empty vector (H/EV) and OPM-2 cells expressing Wnt-1 (O/Wnt1 no. 3, no. 9) or Wnt-4 (O/Wnt4 no. 1, no. 4) or empty vector (O/EV) were starved in serum-free medium for 3 hours and added to chambers containing polycarbonate pore membranes (5-μM pore size) on which HS-27A stromal cells were pregrown for 24 hours. Growth medium containing IGF-I (12.5 ng/mL) was added to the lower chamber, and after 4 hours incubation cells in lower chamber were harvested and counted. The results are shown as mean ± SE (n = 3). Results are representative of 3 independent experiments. *P < .05, **P < .01 versus cells transfected with empty vectors.
Expression of Wnt-1, -3a, and -4 enhances IGF-I-mediated migration of MM cells. (A) H929 and OPM-2 cells were transfected with empty vector or plasmid constructs containing Wnt-1, -3a, and -4 cDNAs with an HA-tag as described in “Material and methods.” Cell lysates were resolved on 12% SDS-polyacrylamide gels, proteins transferred to membranes and blotted with anti-HA antibody to confirm Wnt expression (top). Cytosolic fractions from cell lysates of positive clones were resolved on 8% SDS-polyacrylamide gels, transferred to membranes, and blotted with anti-β-catenin antibody (middle). The same fractions were also blotted with antitubulin as a control for protein loading (bottom). (B) Positive clones expressing Wnts (H929/Wnt-3a and OPM-2/Wnt-1, -4) or empty vector were starved in serum-free medium for 12 hours. Cell lysates were prepared and incubated with GST-Rho binding domain glutathione beads. Beads were collected by centrifugation, and the bound proteins were analyzed by Western blotting using RhoA-specific antibodies. The same lysates were subjected to 12% SDS-PAGE and blotted with anti-RhoA as a control for protein loading. (C) H929 cells expressing Wnt-3a (H/Wnt3a) or empty vector (H/EV) and OPM-2 cells expressing Wnt-1 (O/Wnt1 no. 3, no. 9) or Wnt-4 (O/Wnt4 no. 1, no. 4) or empty vector (O/EV) were starved in serum-free medium for 3 hours and added to chambers containing polycarbonate pore membranes (5-μM pore size) on which HS-27A stromal cells were pregrown for 24 hours. Growth medium containing IGF-I (12.5 ng/mL) was added to the lower chamber, and after 4 hours incubation cells in lower chamber were harvested and counted. The results are shown as mean ± SE (n = 3). Results are representative of 3 independent experiments. *P < .05, **P < .01 versus cells transfected with empty vectors.
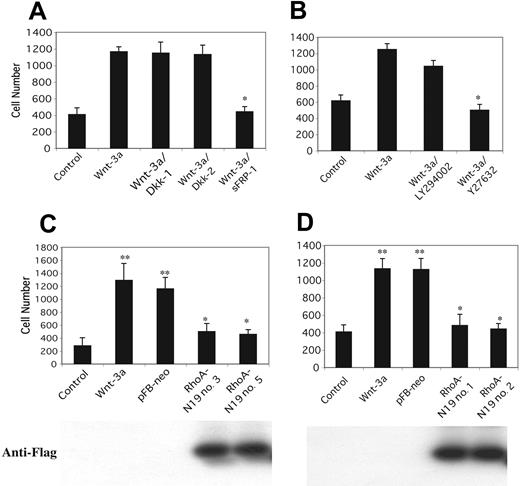
To determine which of the 2 Wnt pathways activated in myeloma cells regulated migration, a series of inhibition studies were performed. Secreted Frizzled related protein-1 (sFRP-1),40 which blocks all Wnt signaling by binding directly to Wnts, completely inhibited transmigration (Figure 3A). In contrast, Dickkopf-1 (Dkk-1) and Dkk-2,41,42 which block signaling only through the Wnt/β-catenin pathway by binding to the LRP coreceptor, showed no effect on migration. Taken together, these results suggest that migration is completely regulated by the Wnt/RhoA pathway. To test this possibility, the Rho-kinase inhibitor Y27632, which has been previously shown to block Wnt-induced morphologic changes in myeloma cells,7 was used. Pretreatment of cells with Y27632 completely blocked Wnt-3a-induced migration, whereas other compounds such as the PI-3K inhibitor LY294002, the mitogen-activated protein kinase (MAPK) inhibitor PD98059, and rapamycin which leads to inhibition of p70S6 kinase had no effect (Figure 3B and data not shown). To confirm the role of RhoA, H929 (Figure 3C) and OPM-2 (Figure 3D) cells were transfected with dominant-negative RhoA-N19. Compared with vector-transfected controls, Wnt-3a-induced migration was almost completely blocked in both cell lines expressing dominant-negative construct. These results indicate that activation of the Wnt/RhoA pathway is requisite for Wnt-induced migration.
Rho-kinase inhibitor and dominant-negative RhoA block Wnt-3a-induced migration. (A) H929 cells pretreated with sFRP-1 or Dickkopf-1/2 (Dkk1/2) or (B) phosphatidylinositol 3-kinase (PI-3K) inhibitor LY294002 (LY, 10 μM) or Rho-kinase inhibitor Y27632 (10 μM) for 1 hour were plated on polycarbonate pore membranes on which the bone marrow stromal cell line HS-5 was pregrown for 24 hours. Wnt-3a CM containing the corresponding inhibitors was added to the bottom chambers followed by incubation for 4 hours. Cells in the lower chamber were harvested and counted as described in Figure 1. Effect of expression of mutant RhoA-N19 on migration of H929 (C) or OPM-2 (D) cells. Wild-type cells or clones expressing vector (PFB-neo) or mutant RhoA (RhoA-N19 nos. 1, 2, 3, and 5) were subjected to migration assay as described for panel A. Results are shown as mean ± SE (n = 3). Figures are representative of 3 separate experiments, respectively. *P < 0.01, **P < .001. Cell lysates isolated from the same clones were subjected to 12% SDS-PAGE and blotted with anti-Flag antibody to confirm expression of constructs (bottom).
Rho-kinase inhibitor and dominant-negative RhoA block Wnt-3a-induced migration. (A) H929 cells pretreated with sFRP-1 or Dickkopf-1/2 (Dkk1/2) or (B) phosphatidylinositol 3-kinase (PI-3K) inhibitor LY294002 (LY, 10 μM) or Rho-kinase inhibitor Y27632 (10 μM) for 1 hour were plated on polycarbonate pore membranes on which the bone marrow stromal cell line HS-5 was pregrown for 24 hours. Wnt-3a CM containing the corresponding inhibitors was added to the bottom chambers followed by incubation for 4 hours. Cells in the lower chamber were harvested and counted as described in Figure 1. Effect of expression of mutant RhoA-N19 on migration of H929 (C) or OPM-2 (D) cells. Wild-type cells or clones expressing vector (PFB-neo) or mutant RhoA (RhoA-N19 nos. 1, 2, 3, and 5) were subjected to migration assay as described for panel A. Results are shown as mean ± SE (n = 3). Figures are representative of 3 separate experiments, respectively. *P < 0.01, **P < .001. Cell lysates isolated from the same clones were subjected to 12% SDS-PAGE and blotted with anti-Flag antibody to confirm expression of constructs (bottom).
Role of PKC family members in Wnt-mediated migration
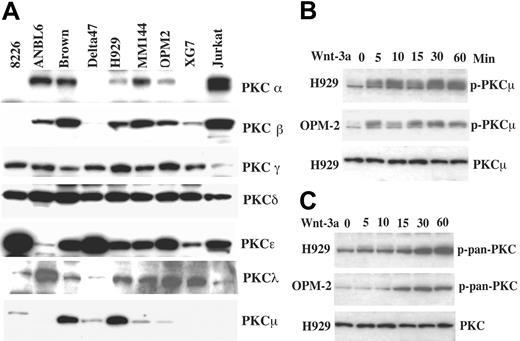
PKC family members have previously been implicated in other systems in both cytoskeletal changes and cell motility.28,29 We have recently shown that IGF-I-mediated MM cell migration is PKC and RhoA dependent.2 We, therefore, next evaluated possible PKC involvement in Wnt-induced myeloma migration. Immunoblotting analysis revealed that most PKC isoforms are expressed at relatively high levels in the majority of myeloma cell lines (Figure 4A). PKCλ is expressed in all MM cell lines at lower levels (exposure time for the film is 10 times longer than other described isoforms) and PKCμ was variably expressed. Note that the 2 lines used as examples in these studies, H929 and OPM-2, have PKCμ expression, ranging from high to barely detectable. In contrast, PKCη, PKCθ, and PKKζ were not observed. We next examined whether Wnt-3a treatment leads to activation of PKC family members using antibodies specific for phosphorylated residues associated with activation and cell localization studies. As seen in Figure 4B, Wnt-3a treatment of both H929 and OPM-2 cells leads to phosphorylation of PKD/PKCμ as detected by antibody specific for p-Ser744/748 (upper band). Phosphorylation is observed by 5 minutes and remains for at least 60 minutes. Similar studies using phospho-specific antibodies to all other expressed PKCs failed to reveal a change in residues associated with activation (not shown). However, phosphorylation detected by a polyclonal pan-PKC (Figure 4C) that recognizes the conserved carboxy terminal Ser660 present in most PKCs (with the exception of atypical PKCs) was increased, suggesting that other PKC isoforms may also be activated.
Expression of PKC isoforms and phosphorylation of PKCμ following Wnt-3a treatment. (A) Cell lysates from the indicated lines were resolved on 8% SDS-PAGE gels, transferred to membranes, and blotted with the indicated antibodies. Lysate from Jurkat cells was included as a control for PKC expression. (B) Myeloma cell lines starved in serum-free medium for 12 hours were treated with Wnt-3a or con-CM for the indicated time. Lysates were resolved on 8% SDS-PAGE gels, transferred to membranes, and blotted with anti-p-PKCμ (B) or anti-p-pan-PKC (C) antibodies.
Expression of PKC isoforms and phosphorylation of PKCμ following Wnt-3a treatment. (A) Cell lysates from the indicated lines were resolved on 8% SDS-PAGE gels, transferred to membranes, and blotted with the indicated antibodies. Lysate from Jurkat cells was included as a control for PKC expression. (B) Myeloma cell lines starved in serum-free medium for 12 hours were treated with Wnt-3a or con-CM for the indicated time. Lysates were resolved on 8% SDS-PAGE gels, transferred to membranes, and blotted with anti-p-PKCμ (B) or anti-p-pan-PKC (C) antibodies.
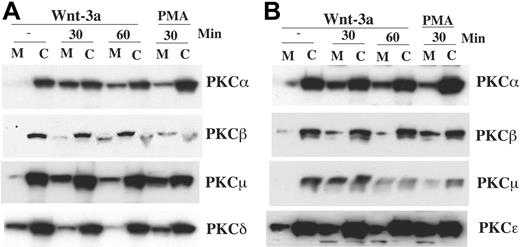
To further investigate the status of PKC family members, cellular localization was assessed as movement from cytosolic to membrane fraction correlates with PKC activation.43 Following Wnt-3a treatment, proteins were isolated from the respective cellular fractions and subjected to immunoblot analysis. Membrane fractions of both H929 and OPM-2 (Figure 5A-B) evidenced increases in PKCα, PKCβ, and PKCμ, whereas no other isozymes were increased in this fraction (not shown) with the exception of PKCϵ that only increased in OPM-2.
Wnt-3a alters cellular localization of PKCs α, β, and μ. H929 (A) and OPM-2 (B) cells starved in serum-free medium for 12 hours were treated with Wnt-3a or con-CM for the indicated time. Cell lysates from cytosolic (C) or membrane (M) fraction were prepared as described in “Material and methods.” Lysates were resolved on 8% SDS-PAGE gels, transferred to membranes, and blotted with indicated antibodies. Treatment of cells with 100 nM PMA (phorbol 12-myristate 13-acetate) for 30 minutes was used as a positive control for PKC membrane translocation.
Wnt-3a alters cellular localization of PKCs α, β, and μ. H929 (A) and OPM-2 (B) cells starved in serum-free medium for 12 hours were treated with Wnt-3a or con-CM for the indicated time. Cell lysates from cytosolic (C) or membrane (M) fraction were prepared as described in “Material and methods.” Lysates were resolved on 8% SDS-PAGE gels, transferred to membranes, and blotted with indicated antibodies. Treatment of cells with 100 nM PMA (phorbol 12-myristate 13-acetate) for 30 minutes was used as a positive control for PKC membrane translocation.
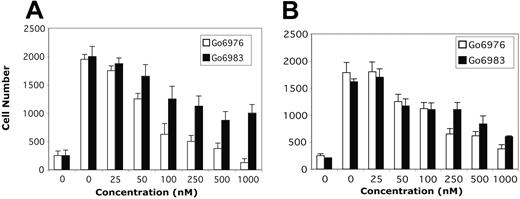
Having demonstrated activation of PKCα, PKCβ, and PKCμ following Wnt treatment, we next sought to determine whether these isoforms have a role in migration. For these experiments, 2 specific PKC inhibitors Go6976 and Go6983 were used.44 Go6976 inhibits the classical PKC isoforms and PKCμ, whereas Go6983 suppresses kinase activity of PKC isoenzymes from all 3 major subgroups but does not effectively inhibit PKCμ. Pretreatment of H929 cells with Go6976 or Go6983 for 1 hour inhibited migration through the HS-27 cell line in a dose-dependent manner (Figure 6A). The effect of Go6983 was only partial even at maximal concentration. Similar results were obtained with OPM-2 (Figure 6B). Taken together, these observations with results from the translocation studies suggest that Wnt-mediated migration is PKC dependent and that PKCμ is one member of this family facilitating the migratory process.
PKC kinase inhibitors block Wnt-3a-induced migration. H929 (A) and OPM-2 (B) cells pretreated with increasing concentrations (0.025-1 μM) of PKC inhibitors were subjected to migration assays as described in Figure 1. Polycarbonate pore membranes were coated with the HS-27A stromal cell line and inhibitor, plus Wnt-3a was included in the lower chamber. Results are shown as mean ± SE (n = 3). Results are representative of 3 independent experiments.
PKC kinase inhibitors block Wnt-3a-induced migration. H929 (A) and OPM-2 (B) cells pretreated with increasing concentrations (0.025-1 μM) of PKC inhibitors were subjected to migration assays as described in Figure 1. Polycarbonate pore membranes were coated with the HS-27A stromal cell line and inhibitor, plus Wnt-3a was included in the lower chamber. Results are shown as mean ± SE (n = 3). Results are representative of 3 independent experiments.
Wnt signaling complexes
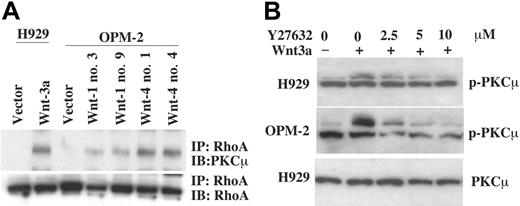
As both RhoA and PKCμ are activated by Wnt treatment, the relationship between these 2 elements was examined. Lysates were prepared from the Wnt-1-, -3a-, and -4-transfected clones and subjected to IP with anti-RhoA antibody followed by blotting with anti-PKCμ. PKCμ was found associated with RhoA in all clones except the empty vector controls (Figure 7A). Furthermore, pretreatment of H929 or OPM-2 cells with the RhoA inhibitor Y27632 led to dose-dependent inhibition of PKCμ phosphorylation (Figure 7B). These results indicate that PKCμ is in a complex including RhoA and places PKCμ downstream of RhoA in the Wnt signaling pathway.
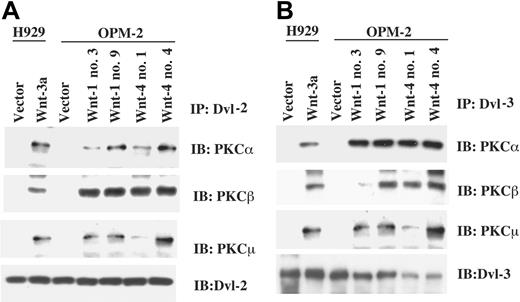
Previous studies have indicated that upon Wnt binding to its receptor, a complex is formed, including the downstream element Dvl and RhoA,17,45 and expression of Dvl-1 activates Rho kinase18 in COS cells. As the present data indicate that RhoA is associated with PKCμ (Figure 7) following Wnt expression, the possibility exists that Dvl, RhoA, and PKCs are all in the same complex. To examine this question, coimmunoprecipitation studies were performed on the Wnt-1, -3a, and -4 transfectants, wherein lysates were subject to immunoprecipitation with anti-Dvl-2 or -3, followed by blotting with antibodies to PKCs. Results of these experiments revealed that PKCα, PCKβ, and PKCμ were associated with both Dvl-2 (Figure 8A) and Dvl-3 (Figure 8B), suggesting that several PKCs are likely included in the Dvl/RhoA complex. The presence of RhoA in Dvl complexes was confirmed by immunoblotting analysis (data not shown).
Expression of Wnts induces RhoA/PKCμ association. (A) Cell lysates from Wnt-transfected clones were incubated with anti-RhoA antibody. Immunoprecipitates (IP) were subjected to 8% SDS-PAGE, transferred to membranes, and blotted with anti-PKCμ antibody. IB indicates immunoblot. (B) Cells starved in serum-free medium and then pretreated with indicated concentrations of Rho-kinase inhibitor Y27632 (2.5-10 μM) were untreated or stimulated with Wnt-3a for 10 minutes. Cell lysates were subjected to SDS-PAGE and blotted with anti-p-PKCμ.
Expression of Wnts induces RhoA/PKCμ association. (A) Cell lysates from Wnt-transfected clones were incubated with anti-RhoA antibody. Immunoprecipitates (IP) were subjected to 8% SDS-PAGE, transferred to membranes, and blotted with anti-PKCμ antibody. IB indicates immunoblot. (B) Cells starved in serum-free medium and then pretreated with indicated concentrations of Rho-kinase inhibitor Y27632 (2.5-10 μM) were untreated or stimulated with Wnt-3a for 10 minutes. Cell lysates were subjected to SDS-PAGE and blotted with anti-p-PKCμ.
Discussion
Migration is one of the important processes fundamental to myeloma cell invasion and dissemination; however, little is know about the mechanisms regulating this phenomenon. Extravasation of myeloma cells from blood vessels into the BM is likely to be controlled by several chemoattractants. Among these, we and others have previously shown that IGF-I is a potent effector of myeloma cell transmigration through vascular endothelium and BM stromal cells.1,2 Interestingly, migration is promoted at low IGF-I concentrations, but inhibited at higher concentrations at which the biologic response is shifted to proliferation. This observation has led to the hypothesis that IGF-I produced by bone marrow stromal cells forms gradients leading initially to attraction of myeloma cells into the BM cavity where higher concentrations promote survival and proliferation. As a result, a need exists for other factors to regulate movement within the BM. We have previously shown that Wnt-3a induces morphologic changes in myeloma cells, suggesting the possibility of altered motility.7 In the present study, we have investigated the function of Wnts as chemoattractants for myeloma plasma cells and characterized the signaling pathways regulating this process.
Initial studies demonstrated that Wnt-3a-induced migration of MM cell lines through vascular endothelial and BM stromal cells (Figure 1) as well as artificial extracellular membrane (matrigel). Furthermore, the same effect was observed using purified plasma cells from patients with myeloma. Additionally, both Wnt-1 and Wnt-4 were able to affect myeloma cell migration (Figure 2). These 2 Wnts were selected as, in addition to Wnt-3 and Wnt-3a, they are the only Wnt family members not expressed in myeloma cell lines. The expression of multiple other Wnt mRNAs (Wnt-2b, -5a, -7A, -10B,-11, and -13) suggests possible autocrine signaling loops, but further studies will be necessary to evaluate possible biologic responses associated with endogenous production of these mRNAs. The present data are consistent with recent reports that Wnts function in inducing cell migration of malignant and normal cells in other systems. Expression of Wnt-5a leads to increased migration and invasion of human melanoma cells.21 Treatment of intestinal epithelial cells with Wnt-11-conditioned medium enhances migration22 and Drosophila Wnt-4 is required for ovarian morphogenesis.46 It is important to note that Wnt-3a did not enhance migration of B-cell lymphomas or normal human B cells, indicating that the effect is restricted to end-stage plasma cells and is thus highly specific within the B-cell lineage.
In contrast to other chemoattractants such as IGF-I, Wnts are largely insoluble and thought to be predominantly bound to cell-surface matrixes following secretion. Matrix-bound Wnts are postulated to form gradients that provide signals promoting directional cell migration. Previous studies have demonstrated that several Wnt family members (Wnt-2, -4, -5A, -7A, and -10) are expressed in human BM stromal cells,47,48 potentially providing an ample source of Wnt proteins to participate in migratory processes. These observations lead us to propose a model wherein soluble factors such as IGF-I diffuse into blood vessels where low concentrations induce migration of myeloma cells into the BM compartment. Here, the IGF-I concentrations are higher and promote survival and proliferation. In this environment the cells become exposed to Wnt gradients which promote interaction with stromal cells and dissemination through the BM, leading to disease progression.
Having demonstrated that Wnts promote migration and invasion by MM cells, additional studies characterized the downstream signaling pathways associated with this biological response. Previously, we have shown that Wnt-3a activates both the canonical Wnt/β-catenin and the Wnt/RhoA pathways in myeloma cells.7 Signaling through the Wnt/β-catenin pathway requires the LRP5/6 coreceptor and can be inhibited by Dkk-1 and Dkk-2 proteins that interact with the coreceptor.41,42 Wnt-3a-induced myeloma cell migration could be blocked by sFRP-1, which binds directly to Wnts (Figure 3), but not by Dkk-1/2, indicating that the Wnt/β-catenin pathway is not involved in migration.
Expression of Wnts induces Dvl/PKC association. Cell lysates from Wnt-transfected clones were incubated with anti-Dvl-2 (A) or anti-Dvl-3 antibody (B). Immunoprecipitates were subjected to 8% SDS-PAGE, transferred to membranes, and blotted with anti-PKCα, -PKCβ, or -PKCμ antibodies.
Expression of Wnts induces Dvl/PKC association. Cell lysates from Wnt-transfected clones were incubated with anti-Dvl-2 (A) or anti-Dvl-3 antibody (B). Immunoprecipitates were subjected to 8% SDS-PAGE, transferred to membranes, and blotted with anti-PKCα, -PKCβ, or -PKCμ antibodies.
Assessment of the Wnt/RhoA pathway revealed that Wnt-1, -3a, and -4 induced activation of RhoA. These findings are consistent with previous reports showing that expression of Wnt-1 and Wnt-3a in COS cells or Wnt-3a treatment of Chinese hamster ovary (CHO) cells led to activation of Rho-associated kinase coiled-coil containing protein kinase (ROCK).18 Several observations in the present study clearly define a critical role for RhoA in Wnt-mediated myeloma cell migration. First, multiple Wnts induce RhoA activation, which correlates with enhanced migration. Second, Wnt-3a-mediated migration is completely blocked by a ROCK inhibitor, whereas MAPK, PI-3K, and p70S6K inhibitors have no effect. Third, a dominant-negative RhoA construct also completely inhibits Wnt-3a-induced migration. The RhoA pathway is known to regulate motility through reorganization of the actin cytoskeleton and stress fiber and focal adhesion formation.49,50 In Drosophila and Xenopus, Wnt-activated RhoA signaling is required for cell movement and planar cell polarity,15,17,45 and RhoA signaling also promotes migration and invasion of hepatoma cells,51,52 as well as in vivo tumor metastasis of murine fibroblast cell lines.53 Thus, the RhoA pathway plays a central role in both normal cell migration and likely numerous forms of malignant cell invasion.
Other cellular proteins frequently involved in motility include members of the PKC family. Evaluation of this family in myeloma cells revealed that PKCα, PKCβ, and PKCμ are downstream targets in the Wnt signaling pathway. Most PKC isoenzymes, including members of the classical, novel, and atypical subgroups, were expressed and constitutively phosphorylated, but they appeared unchanged following Wnt-3a treatment. Only the PKCμ isoenzyme evidenced a clear change in the phosphorylation state (Figure 4). However, because an increase in phosphorylated PKCs was also detected with a pan pPKC reagent, it seemed likely that other PKCs may have been phosphorylated at sites other than those examined. Cellular localization studies revealed that PKCα, PKCβ, and PKCμ were translocated from cytosolic to membrane fractions, indicating activation of all 3 (Figure 5). To ascertain whether these proteins were critical to migration, 2 PKC inhibitors were used in transwell assays. Treatment with Go6976, which inhibits cPKCs and PKCμ, led to nearly complete abrogation of migration, whereas Go6983, which suppresses kinase activity of PKC isoenzymes from the 3 major subgroups but does not effectively inhibit PKCμ kinase activity,44,54 produced only a partial effect (Figure 6). These observations suggest that multiple PKCs are requisite for Wnt-mediated migration and invasion and that PKCμ is a critical participant in this process. Of interest, PKCμ activation is also required for IGF-1-induced migration in myeloma cells,2 and other studies indicate that Wnt-mediated PKC activation is necessary for motility of a variety of cell types, including melanoma21 and epithelial22 cells.
As both RhoA and PKCs are critical to Wnt-mediated migration, it was of interest to examine any potential relationship between these 2 elements. Activation of PKCμ was completely abrogated by treatment with the ROCK inhibitor Y2762 (Figure 7B). In contrast, neither PKC inhibitor affected RhoA-GTP formation (data not shown). These results suggest that PKCμ is downstream of RhoA, and activation of ROCK leads either directly or indirectly to PKCμ phosphorylation, placing at least 1 PKC family member in the Wnt/RhoA pathway. Additionally, RhoA/PKCμ complexes were detected in myeloma clones expressing Wnt-1, -3a, or -4 (Figure 7A). In contrast, complexes of other PKC isozymes with RhoA were not observed. Because PKCμ activation in several cell types is dependent on activation of other PKC isoforms,55 we cannot exclude the possibility that PKCα or PKCβ (which are both activated by Wnt-3a) contribute to activation of PKCμ. PKCμ activation through distinct PKC isoforms probably reflects cell-type-specific function because PKCϵ and PKCη induce activation of PKCμ in COS7 cells.55 A possible role for PKCϵ in myeloma cells is unclear because membrane translocation was observed in only 1 of 2 lines analyzed. PKCη, which is required for activation of PKCμ in other cell types, is not expressed in myeloma cells.
Wnt interaction with Fz receptors results in the activation of PKCs through Dvl proteins thought to be proximal to the receptor,56 and we have previously shown that Wnt-3a activates Dvl-2 and -3 in myeloma cells.7 Having observed that PKCμ associated with RhoA, the question of whether PKCs also associated with Dvls was examined. IP experiments demonstrated that both Dvl-2 and -3 formed complexes with PKCα, PKCβ, and PKCμ, but no other isoforms in myeloma clones expressing Wnt-1, -3a, or -4. Taken together, these results suggest that Wnts induce macromolecular signaling complexes that include Dvl, RhoA, and PKCs. Future studies aimed at understanding the composition and dynamics of such complexes should significantly advance our understanding of the process by which various Wnts activate specific downstream pathways resulting in different biologic responses.
The experiments described in the present study identify for the first time Wnts as promoters of migration and invasion in a lymphoid malignancy and provide a model to address the phenomenon of myeloma plasma cell metastasis and dissemination within the bone marrow. It will be important to determine whether Wnts play a similar role in other lymphoid cancers and whether therapies designed to affect Wnt signaling may prove beneficial in modulating these diseases.
Prepublished online as Blood First Edition Paper, May 10, 2005; DOI 10.1182/blood-2005-01-0049.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Wnt-3a induces migration and invasion by MM cell lines and CD138+ primary plasma cells. MM cells (H929 [A,C] and OPM-2 [B,D]) were starved in serum-free medium for 3 hours and added to transwell chambers containing a polycarbonate pore membrane (5-μM pore size) on which HUVECs (A-B) or bone marrow stromal cell lines (C-D) were pregrown for 24 hours. Wnt-3a CM diluted in con-CM (vol/vol) (A-B) or undiluted (C-D,E) was added to the lower chamber and after 4 hours of incubation cells in lower chamber were harvested and counted. The results are shown as mean ± SE (n = 3). Results are representative of 3 independent experiments. Primary plasma cells purified from bone marrow of patients with myeloma (E) were assayed for transmigration through HS-5 bone marrow stromal cell as described for panel C using IGF-I as a positive control for migration. The results are shown as mean ± SE (n = 3). *P < .05, **P < .01, ***P < .001 versus control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/5/10.1182_blood-2005-01-0049/6/m_zh80170583390001.jpeg?Expires=1769849294&Signature=fEFRQFgek2FXHU9LF9B-2R4OHlBbp~jh~P1O-o9l60fWuILggZwsHTO4PemdtA6~qTo7aBaCAyPrhr8dcC~o6u1~DTOnxq0MGb04~UhkYRkI16uWxwrI31DZ1WMVLawrbz5J159o0zt~HzDGVndq-NK2haH72ON9RCcnCsDOdUj8ugBtYl9WTuDuTGtFakjcVGgy1BJnq~Tcxa86GeXuzyvZRikT65rdzbQlD0MEFWTa52tfOlgqHM37trHiPsTwtWRJqdf1nRK49xpMKJaEsnnZ-1qS-VejUV5RjQO~lV6RAwkhbrSRVN13QzXWjdX~~ZdjKXKEt1fniqL15Wq06w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)