Abstract
The long-term hepatic complications after allogeneic hematopoietic stem cell transplantation (HSCT) in hepatitis B virus (HBV) endemic area are unknown. We examined the serological and liver-related outcome of 803 consecutive patients who received allogeneic HSCTs, with a median follow-up period of 83 months (range, 0.5-155 months). Late HBV-related hepatitis occurred in 2 of the 721 hepatitis B surface antigen–negative (HBsAg–) recipients compared with 16 of the 82 HBsAg+ recipients after HSCT (0.3% vs 19.5%; P < .001 by log-rank). Liver cirrhosis developed in 8 of the 82 HBsAg+ recipients compared with none of the 721 HBsAg– recipients (9.8% vs 0%; P < .001 by log-rank). Twenty of the 31 (64.5%) HBsAg+ recipients of hematopoietic stem cells from donors with natural immunity to HBV had sustained serologic clearance of HBsAg after HSCT. Eight of the 62 recipients without sustained HBsAg clearance compared with none of the 20 recipients with sustained HBsAg clearance developed liver cirrhosis (12.9% vs 0%; P = .02 by log-rank). Our study showed that long-term hepatic complications occur in a significant proportion of HBsAg+ patients after HSCT and the incidence of liver cirrhosis is reduced in those with sustained serologic clearance of HBsAg after HSCT.
Introduction
Chronic hepatitis B virus (HBV) infection is one of the most common viral infections in humans, with more than 350 million HBV carriers worldwide.1,2 It is endemic in Asia, Southern Europe, and Latin America, where the prevalence of chronic HBV infection in the general population ranges from 2% to 20%. The most important sequelae of chronic HBV infection are liver cirrhosis and hepatocellular carcinoma (HCC). In Southern China and Taiwan, chronic HBV infection is responsible for approximately 80%-90% of cases of chronic liver diseases and HCC. It is in the fourth to fifth decade of life that these late or long-term complications begin to emerge.3 In patients with chronic HBV infection, progression from chronic hepatitis to cirrhosis and from compensated cirrhosis to hepatic decompensation and HCC has been estimated to be around 12%-23% and 6%-15% at 5 years of follow-up, respectively.4-6
The increased use of immunosuppressive or cytotoxic therapy, such as in allogeneic hematopoeitic stem cell transplantation (HSCT), has led to increase concern of the impact of immunosuppression on the natural history of chronic HBV infection.7 With the introduction of preemptive lamivudine treatment of hepatitis B surface antigen–positive (HBsAg+) patients treated with intense immunosuppression or chemotherapy, the incidence of hepatitis due to HBV reactivation in the immediate post-HSCT period has been drastically reduced.8,9
Previously, cirrhosis has been demonstrated to be an important late complication of HSCT and is most often due to chronic hepatitis C virus.10 Furthermore, patients infected with hepatitis C virus during the process of allogeneic HSCT progress more rapidly to cirrhosis than those chronic HCV patients who have not undergone allogeneic HSCT.11 However, the long-term outcome of these HBsAg+ recipients of allogeneic HSCT is unknown.12-14
On the other hand, HSCT provides a unique model to understand the virus-specific T-cell reactivity associated with the phenomenon of hepatitis B surface antigen (HBsAg) clearance and seroconversion to hepatitis B surface antibody (anti-HBs). Previously, it has been shown that serologic clearance of HBsAg after allogeneic HSCT is associated with adoptive transfer of donors' CD4+ T-lymphocyte reactivity to hepatitis B core antigen (HBc), rather than to HBV envelope proteins. This explained earlier clinical observations that HBsAg clearance occurs only in recipients with a donor who had natural immunity to HBV (anti-HBs+ and anti-HBc+ donors) and not those who received marrow with a vaccine-induced immunity (anti-HBs alone).15,16 Serologic clearance of HBsAg is usually observed between 3 to 10 months after transplantation, coinciding with the expected time of T- and B-cell immune recovery in the recipient.15,17 However, the long-term outcome of these recipients with serologic clearance of HBsAg is unknown.
In our present study, we examine the long-term hepatic complications in the recipients of allogeneic HSCT in a HBV-endemic area, and attempt to determine whether HBsAg clearance by adoptive transfer of immunity is associated with a reduced risk of developing hepatic complications.
Patients, materials, and methods
Patients
From October 1990 to December 2003, 803 consecutive recipients without hepatitis C infection were treated with allogeneic HSCT at the Bone Marrow Transplantation Unit, Department of Medicine, Queen Mary Hospital, Hong Kong SAR, China. All donors and recipients were screened for HBsAg, anti-HBs (enzyme-linked immunosorbent assay II; Abbott Laboratories, Chicago, IL), and human immunodeficiency virus antibody (Abbott Laboratories). Hepatitis C virus antibody (Ortho Diagnostics System, Raritan, NJ) was screened from July 1993 onwards while the donors and recipients before this period were retrospectively screened for hepatitis C virus (HCV) antibody using stored serum samples. Further testing for hepatitis B e antigen (HBeAg), hepatitis B e antibody (anti-HBe; Abbott Laboratories), and serum HBV DNA (Digene Hybrid Capture II assay; Murex Diagnostics, Dartford, United Kingdom) was performed within 4 to 8 weeks of marrow harvest or HSCT in HBsAg+ donors and recipients, respectively. Hepatitis B core antibody (Anti-HBc; Abbott Laboratories) was assayed on donors and recipients who were tested negative for HBsAg. Between January 1996 and February 1998, all HBsAg+ recipients were treated with preemptive famciclovir starting 1 week before HSCT for at least 6 months after HSCT; from March 1998 onwards, all HBsAg+ recipients were treated with preemptive lamivudine starting one week before HSCT for at least 12 months after transplantation.8,18
All HSCT recipients were prospectively followed up at weekly intervals for the first 12 weeks after transplantation, and then every 4 to 8 weeks until the time of analysis (July 2004) or death. At each follow-up visit, the clinical status of the recipients was recorded and blood was tested for liver biochemistry (serum alanine aminotransaminase, aspartate aminotransaminase, bilirubin, and albumin). For recipients who were HBsAg+, HBeAg, anti-HBs, and anti-HBe, and serum HBV DNA were tested. α-Fetoprotein and ultrasonography of the liver were also performed on all HBsAg+ recipients every 6 months intervals.
Definition of hepatic events
Hepatitis was defined as a more than 3-fold elevation of serum aminotransaminase above the upper limit of normal, on 2 consecutive determinations at least 5 days apart, in the absence of clinical features suggestive of veno-occlusive disease (VOD) or infection by cytomegalovirus or herpes simplex virus. Hepatitis was defined as HBV-related when, if preceded or accompanied by an elevation of serum HBV DNA to more than 10 times that of the pre-exacerbation baseline, the serum HBV DNA turned from negative to positive (by Digene Hybrid Capture II assay), or if HBsAg became positive and remained so for 2 consecutive readings 5 days apart. As graft-versus-host disease (GVHD) can present with markedly increased serum aminotransaminase, a liver biopsy was performed when clinically indicated to look for histologic evidence of active HBV-related hepatitis such as prominent lymphocyte infiltrate and acidophilic bodies in liver acini, interface hepatitis and intrahepatic expression of HBsAg, and hepatitis B core antigen expression.19-21 Late HBV-related hepatitis was defined as HBV-related hepatitis occurring 12 months after HSCT. Hepatic failure was defined as the presence of hepatic encephalopathy and deranged blood coagulation (prothrombin time exceeding 10 seconds of control). The clinical criteria used for the diagnosis of VOD were having a bilirubin level of 34.2 μM or higher, hepatomegaly or right upper quadrant pain of liver origin, and more than 2% weight gain due to fluid accumulation. The diagnosis required 2 of 3 criteria, occurring within 20 days of transplantation. The diagnosis was made only when acute GVHD, sepsis, cardiac failure, and tumor infiltration had been ruled out.22 Liver cirrhosis was diagnosed by either radiologic evidence of cirrhosis on ultrasound/computerized tomography23 or histologically by liver biopsy. Decompensated cirrhosis was diagnosed in the presence of at least one complication of cirrhosis, including radiologic or clinical evidence of ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, or bleeding esophageal varices. The diagnosis of HCC was made histologically or was based on image findings (including ultrasonography, computerized tomography, and arteriography) consistent with HCC, plus an α-fetoprotein level greater than 400 ng/mL.24-28 This study was approved by the Institutional Review Board of the Queen Mary Hospital. Informed consent was obtained from all donors and recipients for their recruitment into the study.
Statistical analysis
All statistical analyses were performed using the Statistical Program for Social Sciences (SPSS 12.5 for Windows; SPSS, Chicago, IL). The Mann-Whitney U test was used for comparing 2 continuous variables and the χ2 with Yates correction for continuity or Fisher exact test was used for comparing 2 categoric variables. The primary outcome measure was the occurrence of liver cirrhosis during follow-up. The secondary outcome measure was the occurrence of late HBV-related hepatitis. Variables such as age, sex, liver biochemistry, and recipients' pre-HSCT serum HBV DNA were analyzed in a univariate analysis in order to determine any factors associated with liver cirrhosis, late HBV-related hepatitis, or durability of HBsAg clearance. The Kaplan-Meier method was used for calculation of the cumulative probability of liver cirrhosis, late HBV-related hepatitis, and durability of HBsAg clearance. Cox proportional hazards regression analysis was used to examine the relationship of liver cirrhosis and durability of HBsAg clearance to HBsAg clearance, antiviral therapy, and HBeAg status. The 95% confidence interval (95%CI) for all estimates was also provided where appropriate. Continuous variables were expressed as medians (range). All statistical analyses were performed on an intention-to-treat basis. Statistical significance was defined as a P value below .05 (2-tailed).
Results
Of the 803 patients who received allogeneic HSCT, 82 (10.2%) were HBsAg+ and 721 (89.9%) were HBsAg– before HSCT. The median duration of follow-up was 83.4 months (range, 0.5-154.8 months). The characteristics of HBsAg+ and HBsAg– patients are shown in Table 1.
At the time of analysis, 376 patients had died (58.7% from relapse of disease, 15.0% from GVHD, 12.8% from infection, 6.4% from graft failure, and 7.1% from other causes). There was no significant difference in the cumulative mortality between HBsAg+ and HBsAg– patients (39 [47.6%] of 82 patients vs 337 [46.7%] of 721 patients; P = .891 by log-rank).
Long-term HBV-related hepatic complication after HSCT
Two (0.8%) of 239 anti-HBc+ and anti-HBs+ HSCT recipients developed late HBV-related hepatitis at 18.5 and 24.6 months post-HSCT, respectively. Both donors were HBsAg– but anti-HBc+ with negative anti-HBs. The 2 patients responded to lamivudine therapy with normalization of serum aminotransaminase levels. However, they remained serologically positive for HBsAg after the episode of hepatitis (Table 2). None of the remaining 482 pre-HSCT HBsAg– patients developed late HBV-related hepatitis.
Sixteen (19.5%) of the 82 pre-HSCT HBsAg positive patients developed late HBV-related hepatitis. Eleven (68.8%) of these 16 patients with late HBV-related hepatitis had a liver biopsy performed at the time of hepatitis, with histology demonstrating the presence of active HBV-related hepatitis.
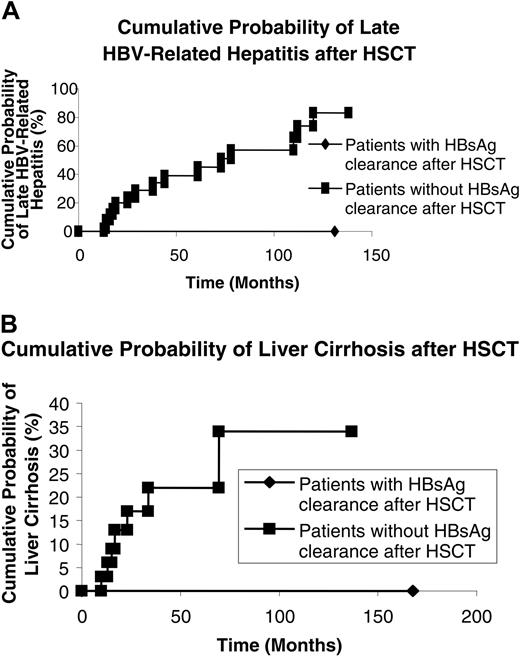
Thirteen patients were reinitiated on lamivudine after late HBV-related hepatitis (Table 2). Three patients did not receive lamivudine treatment, as the drug was unavailable at that time. For those who received lamivudine, 1 patient developed fulminant hepatic failure requiring an orthotropic liver transplantation (Table 2). The HBV-related hepatitis was controlled with lamivudine, with normalization of serum aminotransaminase in the remaining 12 patients. For those who did not receive lamivudine for their late HBV-related hepatitis, 2 patients died of fulminant hepatic failure while the remaining 1 had a spontaneous recovery (Table 2).The cumulative probability of late HBV-related hepatitis was lower in patients who were HBsAg– before HSCT when compared with those who were HBsAg+ before HSCT (0.3% vs 19.5%; P < .001 on log-rank) (Figure 1A).
At the time of analysis, 8 of 82 HBsAg+ patients had developed liver cirrhosis, compensated liver cirrhosis (n = 4), decompensated liver cirrhosis due to HBV reactivation (n = 3), and HCC with liver cirrhosis (n = 1, 0.1%). Liver transplantation was performed in 1 of the patients with decompensated liver cirrhosis. The patient with HCC had a left segmentectomy and was still alive at the time of analysis. The clinical features of these 7 patients are shown in Table 3. None of the 721 HBsAg– patients developed liver cirrhosis. The cumulative probability of liver cirrhosis was significantly higher in HBsAg+ recipients when compared with HBsAg– recipients (9.8% vs 0%; P < .001 on log-rank) (Figure 1B).
Durability of serologic clearance of HBsAg by adoptive immune transfer
Fifty-eight (70.7%) of the 82 HBsAg+ recipients were treated with preemptive nucleoside analog before HSCT, 16 patients (19.5%) with famciclovir, and 42 patients (51.2%) with lamivudine.
Twenty-four (29.3%) of the 82 HBsAg+ recipients had serologic clearance of HBsAg after HSCT. Serologic clearance of HBsAg occurred in 24 (77.4%) of the 31 recipients of hematopoietic stem cells (HSCs) from donors with natural immunity to HBV compared with none (0%) of the 51 recipients of HSCs from donors without natural immunity to HBV (P < .001). Twenty (64.5%) of these 31 patients receiving HSCs from donors with natural immunity to HBV were persistently HBsAg– and anti-HBs+ after a median follow-up time of 83.4 months (range, 0.5-154.8 months).
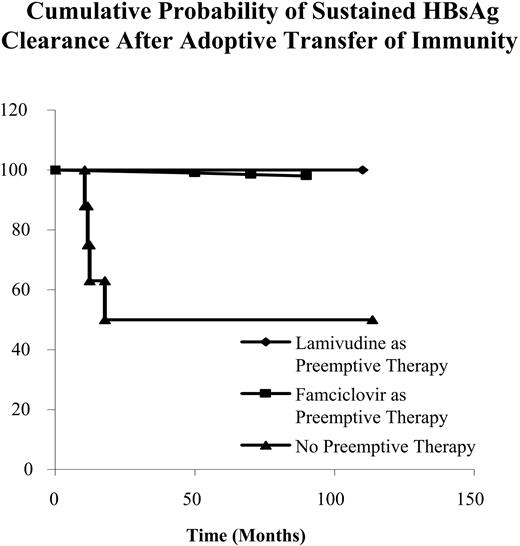
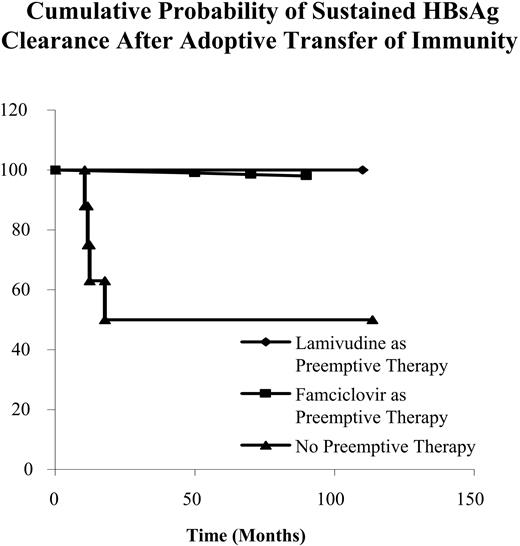
Four (12.9%) patients reverted to HBsAg positivity again at 10.5, 12.5, 13.2, and 16.7 months after HSCT, respectively. The 4 patients with transient HBsAg clearance did not receive either lamivudine or famciclovir as preemptive antiviral therapy (Table 4). Recipients receiving lamivudine for preemptive therapy had a much higher cumulative incidence of sustained HBsAg clearance when compared with recipients without preemptive therapy (P = .011) (Figure 2). There was no difference in sustained HBsAg clearance when recipients receiving famciclovir for preemptive therapy were compared with recipients without preemptive therapy (P = .156) [Table 4; Figure 2].
Cumulative probability of late HBV-related hepatitis and liver cirrhosis in patients who are HBsAg+ and HBsAg– before hematopoietic stem cell transplantation. (A) HBV-related hepatitis. (B) Liver cirrhosis.
Cumulative probability of late HBV-related hepatitis and liver cirrhosis in patients who are HBsAg+ and HBsAg– before hematopoietic stem cell transplantation. (A) HBV-related hepatitis. (B) Liver cirrhosis.
In recipients receiving HSCs from donors with natural immunity to HBV, preemptive lamivudine therapy was independently associated with sustained HBsAg clearance via adoptive transfer of immunity by multivariate Cox proportional hazards regression analysis (adjusted hazard ratio, 5.788; 95%CI, 1.554-21.561; P = .009).
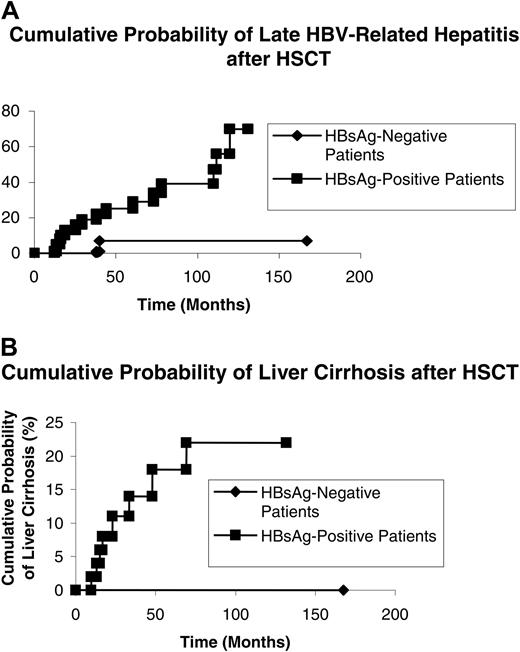
For the HBsAg+ recipients, none of the 20 patients with sustained HBsAg clearance and 16 of the 62 patients without HBsAg clearance developed late HBV-related hepatitis (0% vs 25.8%; P = .002 on log-rank) (Figure 3A). None of the 20 patients with sustained HBsAg clearance and 8 of the 62 patients without HBsAg clearance developed liver cirrhosis (0% vs 12.9%, P = .02 on log-rank) (Figure 3B).
By multivariate Cox regression analysis, a sustained HBsAg clearance after HSCT was an independent factor in reducing the risk of developing liver cirrhosis (adjusted hazard ratio, 0.15; 95%CI, 0.022-1.405; P = .044).
Hepatitis serology after allogeneic HSCT
Four of 23 HBeAg+ recipients had HBeAg seroconversion. The preemptive use of nucleoside analog was not associated with a higher cumulative probability of HBeAg seroconversion at 12, 24, or 36 months. The HBeAg seroconversion in all 4 patients was stable and none of them reverted back to HBeAg positivity at the time of analysis. All the donors for these 4 recipients were negative for all HBV markers.
Five (8.5%) of the 59 HBeAg–, anti-HBe+ patients had HBeAg seroreversion during the period of immunosuppression. This occurred at 1.4, 5.3, 6.8, 9.7, and 12.5 months after HSCT, respectively. None of these 5 patients had received preemptive nucleoside analog. No HBeAg seroreversion was seen after 13 months following HSCT. The donors for these 5 recipients were negative for all HBV markers.
Discussion
In the present study, HBsAg+ recipients developed more long-term HBV-related hepatic complications after HSCT when compared with HBsAg– recipients. The frequency of liver cirrhosis in this series was 9.8% at a median follow-up time of 7 years. For chronic HBV-infected immunocompetent patients, a study in Asians has shown that with a follow-up of 35.3 months, liver cirrhosis occurred in 6.1% of HBsAg+ patients, with a calculated annual incidence of 2.1%. The cumulative probability of liver cirrhosis increases linearly over time and is close to 15% at the end of 66 months.4 Thus, the rate of development of liver cirrhosis in HBsAg+ recipients following allogeneic HSCT appears to be similar to that in immunocompetent patients. This is in contrast to patients infected with hepatitis C virus during allogeneic HSCT, where the median time to cirrhosis in transplant recipients was 18 years compared with 40 years in the control population.10 The underlying mechanisms explaining the different outcome in chronic HBV- and HCV-infected patients after HSCT remains to be explored.
From a prospective study, the incidence of HCC among HBsAg+ immunocompetent patients has been reported to be around 1.2% (40 of 3454) and 1 out of 19 253 in HBsAg– controls at an average follow-up time of 3.3 years, resulting in a relative risk of 233-fold.29 This is also similar to the occurrence rate of HCC in our HBsAg+ HSCT recipients (1 of 82, or 1.2%). Our results demonstrate that liver cirrhosis and HCC remains an important issue in HBsAg+ HSCT recipients and long-term survivors of HSCT with positive HBsAg should be monitored closely for the development of such complications after HSCT.
Two (0.8%) HBsAg–, anti-HBc+, and anti-HBs+ patients developed late HBV-related hepatitis following HSCT. Whether the late HBV-related hepatitis is due to de novo hepatitis B infection or transmission from donors is uncertain. Alternatively, this could be related to reactivation of occult HBV in the 2 recipients after HSCT. This is in keeping with the previous findings that HBV can persists for decades after recovery from acute HBV despite the presence of anti-HBs30 and can be reactivated to develop fulminant hepatitis with immunosuppression.31-33 Hence, further studies need to be performed to explore the usefulness of detecting such low levels of HBV by more sensitive polymerase chain reaction (PCR)–based methods and whether antiviral therapy need to be used in these HBsAg– but HBV DNA+ HSCT recipients in a preemptive manner.34
There is also an increased risk of HBV-related hepatitis following HSCT in HBsAg+ recipients. After the discontinuation of immunosuppressive therapy and immunity recovery, HBsAg+ patients may still develop HBV-related hepatitis. The cumulative probability of late HBV-related hepatitis is 20% at 50 months after HSCT. This increases to 70% at 120 months after HSCT, supporting the need for long-term monitoring of these patients following HSCT.
Cumulative probability of sustained HBsAg clearance after adoptive transfer of immunity.
Cumulative probability of sustained HBsAg clearance after adoptive transfer of immunity.
Similar to spontaneous HBsAg clearance, HBsAg clearance by adoptive transfer of immunity is durable in 64.5% of the cases. Recipients with preemptive lamivudine therapy have a higher sustained HBsAg clearance by adoptive transfer of immunity. Since lamivudine can at least partially overcome HBV-specific T-cell hyporeactivity, it may augment the stability of immune control of HBV by adoptive transfer of HBV immunity leading to disease resolution in recipients chronically infected with the virus.35
HBsAg+ patients can have an improvement in both liver histology and biochemistry after spontaneous HBsAg clearance.36 The frequency of developing liver cirrhosis in these patients has been found to be reduced to only 3.0% in 6 years.37 Even for those patients with compensated liver cirrhosis, an improvement in outcome has been demonstrated after HBsAg clearance.38 Similar improvement in long-term hepatic outcome can be observed after serologic clearance of HBsAg by adoptive transfer of immunity. In fact, the chance of developing liver cirrhosis in patients with HBsAg clearance by adoptive transfer of immunity in our cohort is lower than previously reported in patients with spontaneous HBsAg clearance.
Cumulative probability of late HBV-related hepatitis and liver cirrhosis in those with and without sustained HBsAg clearance after hematopoietic stem cell transplantation. (A) HBV-related hepatitis. (B) Liver cirrhosis.
Cumulative probability of late HBV-related hepatitis and liver cirrhosis in those with and without sustained HBsAg clearance after hematopoietic stem cell transplantation. (A) HBV-related hepatitis. (B) Liver cirrhosis.
In our area where HBV infection is endemic, the most common mode of infection is perinatal, from chronically infected mother to baby.39 Thus, many of our studied HBsAg+ recipients were likely to have been infected for 4 to 5 decades before HSCT. As the baseline serum alanine aminotransaminase levels of the HBsAg+ recipients were mostly within the normal range, it is unlikely that these recipients had severe hepatic necroinflammation.40 On the other hand, it is possible that some of these patients had a more advanced stage of fibrosis at presentation for HSCT accounting for the relatively early development of liver cirrhosis in 8 of the recipients. This would be better characterized if one could have a baseline liver biopsy for these patients. However, it would be difficult to justify such a procedure in high-risk patients with no specific clinical indication, such as derangement of liver function test.
In conclusion, a significant proportion of our HBsAg+ patients suffered from liver cirrhosis and late HBV-related hepatitis after allogeneic HSCT. Serologic clearance of HBsAg by adoptive transfer of immunity reduces the risk of late HBV-related hepatitis and liver cirrhosis, and the durability of the clearance appears to be enhanced with the use of nucleoside analogs. Those with persistent HBsAg positivity after HSCT should be closely monitored for the development of liver cirrhosis or HCC and late HBV-related hepatitis, so that appropriate treatments may be initiated early.
Prepublished online as Blood First Edition Paper, March 29, 2005; DOI 10.1182/blood-2005-02-0698.
Supported by a grant from the Cheng Si-yuan (China-International) Hepatitis Research Foundation (to the University of Hong Kong), and a China National 973 research grant (G 1999 054105 to G.K.K.L.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.