Abstract
Indole-3-carbinol, found in Brassica species vegetables (such as cabbage, cauliflower, and brussels spouts), exhibits antitumor effects through poorly defined mechanisms. Because several genes that regulate apoptosis, proliferation, and metastasis are regulated by nuclear factor-κB (NF-κB), we postulated that indole-3-carbinol must mediate its activity through NF-κB modulation. We demonstrated that indole-3-carbinol suppressed constitutive NF-κB activation and activation induced by tumor necrosis factor (TNF), interleukin-1β (IL-1β), phorbol 12-myristate 13-acetate (PMA), lipopolysaccharide (LPS), and cigarette smoke; the suppression was not cell type specific, because activation was inhibited in myeloid, leukemia, and epithelial cells. This activation correlated with the sequential suppression of the IκBα kinase, IκBα phosphorylation, IκBα ubiquitination, IκBα degradation, p65 phosphorylation, p65 nuclear translocation, p65 acetylation, and NF-κB-dependent reporter gene expression. The NF-κB-regulated gene products cyclin D1, cyclooxygenase-2 (COX-2), matrix metalloproteinase-9 (MMP-9), survivin, inhibitor-of-apoptosis protein-1 (IAP1), IAP2, X chromosome-linked IAP (XIAP), Bcl-2, Bfl-1/A1, TNF receptor-associated factor-1 (TRAF1), and Fas-associated death domain protein-like interleukin-1β-converting enzyme inhibitory protein (FLIP) were all down-regulated by indole-3-carbinol. This down-regulation led to the potentiation of apoptosis induced by cytokines and chemotherapeutic agents. Indole-3-carbinol suppressed constitutive NF-κB activation in mononuclear cells derived from bone marrow of acute myelogenous leukemia patients, and this correlated with inhibition of cell growth. Overall, our results indicated that indole-3-carbinol inhibits NF-κB and NF-κB-regulated gene expression and that this mechanism may provide the molecular basis for its ability to suppress tumorigenesis. (Blood. 2005;106:641-649)
Introduction
Indole-3-carbinol (I3C) is an autolysis product of a glucosinolate, glucobrassicin, found in Brassica species or cruciferous vegetables (cabbage, broccoli, cauliflower, and brussels spouts).1,2 In vitro, I3C was found to suppress the proliferation of various tumor cells, including breast,3 prostate,4 colon,5 and endometrial cancer cells. In most cells, I3C induces G1/S cell cycle arrest.6 In vivo, I3C has been shown to suppress tumorigenesis of the colon,7,8 lung,9-11 breast,12 cervix,13,14 and liver.15
This molecule has been found to be effective clinically in treating precancerous lesions of the cervix and laryngeal papillomas, both of which have a human papillomavirus (HPV) component to their etiologies.16-19 I3C has also been shown to alter immune function,7,20 inhibit cigarette smoke-induced DNA-adduct formation,21 and reverse multidrug resistance in vivo. Rats fed I3C daily for 7 weeks had significantly reduced natural killer cell activity and elevated T-cell-mediated delayed-type hypersensitivity but no change in antibody production.20
Numerous reports indicate that I3C mediates its effects through alteration of gene expression in different cells.22 This indole down-regulates the expression of various genes, including inducible nitric oxide synthase (iNOS),23 cyclin-dependent kinase 6 (CDK6),3,6 prostate-specific antigen (PSA),24 and Bcl-2.4 It has also been shown to inhibit Akt25 and CDK6 expression in human MCF-7 breast cancer cells by disrupting Sp1 transcription factor interactions with a composite element in the CDK6 gene promoter.26 That I3C directly binds to estrogen receptor-α,27-30 aryl hydrocarbon (Ah) receptor,31 and androgen receptor 32 has been reported. Besides antiproliferative and immunomodulatory effects, I3C also has been demonstrated to suppress the invasion and migration of human breast cancer cells.29,33
Although this wide range of activities has been assigned to I3C, its basic mechanism of action remains unclear. Based on the confluence of pathways affected by I3C and nuclear factor-κB (NF-κB), we postulated that I3C interferes with the NF-κB activation pathway to exert its effects on gene expression. For example, several genes that mediate proliferation, apoptosis, and metastasis are regulated by NF-κB.34 This transcription factor is activated by various carcinogens and inflammatory stimuli, including cigarette smoke, tumor necrosis factor (TNF), interleukin-1 (IL-1), receptor of receptor activator of NF-κB ligand (RANKL), phorbol 12-myristate 13-acetate (PMA), and lipopolysaccharide (LPS). NF-κB consists of p50 and p65 heterodimer retained in the cytoplasm by masking nuclear localization signal (NLS) by IκBα, the inhibitor. On activation, IκBα kinase (IKK) is activated, leading to IκBα phosphorylation, ubiquitination, and degradation, thus releasing p50-p65 to translocate to the nucleus, bind to its consensus sequence, and induce gene transcription. Although NF-κB activation has been shown to regulate apoptosis and proliferation in leukemic cells,35-37 there is no report on the effect of I3C in these cells. Therefore, the aim of the current study was to investigate the effect of I3C on the NF-κB activation pathway and on apoptosis in leukemic cells.
Materials and methods
Reagents
I3C was purchased from Sigma-Aldrich (St Louis, MO). A 50 mM solution was prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich), stored as small aliquots at -20°C, and then thawed and diluted as needed in cell culture medium as described.4,6 No loss of activity of I3C was noted after storage for 6 months. Throughout, 0.1% DMSO (equivalent to 50 μM I3C) was used as control. Bacteria-derived human recombinant TNF, purified to homogeneity with a specific activity of 5 × 107 U/mg, was kindly provided by Genentech (South San Francisco, CA). Bacteria-derived human recombinant IL-1β, purified to homogeneity with a specific activity of 1.0 EU/μg, was purchased from R&D Systems (Minneapolis, MN). Cigarette smoke condensate (CSC), prepared as described,38 was kindly supplied by Dr G. Gairola (University of Kentucky, Lexington, KY). Doxorubicin was kindly provided by Dr Waldemar Priebe (M. D. Anderson Cancer Center, The University of Texas, Houston). Penicillin, streptomycin, RPMI 1640, Iscove modified Dulbecco medium (IMDM), Dulbecco modified Eagle medium (DMEM), nonessential amino acids, pyruvate, glutamine, vitamins, and fetal bovine serum (FBS) were obtained from Invitrogen (Grand Island, NY). PMA, LPS, okadaic acid, H2O2, cisplatin, anti-β-actin antibody, and basic chemical reagents were obtained from Sigma-Aldrich. Antibodies against p65, p50, IκBα, cyclin D1, matrix metalloproteinase-9 (MMP-9), poly(adenosine diphosphate-ribose) polymerase (PARP), inhibitor-of-apoptosis protein-1 (IAP1), IAP2, Bcl-2, Bfl-1/A1, and TNF receptor-associated factor-1 (TRAF1) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-cyclooxygenase-2 (anti-COX-2) and anti-X chromosome-linked IAP (anti-XIAP) antibodies were obtained from BD Biosciences (San Diego, CA). Phosphospecific anti-IκBα (Ser32) and anti-acetyl-lysine antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-IKK-α, anti-IKK-β, and anti-Fas-associated death domain protein-like interleukin-1β-converting enzyme inhibitory protein (anti-FLIP) antibodies were kindly provided by Imgenex (San Diego, CA). A polyclonal antibody that recognizes the serine 529 phosphorylated form of p65 was obtained from Rockland Laboratories (Gilbertsville, PA). N-acetyl-leucyl-leucyl-norleucinal (ALLN) was purchased from Calbiochem (San Diego, CA).
Cell lines
Jurkat (human T-cell leukemia), KBM-5 (human myeloid), H1299 (human lung adenocarcinoma), U266 (human multiple myeloma), A293 (human embryonic kidney) and SCC-4 (human squamous cell carcinoma) cells were obtained from American Type Culture Collection (Manassas, VA). MM.1 (human multiple myeloma) cells were kindly provided by Dr Steven T. Rosen (Robert H. Lurie Comprehensive Cancer Center, Feinberg School of Medicine, Northwestern University, Chicago, IL). KBM-5 cells were cultured in IMDM with 15% FBS; Jurkat, H1299, MM.1, and U266 cells were cultured in RPMI 1640 medium with 10% FBS; A293 cells were cultured in DMEM supplemented with 10% FBS; and SCC-4 cells were cultured in DMEM containing 10% FBS, nonessential amino acids, pyruvate, glutamine, and vitamins. All media were supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin.
Clinical samples
Bone marrow specimens from acute myelogenous leukemia (AML) patients with high (more than 70%) blast counts (Table 1) were obtained after signed informed content, according to institutional guidelines. All studies were approved by the Institutional Review Board at the University of Texas M.D. Anderson Cancer Center, Houston, TX. Mononuclear cells were purified by Ficoll-Hypaque (Sigma-Aldrich) density-gradient centrifugation.
Electrophoretic mobility shift assay (EMSA)
To measure NF-κB activation, we prepared the nuclear extracts and performed electrophoretic mobility shift assay (EMSA) as described previously.39 The dried gels were visualized and radioactive bands quantitated by a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) using Imagequant software.
Western blot analysis
To determine the levels of protein expression in the cytoplasm or nucleus, we prepared extracts and fractionated them by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously.39
IKK assay
To determine the effect of I3C on IKK activation, IKK assay was performed by a method described previously.39 Briefly, whole-cell extract was prepared, incubated with anti-IKK-α antibody, and precipitated immunocomplex by protein A/G-Sepharose beads (Pierce, Rockford, IL). Immunocomplex was subjected for kinase assay using glutathione S-transferase (GST)-IκBα (1-79) as substrate.
NF-κB-dependent reporter gene expression assay
The effect of I3C on TNF- and TNF receptor-1 (TNFR1)-induced NF-κB-dependent reporter gene transcription was analyzed by secretory alkaline phosphatase (SEAP) assay as described previously.39 Briefly, A293 cells were transiently transfected with the NF-κB-regulated SEAP reporter construct, incubated with I3C, and then stimulated them with TNF. Cells were also cotransfected with TNFR1-expressing plasmids, and then NF-κB-dependent SEAP expression was monitored.
Immunoprecipitation of p65 for p65 acetylation
The effect of I3C on TNF-induced acetylation of p65 was examined as described previously.40 Briefly, whole-cell extract was prepared, incubated with anti-p65 antibody, and precipitated immunocomplex by protein A/G-Sepharose beads. Immunecomplex was subjected for SDS-PAGE, and Western blot analysis was performed using anti-acetyl-lysine antibody.
Immunocytochemistry for NF-κB p65 localization
The effect of I3C on the nuclear translocation of p65 was examined by immunocytochemistry as described previously.39 Briefly, cells were fixed with paraformaldehyde, permeabilized with Triton X-100, blocked with normal goat serum, incubated with rabbit polyclonal anti-p65 antibody, incubated with goat antirabbit immunoglobulin G (IgG)-Alexa 594 (Molecular Probes, Eugene, OR), and counterstained for nuclei with Hoechst 33342 (Sigma-Aldrich).
Cytotoxicity assay
The effect of I3C on the cytotoxic effects of TNF was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) uptake method as described.41 Briefly, 5000 cells were seeded in triplicate in a 96-well plate and treated as indicated. Thereafter, an MTT solution was added, lysed, and then the optic density (OD) was measured at 570 nm.
TUNEL assay
We also assayed cytotoxicity by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) method as described previously.39 Briefly, cells were fixed with paraformaldehyde, permeabilized with Triton X-100, and incubated with an in situ cell death detection reagent (Roche Applied Science, Indianapolis, IN).
Live and dead assay
Apoptosis was measured by the Live and Dead assay (Molecular Probes), which determines intracellular esterase activity and plasma membrane integrity, performed as described previously.39
Results
The current study was undertaken to investigate the effect of I3C on the NF-κB activation pathway, NF-κB-regulated gene expression, and apoptosis in leukemic cells. The concentration of I3C and NF-κB activators used and the time of exposure had minimal effect on the viability of these cells (data not shown).
I3C blocks NF-κB activation induced by TNF, PMA, IL-1β, LPS, and CSC
Because TNF, IL-1β, PMA, LPS, and CSC are potent activators of NF-κB, perhaps by different mechanisms,38,42,43 we examined the effect of I3C on the activation of NF-κB by these agents in Jurkat cells. Pretreatment of cells with I3C suppressed the activation of NF-κB induced by all 5 agents (Figure 1A). These results suggest that the I3C acts at a step in the NF-κB activation pathway that is common to all 5 agents.
I3C inhibits TNF-dependent NF-κB activation in a time-dependent manner
Because TNF is one of the most potent activators of NF-κB and the mechanism of activation of NF-κB is relatively well established, we examined the effect of I3C on TNF-induced NF-κB activation. Jurkat cells were incubated with I3C for various times and then exposed to TNF. I3C by itself did not activate NF-κB, but TNF-induced NF-κB activation was inhibited by I3C at 24 hours (Figure 1B). Under these conditions, cells treated with I3C were fully viable.
Effect of I3C on TNF-induced NF-κB activation. (A) I3C blocks NF-κB activation induced by TNF, PMA, LPS, and CSC. Jurkat cells were preincubated with 50 μM I3C for 24 hours; treated with 0.1 nM TNF and 10 μg/mL LPS for 30 minutes; treated with 100 ng/mL IL-1β, 15 ng/mL PMA, and 1 μg/mL CSC for 1 hour; and then analyzed for NF-κB activation as described in “Materials and methods.” (B) Jurkat cells were preincubated at 37°C with 50 μM I3C for the indicated times and then treated with 0.1 nM TNF at 37°C for 30 minutes. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA. Cell viability was determined by the trypan blue dye exclusion method. (C) I3C suppresses TNF-induced NF-κB in a dose-dependent manner in Jurkat, KBM-5, and H1299 cells. Cells were incubated with different concentrations of I3C for 24 hours, followed by an incubation with 0.1 nM TNF for 30 minutes. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA. (D) I3C inhibits constitutive NF-κB activation. MM.1, U266, and SCC-4 cells were incubated with different concentrations of I3C for 24 hours. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA.
Effect of I3C on TNF-induced NF-κB activation. (A) I3C blocks NF-κB activation induced by TNF, PMA, LPS, and CSC. Jurkat cells were preincubated with 50 μM I3C for 24 hours; treated with 0.1 nM TNF and 10 μg/mL LPS for 30 minutes; treated with 100 ng/mL IL-1β, 15 ng/mL PMA, and 1 μg/mL CSC for 1 hour; and then analyzed for NF-κB activation as described in “Materials and methods.” (B) Jurkat cells were preincubated at 37°C with 50 μM I3C for the indicated times and then treated with 0.1 nM TNF at 37°C for 30 minutes. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA. Cell viability was determined by the trypan blue dye exclusion method. (C) I3C suppresses TNF-induced NF-κB in a dose-dependent manner in Jurkat, KBM-5, and H1299 cells. Cells were incubated with different concentrations of I3C for 24 hours, followed by an incubation with 0.1 nM TNF for 30 minutes. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA. (D) I3C inhibits constitutive NF-κB activation. MM.1, U266, and SCC-4 cells were incubated with different concentrations of I3C for 24 hours. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA.
Inhibition of NF-κB activation by I3C is dose dependent and is not cell type specific
Because distinct signal transduction pathways can mediate NF-κB induction in different cell types (for references, see Shishodia et al44 ), we also investigated whether I3C could block TNF-induced NF-κB activation in Jurkat, KBM-5, and H1299 cells. Cells were pretreated with different concentrations of I3C and then treated with TNF. TNF activated NF-κB in all cell types, and I3C inhibited the activation in a dose-dependent manner (Figure 1C). The results also indicate that 50 μM I3C is needed for maximum suppression of NF-κB activation.
I3C inhibits constitutive NF-κB activation in a dose-dependent manner
Whether I3C could suppress constitutive NF-κB activation was also examined. We treated human multiple myeloma MM.1 and U266 cells and squamous cell carcinoma SCC-4 cells, which are known to express constitutive active NF-κB,36,37,45 with various concentrations of I3C. Constitutive NF-κB activation was suppressed above 50 μM (Figure 1D).
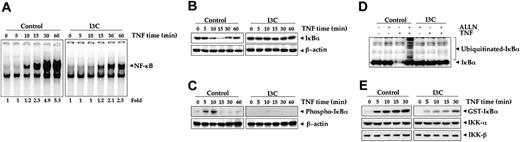
Effect of I3C on IκBα phosphorylation and degradation induced by TNF. (A) I3C inhibits TNF-induced activation of NF-κB. Jurkat cells were incubated with 50 μM I3C for 24 hours, treated with 0.1 nM TNF for indicated times, and then analyzed for NF-κB activation by EMSA. (B) Effect of I3C on TNF-induced degradation of IκBα. Jurkat cells were incubated with 50 μM I3C for 24 hours and treated with 0.1 nM TNF for the indicated times. Cytoplasmic extracts were prepared, fractionated on 10% SDS-PAGE, and electrotransferred to nitrocellulose membrane. Western blot analysis was performed with anti-IκBα antibody. Anti-β-actin antibody was the loading control. (C) Effect of I3C on the phosphorylation of IκBα by TNF. Jurkat cells were incubated with of 50 μM I3C for 24 hours and treated with 0.1 nM TNF for the indicated times. Cytoplasmic extracts were fractionated and then blotted using phosphospecific anti-IκBα antibody. (D) Effect of I3C on TNF-induced ubiquitination of IκBα. Jurkat cells were pretreated with 50 μM I3C for 24 hours, then with 100 μg/mL N-acetyl-leucyl-leucyl-norleucinal (ALLN) for 1 hour, and finally with 0.1 nM TNF for 15 minutes. Whole-cell extracts were prepared, fractionated, and examined by Western blot analysis using anti-IκBα antibody. (E) Effect of I3C on the activation of IKK by TNF. Jurkat cells were incubated with 50 μM I3C for 24 hours and then activated with 1 nM TNF for different times. Whole-cell extracts were immunoprecipitated with antibody against IKK-α and analyzed by immunocomplex kinase assay. To examine the effect of I3C on the level of expression of IKK proteins, whole-cell extracts were fractionated on SDS-PAGE and examined by Western blot analysis using anti-IKK-α and anti-IKK-β antibodies.
Effect of I3C on IκBα phosphorylation and degradation induced by TNF. (A) I3C inhibits TNF-induced activation of NF-κB. Jurkat cells were incubated with 50 μM I3C for 24 hours, treated with 0.1 nM TNF for indicated times, and then analyzed for NF-κB activation by EMSA. (B) Effect of I3C on TNF-induced degradation of IκBα. Jurkat cells were incubated with 50 μM I3C for 24 hours and treated with 0.1 nM TNF for the indicated times. Cytoplasmic extracts were prepared, fractionated on 10% SDS-PAGE, and electrotransferred to nitrocellulose membrane. Western blot analysis was performed with anti-IκBα antibody. Anti-β-actin antibody was the loading control. (C) Effect of I3C on the phosphorylation of IκBα by TNF. Jurkat cells were incubated with of 50 μM I3C for 24 hours and treated with 0.1 nM TNF for the indicated times. Cytoplasmic extracts were fractionated and then blotted using phosphospecific anti-IκBα antibody. (D) Effect of I3C on TNF-induced ubiquitination of IκBα. Jurkat cells were pretreated with 50 μM I3C for 24 hours, then with 100 μg/mL N-acetyl-leucyl-leucyl-norleucinal (ALLN) for 1 hour, and finally with 0.1 nM TNF for 15 minutes. Whole-cell extracts were prepared, fractionated, and examined by Western blot analysis using anti-IκBα antibody. (E) Effect of I3C on the activation of IKK by TNF. Jurkat cells were incubated with 50 μM I3C for 24 hours and then activated with 1 nM TNF for different times. Whole-cell extracts were immunoprecipitated with antibody against IKK-α and analyzed by immunocomplex kinase assay. To examine the effect of I3C on the level of expression of IKK proteins, whole-cell extracts were fractionated on SDS-PAGE and examined by Western blot analysis using anti-IKK-α and anti-IKK-β antibodies.
We have shown that high concentrations of TNF induce NF-κB more intensely than lower doses given for long times,46 so we decided to establish TNF dose effects for I3C inhibition. TNF at a concentration of 10 nM activated NF-κB activity strongly; however, cells pretreated with I3C abolished TNF-induced NF-κB activation (data not shown). These results show that I3C is a very potent inhibitor of TNF-induced NF-κB activation.
To determine whether I3C directly modifies the binding of the NF-κB complex to the DNA, we incubated nuclear extracts from TNF-treated cells with various concentrations of I3C and then analyzed DNA binding activity by EMSA. Our results show that I3C did not modify the DNA binding ability of the NF-κB complex (data not shown). We conclude that I3C inhibits NF-κB activation through an indirect mechanism.
Various combinations of Rel/NF-κB protein constitute active NF-κB heterodimers that bind to a specific DNA sequence.47 To show that the retarded band visualized by EMSA in TNF-treated cells was indeed NF-κB, we incubated nuclear extracts from TNF-stimulated cells with antibodies to either the p50 (NF-κB1) or the p65 (RelA) subunit of NF-κB. Both shifted the band to a higher molecular mass (data not shown), thus suggesting that the TNF-activated complex consisted of p50 and p65 subunits. Neither preimmune serum nor the irrelevant antibody anticyclin D1 had any effect. Excess unlabeled NF-κB (100-fold) caused complete disappearance of the band, but a mutant oligonucleotide of NF-κB did not affect NF-κB binding activity.
I3C inhibits TNF-dependent IκBα degradation
To determine whether I3C's inhibitory activity was due to inhibition of IκBα degradation, we pretreated cells with I3C, exposed them to TNF for various times, and examined them for NF-κB activation in the nucleus by EMSA and for IκBα status in the cytoplasm by Western blot analysis. NF-κB was progressively activated with increase in incubation times with TNF. The I3C-pretreated cells showed a dramatic decrease in activation of NF-κB even after 60 minutes of TNF stimulation (Figure 2A).
The translocation of NF-κB to the nucleus is preceded by the proteolytic degradation of IκBα.47 We found that TNF induced IκBα degradation in control cells within 10 minutes, but in I3C-pretreated cells TNF had no effect on IκBα degradation (Figure 2B). These results indicate that I3C inhibits both TNF-induced NF-κB activation and IκBα degradation.
I3C inhibits TNF-dependent IκBα phosphorylation
To determine whether inhibition of TNF-induced IκBα degradation was due to inhibition of IκBα phosphorylation, we pretreated cells with I3C, exposed them to TNF for various times, and then examined them for IκBα phosphorylation status in the cytoplasm by Western blot analysis using antibody that recognizes the serine-phosphorylated form of IκBα. Figure 2C shows that TNF-induced IκBα phosphorylation was almost completely suppressed by I3C.
I3C inhibits TNF-dependent IκBα ubiquitination
Phosphorylation of IκBα by TNF leads to ubiquitination and degradation of IκBα.47 To investigate whether I3C affects TNF-induced IκBα ubiquitination, we performed Western blot analysis. We used the proteasome inhibitor ALLN to block the degradation of IκBα.48 TNF treatment alone slightly induced ubiquitination of IκBα, but when cells were pretreated with ALLN, TNF-induced ubiquitination of IκBα was enhanced (Figure 2D). I3C almost completely suppressed IκBα ubiquitination induced by TNF, even in the presence of the proteasome inhibitor.
I3C inhibits TNF-induced IKK activation
It has been shown that IKK is required for TNF-induced phosphorylation of IκBα.47 Because I3C inhibits the phosphorylation of IκBα, we determined its effect on TNF-induced IKK activation. In immunecomplex kinase assays, TNF activated IKK as early as 5 minutes after TNF treatment, but I3C completely suppressed this activation (Figure 2E). Neither TNF nor I3C had any effect on the expression of IKK-α or IKK-β proteins.
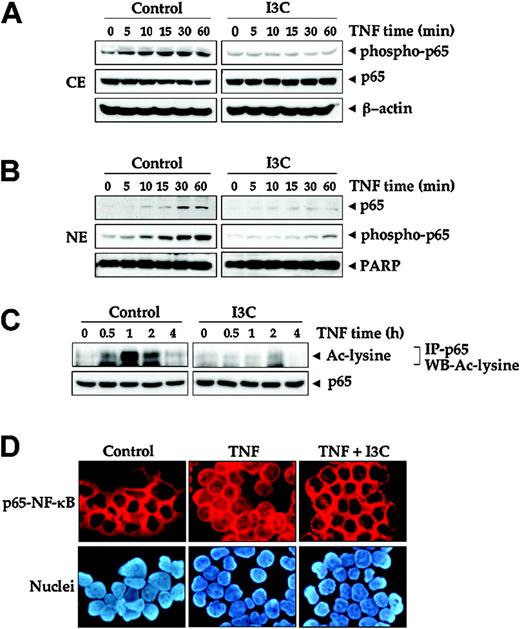
Effect of I3C on the TNF-induced translocation of p65 into the nucleus. (A) Western blot analysis of phospho-p65 and p65 using cytoplasmic extracts (CE). Jurkat cells were incubated with 50 μM I3C for 24 hours and treated with 0.1 nM TNF for the indicated times in minutes. Cytoplasmic extracts were prepared and subjected to Western blot analysis using anti-p65 antibody and phosphospecific anti-p65 antibody. (B) Western blot analysis of phospho-p65 and p65 using nuclear extracts (NE). Jurkat cells were incubated with 50 μM I3C for 24 hours and treated with 0.1 nM TNF for the indicated times. Nuclear extracts were prepared and subjected to Western blot analysis using anti-p65 and phosphospecific anti-p65 antibodies. For loading control of nuclear protein, the membrane was blotted with anti-PARP antibody. (C) Effect of I3C on TNF-induced acetylation of p65. Jurkat cells were incubated with 50 μM I3C for 24 hours and then treated with 1 nM TNF for the indicated times. Whole-cell extracts were prepared, immunoprecipitated (IP) with anti-p65 antibody, and then examined by Western blot analysis (WB) using anti-acetyl-lysine antibody. Whole-cell extracts were subjected to Western blot analysis using anti-p65 antibody. (D) Immunocytochemical analysis of p65 localization after treatment with 1 nM TNF in the absence or presence of 50 μM I3C. Jurkat cells were incubated with I3C for 24 hours and then treated with 1 nM TNF for 30 minutes. Cells were subjected to immunocytochemistry as described in “Materials and methods.” Stained slides were mounted with mounting medium (Sigma-Aldrich) and analyzed under an epifluorescence microscope (Labophot-2; Nikon, Tokyo, Japan). Pictures were captured using a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX) and MetaMorph Version 4.6.5 software (Universal Imaging, Downingtown, PA). Original magnification, ×200.
Effect of I3C on the TNF-induced translocation of p65 into the nucleus. (A) Western blot analysis of phospho-p65 and p65 using cytoplasmic extracts (CE). Jurkat cells were incubated with 50 μM I3C for 24 hours and treated with 0.1 nM TNF for the indicated times in minutes. Cytoplasmic extracts were prepared and subjected to Western blot analysis using anti-p65 antibody and phosphospecific anti-p65 antibody. (B) Western blot analysis of phospho-p65 and p65 using nuclear extracts (NE). Jurkat cells were incubated with 50 μM I3C for 24 hours and treated with 0.1 nM TNF for the indicated times. Nuclear extracts were prepared and subjected to Western blot analysis using anti-p65 and phosphospecific anti-p65 antibodies. For loading control of nuclear protein, the membrane was blotted with anti-PARP antibody. (C) Effect of I3C on TNF-induced acetylation of p65. Jurkat cells were incubated with 50 μM I3C for 24 hours and then treated with 1 nM TNF for the indicated times. Whole-cell extracts were prepared, immunoprecipitated (IP) with anti-p65 antibody, and then examined by Western blot analysis (WB) using anti-acetyl-lysine antibody. Whole-cell extracts were subjected to Western blot analysis using anti-p65 antibody. (D) Immunocytochemical analysis of p65 localization after treatment with 1 nM TNF in the absence or presence of 50 μM I3C. Jurkat cells were incubated with I3C for 24 hours and then treated with 1 nM TNF for 30 minutes. Cells were subjected to immunocytochemistry as described in “Materials and methods.” Stained slides were mounted with mounting medium (Sigma-Aldrich) and analyzed under an epifluorescence microscope (Labophot-2; Nikon, Tokyo, Japan). Pictures were captured using a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX) and MetaMorph Version 4.6.5 software (Universal Imaging, Downingtown, PA). Original magnification, ×200.
To evaluate whether I3C suppresses the IKK activity directly by binding to the IKK protein or by suppressing the activation of IKK, we incubated whole-cell extracts from untreated and TNF-treated cells with various concentrations of I3C. Immunecomplex kinase assay showed that I3C did not directly bind IKK, suggesting that I3C modulates TNF-induced IKK activation (data not shown).
I3C inhibits TNF-induced nuclear translocation of p65 in lymphoma cells
TNF induces the phosphorylation of p65, which is required for its transcriptional activity.49 Following phosphorylation, the p65 subunit is translocated to the nucleus. As shown in Figure 3A, TNF induced phosphorylation of p65 in a time-dependent manner; as early as 5 minutes after TNF stimulation, p65 was phosphorylated and its levels in the cytoplasm increased up to 30 minutes. On pretreatment of cells with I3C, TNF failed to induce phosphorylation of p65. We also showed that I3C suppressed TNF-induced nuclear translocation of p65 in a time-dependent manner (Figure 3B).
The acetylation of p65 plays a key role in NF-κB transcriptional activity.50 To examine the effect of I3C on the acetylation of p65 by TNF, cells were pretreated with I3C and then treated with TNF for the indicated times. Whole-cell extracts were prepared and immunoprecipitated with anti-p65 antibody, and then Western blot analysis was performed using anti-acetyl-lysine antibody. Whereas TNF induced acetylation of p65 in a time-dependent manner, I3C suppressed it (Figure 3C).
An immunocytochemistry assay confirmed the effect of I3C on the suppression of nuclear translocation of p65: In untreated cells p65 localized in the cytoplasm, TNF induced its nuclear translocation, and I3C clearly suppressed this translocation (Figure 3D).
I3C represses TNF-induced NF-κB-dependent reporter gene expression
Although we have shown by EMSA that I3C blocks NF-κB activation, DNA binding alone does not always correlate with NF-κB-dependent gene transcription, suggesting that there are additional regulatory steps.51 TNF-induced NF-κB activation is mediated through sequential interaction of the TNF receptor. To determine the effect of I3C on TNF-induced NF-κB-dependent reporter gene expression, we transiently transfected the cells with the NF-κB-regulated SEAP reporter construct, incubated them with I3C, and then stimulated them with TNF. Cells were also cotransfected with TNFR1-expressing plasmids, and then NF-κB-dependent SEAP expression was monitored. As shown in Figure 4A, cells showed NF-κB-regulated reporter gene expression whether they were activated with TNF or transfected with TNFR1 plasmid, but I3C suppressed activation.
I3C inhibits the TNF-induced expression of NF-κB-dependent genes. (A) I3C inhibits the NF-κB-dependent reporter gene expression induced by TNF and TNFR1. A293 cells were transiently transfected with an NF-κB-containing plasmid alone or with TNFR1-expressing plasmids for 24 hours. After transfection, cells were washed and treated with the indicated concentrations of I3C for 24 hours. For TNF-treated cells, cells were washed and treated with 1 nM TNF for an additional 24 hours. The supernatants of the culture medium were assayed for SEAP activity as described in “Materials and methods.” Values represent the mean ± SD of triplicate cultures. (B) I3C inhibits TNF-induced cyclin D1, COX-2, and MMP-9 expression. Jurkat cells were incubated with 25 μM I3C and then treated with 1 nM TNF for the indicated times. Whole-cell extracts were prepared and analyzed by Western blot analysis using antibodies against cyclin D1, COX-2, and MMP-9. (C) I3C inhibits the expression of TNF-induced antiapoptotic proteins. Jurkat cells were incubated with 25 μM I3C and then treated with 1 nM TNF for the indicated times. Whole-cell extracts were prepared and analyzed by Western blot analysis using antibodies against survivin, IAP1, IAP2, XIAP, Bcl-2, Bfl-1/A1, TRAF1, FLIP, and β-actin.
I3C inhibits the TNF-induced expression of NF-κB-dependent genes. (A) I3C inhibits the NF-κB-dependent reporter gene expression induced by TNF and TNFR1. A293 cells were transiently transfected with an NF-κB-containing plasmid alone or with TNFR1-expressing plasmids for 24 hours. After transfection, cells were washed and treated with the indicated concentrations of I3C for 24 hours. For TNF-treated cells, cells were washed and treated with 1 nM TNF for an additional 24 hours. The supernatants of the culture medium were assayed for SEAP activity as described in “Materials and methods.” Values represent the mean ± SD of triplicate cultures. (B) I3C inhibits TNF-induced cyclin D1, COX-2, and MMP-9 expression. Jurkat cells were incubated with 25 μM I3C and then treated with 1 nM TNF for the indicated times. Whole-cell extracts were prepared and analyzed by Western blot analysis using antibodies against cyclin D1, COX-2, and MMP-9. (C) I3C inhibits the expression of TNF-induced antiapoptotic proteins. Jurkat cells were incubated with 25 μM I3C and then treated with 1 nM TNF for the indicated times. Whole-cell extracts were prepared and analyzed by Western blot analysis using antibodies against survivin, IAP1, IAP2, XIAP, Bcl-2, Bfl-1/A1, TRAF1, FLIP, and β-actin.
I3C represses the TNF-induced NF-κB-dependent reporter gene products COX-2, cyclin D1, and MMP-9 Whether I3C can modulate certain NF-κB-regulated gene products induced by TNF was also examined. That COX-2, cyclin D1, and MMP-9 expression are regulated by NF-κB has been reported.34 TNF has been shown to induce cyclin D1, COX-2, and MMP-9, which have an NF-κB binding site in their promoter. Western blot analysis of whole-cell extracts showed that TNF induced cyclin D1, COX-2, and MMP-9 expression in a time-dependent manner and I3C blocked these expressions (Figure 4B). The results further support the role of I3C in blocking TNF-induced NF-κB-regulated gene products.
I3C represses TNF-induced NF-κB-dependent antiapoptotic gene products
NF-κB regulates the expression of the antiapoptotic proteins survivin, inhibitor-of-apoptosis protein-1/2 (IAP1/2), X chromosome-linked inhibitor-of-apoptosis protein (XIAP), Bcl-2, Bfl-1/A1, TRAF1, and FLIP.34 Whether I3C can modulate the expression of these antiapoptotic gene products induced by TNF was also examined. As shown in Figure 4C, TNF induced these antiapoptotic proteins in a time-dependent manner, and I3C blocked this induction.
I3C potentiates apoptosis induced by TNF and chemotherapeutic agents
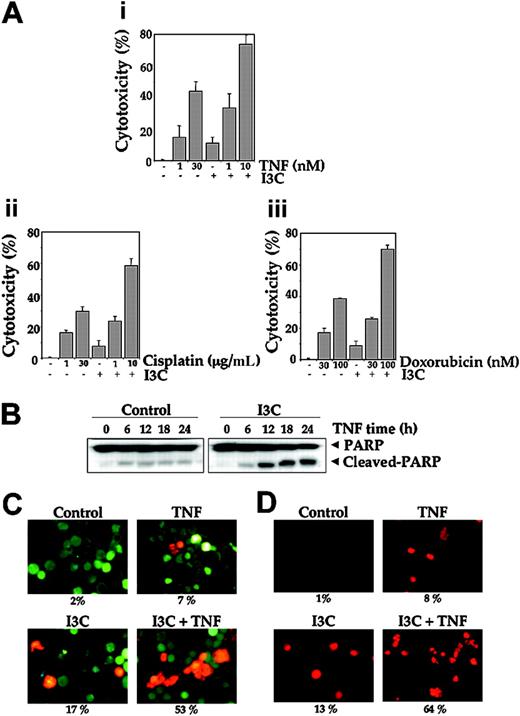
The activation of NF-κB has been shown to inhibit TNF-induced apoptosis.52 Results from Figure 4C suggest the potential of I3C in enhancing apoptosis induced by TNF and other cytotoxic agents through suppression of NF-κB-regulated antiapoptotic gene products. Whether suppression of NF-κB by I3C affects TNF-induced apoptosis was investigated by MTT, PARP cleavage, Live and Dead assay, and TUNEL staining methods. I3C enhanced TNF-induced cytotoxicity (Figure 5A1) as well as doxorubicin- (Figure 5A2) and cisplatin-induced cytotoxicity (Figure 5A3). I3C by itself had little cytotoxic effect. Whether enhanced cytotoxicity was due to apoptosis was further investigated. As shown in Figure 5B, TNF activated caspases as indicated by PARP cleavage, and I3C potentiated TNF-induced activity. The Live and Dead assay indicated that I3C up-regulates TNF-induced apoptosis from 7% to 53% (Figure 5C), and TUNEL staining showed that TNF-induced apoptosis was enhanced from 8% with TNF alone to 64% with I3C plus TNF (Figure 5D). In this assay I3C alone exhibited significant toxicity. Nevertheless, all assays together suggest that I3C enhanced the apoptotic effects of TNF and chemotherapeutic agents.
I3C inhibits constitutive NF-κB activation and proliferation of mononuclear cells derived from AML patients
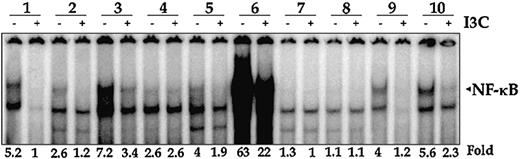
It has been reported that NF-κB is constitutively active in AML cells53 and that the active NF-κB mediates proliferation of cells.35 Whether I3C could suppress constitutively active NF-κB in AML cells was investigated in AML cells derived from 10 different patients (Table 1). The cells were treated with I3C, nuclear extracts were prepared, and then NF-κB activity was analyzed by EMSA. As shown in Figure 6A, NF-κB was found to be active in AML cells from most patients, though to variable extents, and I3C suppressed the activation. We also found that the treatment of AML cells with I3C decreased the proliferation of cells (Table 2).
Discussion
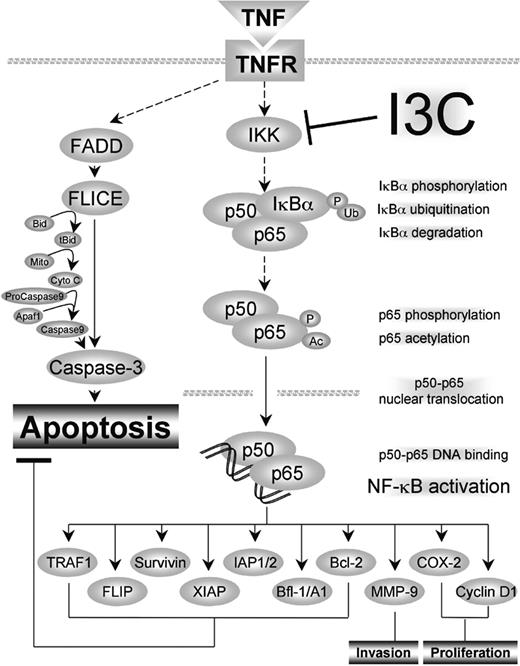
We found that I3C suppressed NF-κB activation induced by various agents irrespective of the cell type. NF-κB inhibition correlated with suppression of IKK and IκBα phosphorylation, ubiquitination, and degradation and with p65 phosphorylation, nuclear translocation, and acetylation. I3C also down-regulated NF-κB-regulated reporter gene transcription and gene products involved in cell proliferation, antiapoptosis, and invasion. This led to the potentiation of apoptosis induced by cytokines and chemotherapeutic agents (Figure 7).
I3C enhances TNF-induced cytotoxicity. (A) I3C enhances TNF- and chemotherapy-induced cytotoxicity. A total of 5000 Jurkat cells were seeded in triplicate in 96-well plates. Cells were pretreated with 1 μM I3C and then incubated with indicated concentrations of TNF (i), cisplatin (ii), or doxorubicin (iii) for 72 hours. Thereafter, cell viability was analyzed by the MTT method as described in “Materials and methods.” Values represent the mean ± SD of triplicate cultures. (B) Jurkat cells were pretreated with 25 μM I3C and then incubated with 1 nM TNF for the indicated times. Whole-cell extracts were prepared, subjected to SDS-PAGE, and blotted with anti-PARP antibody. (C) Jurkat cells were pretreated with 25 μM I3C and then incubated with 1 nM TNF for 16 hours. Cells were stained with Live and Dead assay reagent for 30 minutes and then analyzed under a fluorescence microscope as described in “Materials and methods.” (D) Jurkat cells were pretreated with 25 μM I3C and then incubated with 1 nM TNF for 16 hours. Cells were fixed, stained with TUNEL assay reagent, and then analyzed under a fluorescence microscope as described in “Materials and methods.” Stained slides were mounted with mounting medium (Sigma-Aldrich) and analyzed under an epifluorescence microscope (Labophot-2; Nikon). Pictures were captured using a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX) and Meta Morph version 4.6.5 software (Universal Imaging). Original magnification, ×200.
I3C enhances TNF-induced cytotoxicity. (A) I3C enhances TNF- and chemotherapy-induced cytotoxicity. A total of 5000 Jurkat cells were seeded in triplicate in 96-well plates. Cells were pretreated with 1 μM I3C and then incubated with indicated concentrations of TNF (i), cisplatin (ii), or doxorubicin (iii) for 72 hours. Thereafter, cell viability was analyzed by the MTT method as described in “Materials and methods.” Values represent the mean ± SD of triplicate cultures. (B) Jurkat cells were pretreated with 25 μM I3C and then incubated with 1 nM TNF for the indicated times. Whole-cell extracts were prepared, subjected to SDS-PAGE, and blotted with anti-PARP antibody. (C) Jurkat cells were pretreated with 25 μM I3C and then incubated with 1 nM TNF for 16 hours. Cells were stained with Live and Dead assay reagent for 30 minutes and then analyzed under a fluorescence microscope as described in “Materials and methods.” (D) Jurkat cells were pretreated with 25 μM I3C and then incubated with 1 nM TNF for 16 hours. Cells were fixed, stained with TUNEL assay reagent, and then analyzed under a fluorescence microscope as described in “Materials and methods.” Stained slides were mounted with mounting medium (Sigma-Aldrich) and analyzed under an epifluorescence microscope (Labophot-2; Nikon). Pictures were captured using a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX) and Meta Morph version 4.6.5 software (Universal Imaging). Original magnification, ×200.
I3C inhibited NF-κB activation induced by TNF, IL-1β, PMA, LPS, and CSC, suggesting that I3C must act at a step common to all of these activators. In response to most of these stimuli, NF-κB activation proceeds through sequential activation of IKK, phosphorylation at serines 32 and 36 of IκBα, and ubiquitination at lysines 21 and 22 of IκBα, leading finally to degradation of IκBα and the release of NF-κB.47 We found that I3C blocks NF-κB activation by inhibiting IKK.
Suppression of TNF-induced NF-κB activation by I3C is consistent with a report by Chinni et al,4 who showed the suppression of constitutive NF-κB in PC3 cells. They did not, however, report how I3C suppressed constitutive NF-κB activation. Howells et al54 found that the breast cancer cell lines MDA-MB468 and HBL-100 express constitutive NF-κB as examined by DNA binding and that treatment with I3C decreases DNA binding in MDA-MB468 cells and increases it in HBL-100 cells without changing IKK activity in either cell line. They used up to 20 times (1 mM) the concentration of I3C as we did to suppress NF-κB. It is unlikely that these differences are due to cell type, because we found that I3C suppressed TNF-induced NF-κB activation in all cell types tested. Perhaps the mechanism of constitutive NF-κB activation differs from that of inducible activation examined in our studies.
I3C inhibits constitutively active NF-κB activity and proliferation of AML cells. A total of 1 × 107 cells were resuspended in RPMI 1640 medium and treated with 50 μM I3C for 24 hours, and nuclear extracts were prepared and then analyzed for NF-κB activity by EMSA.
I3C inhibits constitutively active NF-κB activity and proliferation of AML cells. A total of 1 × 107 cells were resuspended in RPMI 1640 medium and treated with 50 μM I3C for 24 hours, and nuclear extracts were prepared and then analyzed for NF-κB activity by EMSA.
Our studies are the first to indicate that I3C also inhibits TNF-induced IKK activation. How IKK is activated is not fully understood, but Akt has been implicated,55 and I3C has been shown to suppress Akt activation.25,54 Thus, it is possible that inhibition of TNF-induced IKK activation is due to inhibition of Akt. The activation of Akt has also been shown to be involved in the phosphorylation of the p65 subunit of NF-κB.55 The suppression of p65 phosphorylation by I3C could also be due to inhibition of Akt.
We showed that I3C inhibited NF-κB-regulated gene transcription and NF-κB-regulated gene products involved in cell proliferation (eg, cyclin D1 and COX-2), antiapoptosis (eg, survivin, IAP1, IAP2, XIAP, Bcl-2, Bfl-1/A1, TRAF1, and FLIP), and invasion (MMP-9). There is no previous report of the regulation of these gene products by I3C. That I3C down-regulates cyclin D1 expression and the latter mediates G1/S transition may explain reports indicating that I3C induces G1/S arrest.3,4 The down-regulation of the expression of iNOS,23 PSA,12 and Bcl-2 4 could also be due to down-regulation of NF-κB as described here.
We found that I3C potentiates the apoptotic effects of cytokines and chemotherapeutic agents through the down-regulation of the antiapoptosis gene products survivin, IAP1, IAP2, XIAP, Bcl-2, Bfl-1/A1, TRAF1, and FLIP. The cytotoxic effects of TNF, cisplatin, and doxorubicin were enhanced by I3C. These results are in agreement with a report that pretreatment with I3C augments TRAIL-induced apoptosis in the prostate cancer cell line LNCaP, as measured by PARP and caspase-3 cleavage.56 Our results are also consistent with a report that an I3C acid-condensation product mixture sensitizes multidrug resistance gene transfectants to the toxicity of vinblastine and doxorubicin.57 Whether enhancement of apoptosis by I3C is also mediated through this mechanism, in addition to down-modulation of NF-κB, is unclear at present.
A schematic diagram of the effect of I3C on TNF-induced NF-κB activation and apoptosis.
A schematic diagram of the effect of I3C on TNF-induced NF-κB activation and apoptosis.
We also found that the expression of such NF-κB-regulated gene products as COX-2 and MMP-9 were down-regulated by I3C. These results might explain the antiinvasive and antimetastatic activities assigned to I3C.33,58 Several chemokines, interleukins, and hematopoietic growth factors are regulated by NF-κB activation.43 It is possible that immunomodulatory effects of I3C 7,20 are mediated through the regulation of these cytokines. I3C has been found to prevent cervical cancer in human HPV16 transgenic mice.13 Because a functional NF-κB binding site exists in the HPV16 long control region,59 it is possible that its effects against cervical cancer are mediated through suppression of NF-κB as described here. Constitutively active NF-κB has also been positively correlated with HPV16 E7 level in laryngeal squamous cell carcinoma.60 Through suppression of NF-κB, I3C may also be effective against this cancer.
Given the pharmacologic safety of I3C (established through centuries of dietary intake), our studies suggest that this compound has great potential as both a chemopreventive and a chemotherapeutic agent, especially when used in combination with existing agents. Whether the concentrations of I3C used in our studies are achievable in vivo remains to be determined. How I3C is metabolized in the cells is also unclear at present. Our results also show that AML cells derived from patients exhibit constitutive NF-κB activation. This NF-κB activation is suppressed by I3C, thus leading to inhibition of proliferation of the cells. Overall, our results demonstrate that I3C is a potent inhibitor of NF-κB activation, which may explain its antiproliferative, proapoptotic, antimetastatic, anti-inflammatory, and immunomodulatory effects.
Prepublished online as Blood First Edition Paper, April 5, 2005; DOI 10.1182/blood-2004-12-4589.
Supported by the Clayton Foundation for Research (B.B.A.), Department of Defense US Army Breast Cancer Research Program grant (BC010610; B.B.A.), a PO1 grant (CA91844) from the National Institutes of Health on lung chemoprevention (B.B.A.), a P50 Head and Neck Specialized Program Of Research Excellence (SPORE) grant from the National Institutes of Health (B.B.A.), and the Theodore N. Law Award for Scientific Achievement Fund from The University of Texas M. D. Anderson Cancer Center (Y.T.).
We thank Mr Walter Pagel for carefully reading the manuscript and providing valuable comments. Y.T. is an Odyssey Program Special Fellow at The University of Texas M. D. Anderson Cancer Center. B.B.A. is a Ransom Horne, Jr, Distinguished Professor of Cancer Research at The University of Texas M. D. Anderson Cancer Center.