Abstract
The phosphoinositide 3-kinase (PI3K)/Akt signaling pathway has been shown to be frequently activated in blast cells from patients with acute myeloid leukemia (AML) and to contribute to survival and proliferation of these cells. Of the 8 distinct mammalian isoforms of PI3K, it is the class I PI3Ks (p110α, p110β, p110γ, and p110δ) that are responsible for Akt activation. It is not known which PI3K isoform is critical in AML. Here we show that the p110δ isoform of PI3K is consistently expressed at a high level in blast cells from AML, in contrast to the other class I isoforms, the expression of which was very variable among patients. IC87114, a p110δ-selective inhibitor, suppressed both constitutive and Flt-3–stimulated Akt activation in blasts to the same extent as Ly294002, an inhibitor of all PI3K isoforms. Moreover, IC87114 inhibited AML cell proliferation without affecting the proliferation of normal hematopoietic progenitor cells. These observations identify p110δ as a potential therapeutic target in AML.
Introduction
AML is a clonal hematologic disease and is due to acquired mutations in immature progenitors, resulting in a block of differentiation leading to an accumulation of myeloid blasts.1 Two classes of mutations, one impairing cell differentiation and the other conferring survival and proliferative benefits, are known to cooperate to cause acute leukemia.2 Phosphoinositide 3-kinase (PI3K) and its downstream target Akt have been reported to be frequently constitutively activated in leukemic blasts from patients with AML and to contribute to cell survival and proliferation.3-5 Moreover, constitutive phosphorylation of Akt or of forkhead in rabdomyosarcoma (FKHR), one of its substrates, correlates with decreased survival in patients with AML.3,6 Among the 8 isoforms of PI3K, the class I PI3Ks are responsible for Akt activation in cells. These PI3Ks are heterodimers composed of a catalytic and a regulatory subunit. The class IA PI3K catalytic subunits (p110α, p110β, and p110δ) associate with Src homology domain 2 (SH2)–containing regulatory subunits and signal downstream of cytokine and tyrosine kinase receptors. p110γ is the only class IB PI3K, and functions in the context of heterotrimeric G-protein–coupled receptor signaling. p110α and -β are widely distributed in mammalian tissues, whereas p110δ and -γ show a more restricted distribution and are mainly but not exclusively expressed in blood cells and their precursors.7 In this study, we examined which of the class I PI3K isoforms is responsible for PI3K activation in AML blasts.
Study design
Patients
All patients were included in the AML2001 trial of chemotherapy initiated by the French Multicenter Group, Groupe Ouest-Est des Leucémies et des Autres Maladies du Sang (GOELAMS). All biologic studies performed in this report were approved by the GOELAMS institutional review board, and signed informed consent was provided according to the Declaration of Helsinki. Classification of AML was based on the French-American-British (FAB) criteria.8 Patients who presented with acute promyelocytic leukemia (AML3) or AML6 and AML7 FAB subtypes were excluded from the study. Blasts from 64 patients with AML were tested for constitutive activation of PI3K by analysis of Akt phosphorylation on Ser473, and constitutive activation of PI3K was detected in 37 patients (58%). The expression of the 4 class I isoforms of the PI3K p110 subunit was tested in 21 patients with primary AML that presented a constitutive activation of PI3K. Due to limitations on the available amount of material, the full set of experiments depicted in Figure 1 and Figure 2 was performed in only 10 of these patients. Their characteristics are presented in Table 1.
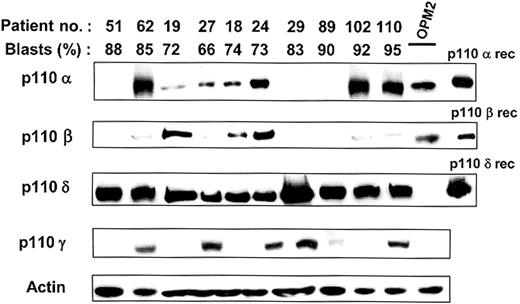
Expression of p110 isoforms in leukemic blasts from patients with AML. Expression of class IA (p110α,-β and -δ) and class IB (p110γ) catalytic subunits of PI3K was tested in leukemic blasts of 21 patients showing a constitutive activation of PI3K. Results from the 10 patients described in Table 1 are presented. Blast analysis of the other patients gave similar results. Proteins from 106 cells were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blot using antibodies specific for the various isoforms of p110. p110α and p110β antibodies were from Cell Signaling Technology (Beverly, MA). p110δ antibodies have been described previously18 and p110γ antibodies were provided by Dr R. Wetzker.19 Purified recombinant p110 proteins and cell lysates from 106 OPM2 cells were used as controls. Western blot using antiactin antibodies (Sigma, St Louis, MO; cat number A5441) was used to assess equal loading of the samples.
Expression of p110 isoforms in leukemic blasts from patients with AML. Expression of class IA (p110α,-β and -δ) and class IB (p110γ) catalytic subunits of PI3K was tested in leukemic blasts of 21 patients showing a constitutive activation of PI3K. Results from the 10 patients described in Table 1 are presented. Blast analysis of the other patients gave similar results. Proteins from 106 cells were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blot using antibodies specific for the various isoforms of p110. p110α and p110β antibodies were from Cell Signaling Technology (Beverly, MA). p110δ antibodies have been described previously18 and p110γ antibodies were provided by Dr R. Wetzker.19 Purified recombinant p110 proteins and cell lysates from 106 OPM2 cells were used as controls. Western blot using antiactin antibodies (Sigma, St Louis, MO; cat number A5441) was used to assess equal loading of the samples.
Cells
Bone marrow cells from newly diagnosed patients with AML were obtained before induction of chemotherapy. Bone marrow samples were subjected to Ficoll-Hypaque density gradient separation to isolate mononuclear cells (BMMCs). CD34+ cells from cord blood were isolated as previously reported.9 The OPM2 cell line was established from the peripheral blood of a patient with multiple myeloma.10
Reagents
The p110δ inhibitor IC87114 was from ICOS Corporation, Bothell, WA.11
p110δ activity is required for Akt phosphorylation and proliferation of AML blasts. (A) IC87114 dose-response relationship and specificity. BMMCs from patient no. 89 (90% leukemic blasts) or OPM2 cells were incubated for 30 minutes with the indicated concentrations of IC87114 or 25 μM LY294002. Cell extracts from 106 cells were analyzed by Western blot using antibodies against phosphoSer473 of Akt (Cell Signaling Technology). After stripping, the blots were reprobed with antiactin antibodies to ascertain equal loading of the samples. (B) IC87114 inhibits Flt-3 ligand–stimulated PI3K activity. BMMCs from patient no. 102 were incubated for 30 minutes with or without 10 μM IC87114 or 25 μM LY294002, followed by stimulation for 15 minutes with 50 ng/mL FLT-3 ligand, and Ser473 phosphorylation of Akt was analyzed by Western blotting. (C) IC87114 inhibits constitutive PI3K activity. BMMCs were incubated for 30 minutes with 10 μM IC87114 or 25 μM LY294002 or with solvent (dimethylsulfoxide [DMSO]) alone. Cell extracts from 106 cells were analyzed by Western blot using anti–p-Akt (Ser473) and antiactin antibodies. Inset blots show the results of a typical experiment using blasts from patient no. 51. Similar experiments were performed with the blasts of each patient presented in Table 1 and Figure 1. The blots were scanned and analyzed using ImageJ software. For each sample, Akt phosphorylation observed in the absence of inhibitor was set at 100% and Akt phosphorylation in the presence of inhibitors was expressed relative to this value (main graph). Error bars represent standard deviations of the 10 patient samples. Significance determined by Student t test: control/LY294002, P < .001; control/IC87114, P < .001; LY294002/IC87114, P > .2 (not significant). (D) IC87114 inhibits AML cell proliferation. BMMCs from each patient presented in Table 1 were incubated for 48 hours with or without 10 μM IC87114 and pulsed for 6 hours with [3H]-thymidine. Moreover, BMMCs from patient nos. 89, 102, and 110 were also incubated for 48 hours with 10 ng/mL FLT-3 ligand in the presence or absence of IC87114. For each BMMC sample, [3H]-thymidine incorporation in the absence of FLT-3 ligand and IC87114 (control) was set at 100% and [3H]-thymidine incorporation in the presence of FLT-3 ligand and/or IC87114 was expressed relative to this value. Significance determined by Student t test: control/IC87114, P < .001; FLT-3L/FLT-3L + IC87114, P < .01. Error bars indicate standard deviation.
p110δ activity is required for Akt phosphorylation and proliferation of AML blasts. (A) IC87114 dose-response relationship and specificity. BMMCs from patient no. 89 (90% leukemic blasts) or OPM2 cells were incubated for 30 minutes with the indicated concentrations of IC87114 or 25 μM LY294002. Cell extracts from 106 cells were analyzed by Western blot using antibodies against phosphoSer473 of Akt (Cell Signaling Technology). After stripping, the blots were reprobed with antiactin antibodies to ascertain equal loading of the samples. (B) IC87114 inhibits Flt-3 ligand–stimulated PI3K activity. BMMCs from patient no. 102 were incubated for 30 minutes with or without 10 μM IC87114 or 25 μM LY294002, followed by stimulation for 15 minutes with 50 ng/mL FLT-3 ligand, and Ser473 phosphorylation of Akt was analyzed by Western blotting. (C) IC87114 inhibits constitutive PI3K activity. BMMCs were incubated for 30 minutes with 10 μM IC87114 or 25 μM LY294002 or with solvent (dimethylsulfoxide [DMSO]) alone. Cell extracts from 106 cells were analyzed by Western blot using anti–p-Akt (Ser473) and antiactin antibodies. Inset blots show the results of a typical experiment using blasts from patient no. 51. Similar experiments were performed with the blasts of each patient presented in Table 1 and Figure 1. The blots were scanned and analyzed using ImageJ software. For each sample, Akt phosphorylation observed in the absence of inhibitor was set at 100% and Akt phosphorylation in the presence of inhibitors was expressed relative to this value (main graph). Error bars represent standard deviations of the 10 patient samples. Significance determined by Student t test: control/LY294002, P < .001; control/IC87114, P < .001; LY294002/IC87114, P > .2 (not significant). (D) IC87114 inhibits AML cell proliferation. BMMCs from each patient presented in Table 1 were incubated for 48 hours with or without 10 μM IC87114 and pulsed for 6 hours with [3H]-thymidine. Moreover, BMMCs from patient nos. 89, 102, and 110 were also incubated for 48 hours with 10 ng/mL FLT-3 ligand in the presence or absence of IC87114. For each BMMC sample, [3H]-thymidine incorporation in the absence of FLT-3 ligand and IC87114 (control) was set at 100% and [3H]-thymidine incorporation in the presence of FLT-3 ligand and/or IC87114 was expressed relative to this value. Significance determined by Student t test: control/IC87114, P < .001; FLT-3L/FLT-3L + IC87114, P < .01. Error bars indicate standard deviation.
Western blot analysis
BMMCs of patients with AML were starved in serum-free medium for 4 hours. Cells were incubated with or without inhibitor for 30 minutes at 37°C. Cells were then boiled in Laemmli sample buffer and proteins were analyzed by Western blot. Enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ) or SuperSignal West Femto (Pierce, Rockford, IL) chemoluminescence kits were used for detection. Western blots were quantified using the ImageJ 1.32 software (National Institutes of Health, Bethesda, MD) after densitometric scanning of the films.
Cell proliferation assays
BMMCs were cultured in α-medium with 5% fetal calf serum (FCS) with or without FLT-3 ligand (10 ng/mL) for 48 hours and with or without 10 μM IC87114. [3H]-thymidine (1 μCi [37 kBq]) was added for a final 6 hours and the amount of radioactivity incorporated was determined by trichloracetic acid precipitation. CD34+ cells from cord blood were cultured in stem cell factor (SCF; 20 ng/mL), FLT-3 ligand (10 ng/mL), and Tpo (20 nM) for 48 hours with or without 10 μM IC87114 and pulsed for 12 hours with [3H]-thymidine.
Results and discussion
p110δ is the only class I PI3K isoform consistently present in AML blasts
Expression of the 4 catalytic subunits of class I PI3K was tested by Western blot of AML blast extracts in 21 patients with constitutive activation of PI3K. Expression of p110α, -β, and -γ was highly variable between patients and did not correlate with the FAB subtype. In contrast, p110δ expression was consistently detected at comparable levels in all samples. Representative expression patterns are presented in Figure 1 for the 10 patients listed in Table 1. We used the OPM2 cell line as a negative control in our experiments. These cells only express p110α and p110β but no detectable p110δ or p110γ (Figure 1).
Inhibition of Akt phosphorylation by IC87114, a p110δ-selective inhibitor
Recently, IC87114, an inhibitor with selectivity for p110δ over the other class I PI3K isoforms, has been developed.11 In AML blast cells, IC87114 decreased Akt phosphorylation in a dose-dependent manner. In 3 patient samples, maximal PI3K inhibition was observed at 10 μM IC87114 (Figure 2A). Constitutive Akt phosphorylation in p110δ-negative OMP2 cells was fully inhibited by 25 μM LY294002 but not by IC87114 (Figure 2A, right panel). We next tested the sensitivity of Akt phosphorylation to 10 μM IC87114 and 25 μM LY294002 in the 10 patient samples shown in Table 1 and Figure 1. The results of a typical experiment are presented in Figure 2C (inset). Blots were analyzed by densitometric scanning for Akt phosphorylation in the presence of either IC87114 or LY294002 and results are presented as a percentage of that seen in control cells treated with vehicle only (Figure 2C, graph). From these data, it is clear that IC87114 was as effective as the pan-PI3K inhibitor LY294002 at inhibiting Akt phosphorylation in AML blasts. This indicates that p110δ is the main contributor of PI3K activity in AML blasts.
Akt phosphorylation in AML blasts increases in response to FLT-3 ligand.12 We tested the impact of p110δ inhibition on Akt phosphorylation. Figure 2B shows that FLT-3 ligand–stimulated Akt phosphorylation was inhibited by IC87114 to the same extent as by LY294002, showing that p110δ can be responsible for PI3K activation after FLT-3 ligand stimulation. At present, it is not clear why p110δ is also the main contributor of PI3K activity in cells that also express p110α and p110β. One possible explanation would be that the expression level of p110δ is significantly higher than that of the other isoforms.
Inhibition of AML cell proliferation by IC87114
Next, we tested the effect of IC87114 on cell proliferation on the blast samples described in the previous paragraph. AML proliferation was found to be almost completely blocked by 10 μM IC87114. IC87114 also strongly reduced the proliferation of cells stimulated with FLT-3 ligand (Figure 2D). We observed that FLT-3 ligand was still able to stimulate both PI3K activity and proliferation of blast cells from patient no. 102, who presented with an activating mutation (ITD) of FLT-3 (Figure 2B,D), confirming the observations previously reported by Bruserud et al.13 In contrast, cell proliferation of OMP2 and cord blood CD34+ cells was not decreased by IC87114 (data not shown).
AML is associated with poor long-term survival. The development of new therapeutic strategies directed against specific targets is an area of intense interest and may prove effective as adjunct treatments in combination with traditional chemotherapy. The PI3K/Akt pathway is often activated in AML blast cells, contributing to their survival4,5 and their proliferation (results described in this manuscript). Blockade of all PI3K isoforms in the organism using nonselective PI3K inhibitors such as LY294002 or wortmannin is very toxic in vivo, possibly due to a general requirement of PI3K for many cellular functions. Mice lacking functional p110δ are viable and fertile, in contrast to mice lacking p110α or p110β, which are embryonic lethal.14-17 This finding suggests that specifically blocking p110δ might be less toxic than inhibiting all PI3K activities. Our data suggest that in patients with AML, pharmacologic inhibition of p110δ may offer clinical benefit.
Prepublished online as Blood First Edition Paper, April 19, 2005; DOI 10.1182/blood-2004-08-3225.
Supported by the Comité de Paris of the Ligue Nationale Contre le Cancer (LNCC; laboratoire associé no. 8), the Association pour la Recherche contre le Cancer (ARC) and the Groupe Ouest-Est des Leucémies et des Autres Maladies du Sang (GOELAMS).
P.S. and V.B. contributed equally to this work.
One of the authors, J.S.H., is employed by the ICOS Corporation company, whose product (IC87114 inhibitor) is used in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr R. Wetzker (University of Jena, Germany) for providing the anti-p110γ antibody.

![Figure 2. p110δ activity is required for Akt phosphorylation and proliferation of AML blasts. (A) IC87114 dose-response relationship and specificity. BMMCs from patient no. 89 (90% leukemic blasts) or OPM2 cells were incubated for 30 minutes with the indicated concentrations of IC87114 or 25 μM LY294002. Cell extracts from 106 cells were analyzed by Western blot using antibodies against phosphoSer473 of Akt (Cell Signaling Technology). After stripping, the blots were reprobed with antiactin antibodies to ascertain equal loading of the samples. (B) IC87114 inhibits Flt-3 ligand–stimulated PI3K activity. BMMCs from patient no. 102 were incubated for 30 minutes with or without 10 μM IC87114 or 25 μM LY294002, followed by stimulation for 15 minutes with 50 ng/mL FLT-3 ligand, and Ser473 phosphorylation of Akt was analyzed by Western blotting. (C) IC87114 inhibits constitutive PI3K activity. BMMCs were incubated for 30 minutes with 10 μM IC87114 or 25 μM LY294002 or with solvent (dimethylsulfoxide [DMSO]) alone. Cell extracts from 106 cells were analyzed by Western blot using anti–p-Akt (Ser473) and antiactin antibodies. Inset blots show the results of a typical experiment using blasts from patient no. 51. Similar experiments were performed with the blasts of each patient presented in Table 1 and Figure 1. The blots were scanned and analyzed using ImageJ software. For each sample, Akt phosphorylation observed in the absence of inhibitor was set at 100% and Akt phosphorylation in the presence of inhibitors was expressed relative to this value (main graph). Error bars represent standard deviations of the 10 patient samples. Significance determined by Student t test: control/LY294002, P < .001; control/IC87114, P < .001; LY294002/IC87114, P > .2 (not significant). (D) IC87114 inhibits AML cell proliferation. BMMCs from each patient presented in Table 1 were incubated for 48 hours with or without 10 μM IC87114 and pulsed for 6 hours with [3H]-thymidine. Moreover, BMMCs from patient nos. 89, 102, and 110 were also incubated for 48 hours with 10 ng/mL FLT-3 ligand in the presence or absence of IC87114. For each BMMC sample, [3H]-thymidine incorporation in the absence of FLT-3 ligand and IC87114 (control) was set at 100% and [3H]-thymidine incorporation in the presence of FLT-3 ligand and/or IC87114 was expressed relative to this value. Significance determined by Student t test: control/IC87114, P < .001; FLT-3L/FLT-3L + IC87114, P < .01. Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/3/10.1182_blood-2004-08-3225/6/m_zh80150581680002.jpeg?Expires=1769830299&Signature=vVFmfFI5-EZkLi09eNWHpxE-6DszwCFSwV6VROFgq23VfiwlVtY-vA-dfd36uhEeNdI~3dFHIglsOhqix-dDslm-nGrmxCf6N3V9oOiBdCnc4eyBzvxwl1yRifssNMmSO4LXq-Zh18rnDqy~A2JmpGMOK7mG4mhLoVBV7dbPhYZUNS6MwwzIv3wAcvrje6Kjw5YN7EPLV2Xw5UZUkOPJg93sOtd3qr1ObQOoA9bHhxu8xu31pzg~E4At8mmpLvlts9ySS9m5JSvKWwEZADzf0W1qggLZsCQpx0L9Eu8UtKhBB2sSO9Rqn9i7P7GUWHeyl0Kb2k6CHyUHWQ7VvCyI8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
