Comment on Maeda et al, page 749
Host γδ T cells are shown to be a critical population for the activation of host antigen-presenting cells and subsequent induction of graft-versus-host disease (GVHD).
The characteristic tissue distribution of acute GVHD has long puzzled researchers and clinicians alike. In this issue of Blood, Maeda and colleagues provide a potential explanation for acute GVHD's predilection for the skin and intestinal tract. The authors demonstrate that host γδ T cells, which predominate in these organs, play an important role in the activation of host antigen-presenting cells (APCs) following total body irradiation. These fully activated host APCs in turn stimulate alloreactive donor αβ T cells, unleashing the subsequent tissue injury characteristic of GVHD.1
So how do γδ T cells differ from αβ T cells and what is their physiologic role in immunity? Although γδ T cells represent only a small proportion (< 5%) of lymphocytes in the blood and peripheral lymph nodes, they are present in much larger numbers in epithelial tissues such as the skin and intestinal tract. They are unique in that they recognize both protein and nonprotein moieties in the absence of major histocompatibility complex (MHC) and thus are not dependent on antigen processing by APCs. The predominant antigens that γδ T cells recognize and the requirement for the γδ T-cell receptor (TCR) in this process remain unclear. To date, the epithelial-associated γδ T cells are known to be capable of recognizing constitutively expressed “self”-antigens such as the MHC class I polypeptide-related sequences and the T10/22 proteins, the latter of which are expressed in stressed epithelial cells in response to inflammation. It is clear that epithelial-based γδ T cells can release proinflammatory cytokines and chemokines to augment mucosal immunity early in the immune response.2 Conversely, they are also a critical immunoregulatory population late in immunity, helping to terminate the immune response through the production of anti-inflammatory cytokines such as interleukin-10 (IL-10).3 FIG1
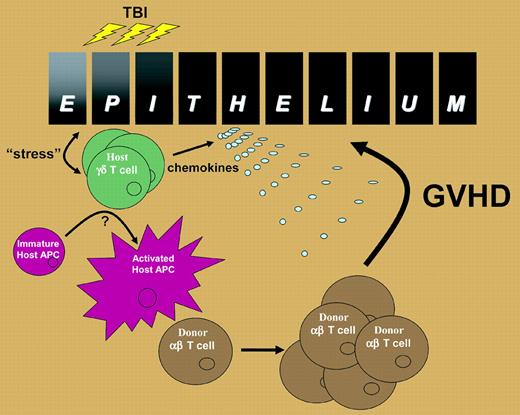
Proposed role of host γδ T cells in the induction of GVHD. Epithelia within the skin and intestinal tract produce “stress” proteins in response to the inflammation associated with total body irradiation that is recognized by resident γδ T cells. The γδ T cells activate host APC trafficking through the tissue and also secrete chemokines such as macrophage inflammatory protein-1 α (MIP1α), MIP1β, and RANTES (regulated on activation, normal T cells expressed and secreted). The activated host APCs then migrate to the regional lymph nodes and stimulate alloreactive αβ T cells, which are subsequently attracted to the site of epithelial injury, perhaps by γδ T-cell–derived chemokines, where they induce GVHD in the target organ.
Proposed role of host γδ T cells in the induction of GVHD. Epithelia within the skin and intestinal tract produce “stress” proteins in response to the inflammation associated with total body irradiation that is recognized by resident γδ T cells. The γδ T cells activate host APC trafficking through the tissue and also secrete chemokines such as macrophage inflammatory protein-1 α (MIP1α), MIP1β, and RANTES (regulated on activation, normal T cells expressed and secreted). The activated host APCs then migrate to the regional lymph nodes and stimulate alloreactive αβ T cells, which are subsequently attracted to the site of epithelial injury, perhaps by γδ T-cell–derived chemokines, where they induce GVHD in the target organ.
Thus, it appears likely that, following total body irradiation, host γδ T cells sense damage to stressed epithelial tissue in the skin and intestine to which they are juxtaposed and subsequently activate host APC trafficking through the tissue (see figure). The mechanism(s) by which host γδ T cells activate host APCs remains to be defined but appears to require cell-to-cell contact, at least in vitro according to Maeda and colleagues. The fully activated recipient APC then traffics to the regional lymph node to initiate the activation and expansion of alloreactive donor αβ T cells, which are in turn attracted back to the intestine, perhaps by virtue of the chemokines secreted by the γδ T cell. The final effector phase of tissue injury is then mediated by a combination of cytolytic pathways and inflammatory cytokines downstream of the donor αβ T cell.4 The mechanisms by which γδ T cells are activated during allogeneic transplantation and their subsequent enhancement of host APC allostimulatory capacity will be important avenues for future study. From a clinical perspective, the current study suggests a novel approach to GVHD prophylaxis: the specific deletion of recipient γδ T cells prior to transplant conditioning. ▪