Leukocyte recruitment to inflammation sites depends on interactions between integrins and extracellular matrix (ECM). In this report we show that mice lacking the ECM protein mindin exhibit severely impaired recruitment of neutrophils and macrophages in 4 different inflammation models. Furthermore, neutrophils directly bind to immobilized mindin, and mindin matrix mediates neutrophil migration in vitro. The adhesion of neutrophils to mindin is blocked by anti–integrin α4, anti–integrin αM, and anti–integrin β2 antibodies. We also show that HEK-293 cells transfected with cDNA encoding these integrins exhibit enhanced binding to immobilized mindin matrix and the increased binding can be blocked by anti-integrin antibodies. Our results suggest that mindin serves as a novel ligand for integrins and mindin-integrin interactions are critical for inflammatory cell recruitment in vivo.
Introduction
Leukocyte recruitment to inflamed sites is a multistep process consisting of cell tethering, rolling, firm adhesion, transmigration, and retention.1,2 Upon inflammatory stimulation, leukocytes in the blood vessel traverse the endothelial cell monolayer and the basement membrane of the blood vessel endothelium and migrate into the interstitial extracellular matrix (ECM). It has been shown that different sets of adhesion receptors are involved at different steps of inflammatory cell recruitment.1,2 Members of the integrin family play important roles in several stages of leukocyte migration during inflammation.3 Integrins are transmembrane receptors composed of α and β heterodimers. To date, 24 distinct integrins assembled from 8 β and 18 α subunits have been described.4 Both β1 and β2 integrins mediate leukocyte adhesion and migration by interacting with endothelial cells and ECM proteins.5-7 Several ECM proteins including fibronectin, vitronectin, collagen, and laminin have been shown to function as ligands for integrins.8 ECM proteins play important roles in the recruitment of inflammatory cells. Monocytes and neutrophils not only adhere to laminin, thrombospondin, and fibronectin in vitro9-11 but also depend on fibronectin, a major ECM component in synovium, for their migration to inflamed sites in rat and mouse arthritis models.12-14
Mindin is a member of the mindin–F-spondin family of ECM proteins. The identified members of this family include murine F-spondin and mindin, zebrafish mindin1 and mindin2, and Drosophila melanogaster M-spondin.15-19 All members of the mindin–F-spondin family share 3 domains: FS1 (for F-spondin), FS2, and thrombospondin type 1 repeats. Mouse mindin is expressed abundantly in lymphoid organs and lungs.19 Mindin functions as a pattern-recognition molecule for microbial pathogens. Mindindeficient mice exhibit an impaired ability to clear bacterial infection, and mindin-deficient macrophages show defective responses to a broad spectrum of microbial stimuli. Moreover, mindin directly binds to bacteria and their components and functions as an opsonin for the phagocytosis of bacteria.19
In this report we have determined the role of mindin in inflammatory cell recruitment in vivo using mindin mutant mice. We found that the recruitment of macrophages and neutrophils was severely impaired in mindin-deficient mice in 4 different inflammation models. We show that neutrophils directly adhere to immobilized mindin matrix. Furthermore, mindin matrix mediates neutrophil migration in response to fMLP (formyl-Met-Leu-Phe), and mindin-mediated migration can be blocked by anti-α4, anti-αM, and anti-β2 integrin monoclonal antibodies (mAbs). Importantly, HEK-293 cells expressing these integrins exhibit enhanced specific adhesion to coated mindin matrix. Our results suggest that mindin functions as a novel ligand for integrins and plays a critical role in inflammatory cell recruitment.
Materials and methods
Mice
Mindin-/- and mindin+/+ mice19 were derived from breeding of heterozygous mice after these mice were backcrossed to C57BL/6 for 7 generations and maintained in a specific pathogen-free facility at Duke Vivarium. Six- to 10-week-old age- and sex-matched mice were used in experiments. All animal experiments were performed according to protocols approved by the Duke University Institutional Animal Care and Use Committee.
Reagents and cell lines
The following blocking antibodies were purchased as listed: CD3 (2C11; Pharmingen, San Diego, CA), CD29 (clone HMβ1-1; Biolegend, San Diego, CA), CD18 (MA-1806; Endogen, Rockford, IL), CD11a (M17/4; e-Bioscience, San Diego, CA), CD11b (MCA711; Biosource, Thousand Oaks, CA), CD49d (9C10; Biolegend), CD49e (5H10-27; Biolegend). Fluorescence-labeled mAbs including anti–granulocyte-differention antigen (Gr-1)–cyanin 5/phycoerythrin (Cy5/PE) or fluorescein isothiocyanate (FITC), CD29-FITC, CD18-FITC, CD61-FITC, CD11a-FITC, CD11b-FITC, CD11c-FITC, CD49d-PE, and CD49e-PE were obtained from either eBioscience or Biolegend. cDNAs encoding full-length CD11b, CD18, CD29, and CD49d were amplified by reverse transcriptase–polymerase chain reaction (RT-PCR), cloned into pcDNA3.1 vector, and sequenced. Recombinant mouse mindin was generated as described19 and is 98% pure as judged by Coomassie staining of a protein gel. Group B streptococcus (GBS) was a clinical isolate provided by J. R. Wright (Duke University Medical Center). Influenza virus strain A/PR/8/34 (H1N1) (PR8) was kindly provided by Dr D. J. Topham (University of Rochester).
HEK-293 cells were cotransfected with pcDNA3.1CD18/pcDNA3.1CD11b, pcDNA3.1CD29/pcDNA3.1CD49d, or vector alone using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Transfection efficiency was determined by fluorescence-activated cell sorter (FACS) analysis at 48 hours after transfection. For binding assay, transfected HEK-293 cells were stimulated with 100 ng/mL phorbol myristate acetate (PMA; Sigma, St Louis, MO) for 15 minutes before they were added into mindin-coated plates. For antibody blocking assay, anti-CD18/CD11b or CD29/CD49d mAbs at 25 μg/mL were added to the binding assays.
Inflammation models
To induce peritonitis, mice were injected peritoneally with 1 mL of 3% thioglycollate broth. At days 1 and 3 after injection, the peritoneal cavity was lavaged. Cytospin slides with peritoneal cells were stained with Diff-Quik Stain Set (Dade Behring, Düdingen, Switzerland) and neutrophils and macrophages were counted in at least 3 different fields according to their morphologic characteristic after staining.
To examine neutrophil response in the lung, mice were anesthetized by peritoneal injection of a mixture of ketamine hydrochloride (100 mg/kg) and xylazine (3 mg/kg). A total volume of 50 μL phosphate-buffered saline (PBS) containing either 2 × 107 colony-forming units (cfu's)/mL GBS or 3 × 104 50% tissue culture infections dose (TCID50)/mL influenza virus (H1N1) was intratracheally inoculated. Twenty-four hours after GBS infection or 48 hours after influenza virus infection, mice were killed. The trachea was exposed and bronchoalveolar lavage (BAL) was performed 3 times with a total of 3 mL cold PBS. Cells in the BAL fluids were analyzed as described in the peritonitis model.
To induce ear dermatitis, mice were anesthetized and 10 μL 3% oxazolone (Sigma) in 4:1 acetone–olive oil was applied to each side of one ear. Mice were killed 6 hours later and ears were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). The untreated ears were used as controls. The number of migrated neutrophils was counted in different fields under light microscopy according to the polynuclear morphologic characteristic. Mindin expression on the ear was detected using frozen sections. Five-micrometer ear sections were prepared, incubated in 3% bovine serum albumin (BSA)–PBS for 1 hour at room temperature, washed 3 times with PBS containing 0.5% BSA and 0.01% Tween 20, and stained with rabbit antimindin antibody or normal rabbit sera for 30 minutes at room temperature. After washing 3 times, the sections were incubated with FITC-labeled antirabbit antibody (Pharmingen) and examined under fluorescence microscopy.
Neutrophil purification and analysis
To determine the number of circulating neutrophils, 50 μL heparinized blood collected from anesthetized mice was lysed of red blood cells (RBCs) and then total numbers of leukocytes and neutrophils were counted. For FACS analysis of circulating neutrophils, 40 μL whole blood or bone marrow (BM) cells were incubated with 50 μL 2.4G2 supernatant for 15 minutes on ice followed by anti–Gr-1–Cy5/PE and –CD11b-FITC or other antibodies as indicated. Granulocytes were gated based on side and forward scatter profiles and analyzed for their Gr-1/CD11b expression by flow cytometry.
Neutrophils were purified by discontinuous Percoll (Amersham Bioscience, Arlington Heights, IL) gradients centrifugation as described.20,21 BM or peritoneal cells were layered onto Percoll gradients of 82%/65%/55% and centrifuged at 4°C for 30 minutes. Neutrophils were recovered between the interface of 82%/65%, washed with cold PBS, and resuspended in serum-free Dulbecco modified Eagle medium (DMEM) medium. The purity of neutrophils was approximately 95% as determined by cytospin and H&E staining. Viability determined by 0.1% trypan blue exclusion was greater than 95%.
Neutrophil binding assay
Neutrophil binding assay was performed according to Lowell et al.21 Mindin, fibronectin (Invitrogen), laminin (Sigma), or BSA (Sigma; 100 μL/well at 20 μg/mL) was coated on 96-well plates at 4°C overnight in sodium bicarbonate buffer (pH 9.6). Purified neutrophils from BM or peritoneal cells of wild-type mice were added into the wells and incubated at 37°C for 1 hour. PBS was gently added to each well and then the plates were reversed and left for 5 minutes, then reversed again. Half of the supernatants were discarded right away to remove nonadherent cells. The plates were washed 3 times and centrifuged for 5 minutes. At least 3 different fields of adherent cells in each well were counted under light microscopy.
Neutrophil migration assay
The polycarbonate membranes (pore size 3.0 μm) of transwell for 24-well plates (Corning, Corning, NY) were precoated with 100 μL rMindin, fibronectin, or BSA at 50 μg/mL in PBS overnight at 4°C. DMEM medium (0.6 mL) with or without 100 nM fMLP (Sigma) was added to the lower well of the plate. BM neutrophils (1 × 106 in 100 μL) were placed onto the upper chamber of the transwell system and incubated at 37°C for 1 hour. Then the upper chambers were removed and the migrated cells in the lower cell culture plate were counted. For antibody blocking assay, neutrophils purified from mindin-/- BM were mixed with mAbs as indicated and added to the precoated transwell upper chambers. After 1-hour incubation at 37°C, the migrated neutrophils were counted.
Results
Impaired recruitment of inflammatory cells in mindin-/- mice
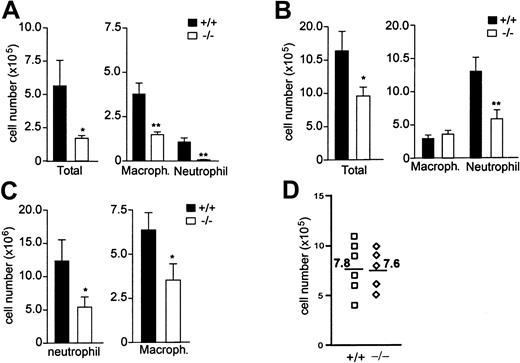
Our previous results show that mindin-/- mice exhibit defective clearance of GBS infection in the lung.19 The defective clearance of GBS in the mutant mice may result from an impaired activation of macrophages since mindin is critical for macrophage activation.19 However, as an ECM protein, mindin may also play a role in leukocyte recruitment into the lung after GBS infection. To address this issue we determined the number of total cells in BAL fluids from wild-type and mindin-/- mice 24 hours after GBS infection. The total number of leukocytes in BAL fluids of mindin-/- mice was reduced by more than 70% when compared with wild-type controls (Figure 1A). This reduction was attributed to markedly lower numbers of both macrophages and neutrophils in BAL fluids from mindin-/- mice (Figure 1A). These results demonstrate an important role for mindin in leukocyte migration in the lung after bacterial infection.
We next determined whether mindin plays a similar role in lung infection by viruses. Wild-type and mindin-/- mice were infected with influenza virus strain PR8 (H1N1) intratracheally, and BAL fluids were collected 48 hours after the infection. The total number of leukocytes in BAL fluids from the virus-infected mindin-/- mice was only 30% to 40% of that in control mice (Figure 1B). Furthermore, the recruitment of both macrophages and neutrophils was significantly impaired (Figure 1B), indicating that mindin is critical for leukocyte recruitment in viral infection.
Impaired inflammatory cell recruitment in mindin-deficient mice. (A) Leukocyte number in BAL fluids of GBS-infected mice. Wild-type and mindin-/- mice (n = 8/group) were infected with GBS intratracheally and BAL fluids were collected 24 hours later for differential cell count. (B) Leukocyte number in BAL fluids of influenza virus–infected mice. BAL fluids were collected at 48 hours after intratracheal influenza virus infection for differential cell count (n = 10/group). (C) Leukocyte number in peritoneal cells of thioglycollate-treated mice. Wild-type and mindin-/- mice (n = 9/group) were intraperitoneally injected with 1 mL of 3% thioglycollate broth. Peritoneal cells were collected at days 1 and 3 for neutrophil and macrophage count, respectively. (A-C) Shown are mean ± SD. *P < .05; **P < .001. (D) Number of resident macrophages in the peritoneal cavity of wild-type and mindin-/- mice. Resident macrophages were harvested from untreated mice and counted. Numbers are the averages of peritoneal macrophages from each group.
Impaired inflammatory cell recruitment in mindin-deficient mice. (A) Leukocyte number in BAL fluids of GBS-infected mice. Wild-type and mindin-/- mice (n = 8/group) were infected with GBS intratracheally and BAL fluids were collected 24 hours later for differential cell count. (B) Leukocyte number in BAL fluids of influenza virus–infected mice. BAL fluids were collected at 48 hours after intratracheal influenza virus infection for differential cell count (n = 10/group). (C) Leukocyte number in peritoneal cells of thioglycollate-treated mice. Wild-type and mindin-/- mice (n = 9/group) were intraperitoneally injected with 1 mL of 3% thioglycollate broth. Peritoneal cells were collected at days 1 and 3 for neutrophil and macrophage count, respectively. (A-C) Shown are mean ± SD. *P < .05; **P < .001. (D) Number of resident macrophages in the peritoneal cavity of wild-type and mindin-/- mice. Resident macrophages were harvested from untreated mice and counted. Numbers are the averages of peritoneal macrophages from each group.
The above results raise the question whether mindin plays a role in leukocyte recruitment in inflammation caused not only by microbes but also by nonmicrobial stimuli. To test this, we examined leukocyte recruitment in thioglycollate-induced peritonitis in wild-type and mindin-/- mice. Peritoneal cells were analyzed for neutrophil migration at day 1 and for macrophage migration at day 3, as neutrophils respond in early phase whereas macrophages respond in late phase of peritonitis. The number of neutrophils in peritoneal cells of mindin-/- mice was reduced by approximately 60% whereas the number of macrophages in mindin-/- mice was reduced by 80% to 90% (Figure 1C). In contrast, the number of resident macrophages in the peritoneal cavity of untreated mindin-/- mice was comparable to that in control mice (Figure 1D). These results suggest that mindin plays a critical role in the recruitment of both neutrophils and macrophages in peritoneal inflammation induced by nonmicrobial stimuli.
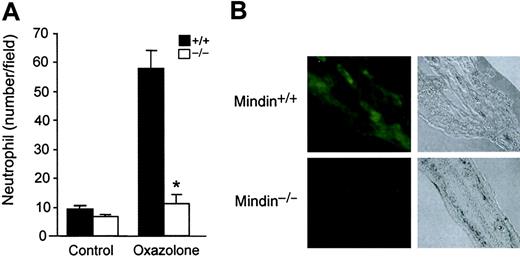
To further examine the role of mindin in inflammatory cell recruitment, we determined neutrophil responses in oxazolone-induced acute dermatitis. Oxazolone was topically applied to the ears of wild-type and mindin-/- mice and neutrophil response was observed 6 hours later. Untreated ears of wild-type and mindin-/- mice contained few migrated neutrophils, whereas oxazolone-treated ears of wild-type mice had an approximately 6-fold increase of neutrophils (Figure 2A). In contrast, few neutrophils migrated into the ear dermis in mindin-/- mice after oxazolone stimulation (Figure 2A). Mindin is abundantly expressed in lymphoid organs including spleen and lymph nodes (LNs) as well as in the lung.19 We examined whether mindin is also expressed in ear dermis using immunofluorescence staining. Mindin was readily detected in the ear dermis (Figure 2B). As a negative control, ear dermis from mindin-/- mice displayed no detectable staining (Figure 2B). Taken together, our results demonstrate that mindin is critical for the recruitment of neutrophils and macrophages to inflammation sites in 4 different inflammation models.
Impaired neutrophil response in oxazolone-induced acute dermatitis in mindin-deficient mice. (A) Neutrophil responses in the ear dermis of oxazolone-treated mice. Oxazolone was applied to the ears of wild-type and mindin-/- mice (n = 3). Six hours later, neutrophil migration to the affected area was examined in tissue sections. Shown are mean ± SD of neutrophils. Data are representative of 2 independent experiments. *P < .001. (B) Immunofluorescence staining of ear dermis of wild-type and mindin-/- mice. Tissue sections were stained with polyclonal antimindin antibody followed by FITC-anti–rabbit immunoglobulin G (IgG). Tissue structure under light microscopy with a 10 ×/0.30 objective lens is also shown (original magnification, × 100).
Impaired neutrophil response in oxazolone-induced acute dermatitis in mindin-deficient mice. (A) Neutrophil responses in the ear dermis of oxazolone-treated mice. Oxazolone was applied to the ears of wild-type and mindin-/- mice (n = 3). Six hours later, neutrophil migration to the affected area was examined in tissue sections. Shown are mean ± SD of neutrophils. Data are representative of 2 independent experiments. *P < .001. (B) Immunofluorescence staining of ear dermis of wild-type and mindin-/- mice. Tissue sections were stained with polyclonal antimindin antibody followed by FITC-anti–rabbit immunoglobulin G (IgG). Tissue structure under light microscopy with a 10 ×/0.30 objective lens is also shown (original magnification, × 100).
Normal neutrophil development in mindin-/- mice
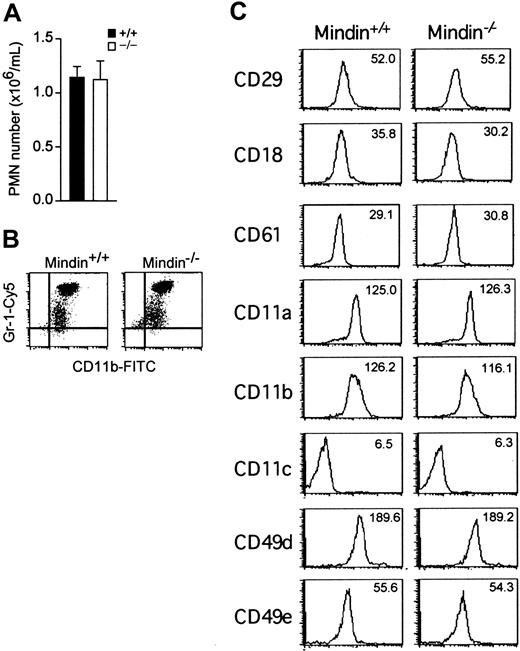
The lack of recruited macrophages in inflammation sites in mindin-/- mice is not due to defective macrophage development (Figure 1D and He et al19 ). We also examined neutrophil development in mindin-/- mice. The number of neutrophils in the peripheral blood of mindin-/- mice was comparable to that of control mice (Figure 3A). Phenotypic analysis revealed that mindin-/- and control neutrophils expressed similar levels of Gr-1, a neutrophil surface marker (Figure 3B). Furthermore, the expression levels of various integrins involved in leukocyte migration were not obviously changed on mindin-/- neutrophils (Figure 3C). Taken together, these results demonstrate that mindin is not required for neutrophil development and the normal expression of various integrins.
Normal neutrophil development in mindin-deficient mice. (A) Number of circulating polymorphonuclear neutrophils (PMNs) in the blood of mindin-/- mice. Blood drawn from orbital sinus of wild-type and mindin-/- mice (n = 4) was examined for neutrophils by differential cell count. Shown are mean ± SD. (B) FACS analysis of circulating neutrophils in mindin-/- mice. Shown is the expression of CD11b and Gr-1 on granulocytes gated on their forward and side scatter. (C) Expression of adhesion molecules on BM neutrophils from mindin-/- mice. Numbers are the mean fluorescent intensity (MFI) value for each file.
Normal neutrophil development in mindin-deficient mice. (A) Number of circulating polymorphonuclear neutrophils (PMNs) in the blood of mindin-/- mice. Blood drawn from orbital sinus of wild-type and mindin-/- mice (n = 4) was examined for neutrophils by differential cell count. Shown are mean ± SD. (B) FACS analysis of circulating neutrophils in mindin-/- mice. Shown is the expression of CD11b and Gr-1 on granulocytes gated on their forward and side scatter. (C) Expression of adhesion molecules on BM neutrophils from mindin-/- mice. Numbers are the mean fluorescent intensity (MFI) value for each file.
Neutrophil adhesion to mindin matrix. (A) Static adhesion of BM neutrophils to coated mindin. BM neutrophils (2 × 105/well) were incubated with coated proteins as indicated. The number of adherent cells was counted in at least 3 different fields after washing. FN indicates fibronectin. (B) Static adhesion of activated BM neutrophils to coated mindin. Purified BM neutrophils (3 × 105/well) were stimulated with PMA (100 ng/mL) for 15 minutes and incubated with coated proteins as in panel A. (C) Static adhesion of peritoneal neutrophils to coated mindin. Peritoneal neutrophils (5 × 104/well) from thioglycollate-induced cells were incubated and counted as in panel A. (D) Mindin-mediated neutrophil migration. BM neutrophils were induced to migrate by fMLP with or without coated protein matrix on transwell membranes. Shown are the numbers of neutrophils migrated to the lower chambers. All data are mean ± SD from triplicate samples and representative of 3 experiments. *P < .001; **P < .05.
Neutrophil adhesion to mindin matrix. (A) Static adhesion of BM neutrophils to coated mindin. BM neutrophils (2 × 105/well) were incubated with coated proteins as indicated. The number of adherent cells was counted in at least 3 different fields after washing. FN indicates fibronectin. (B) Static adhesion of activated BM neutrophils to coated mindin. Purified BM neutrophils (3 × 105/well) were stimulated with PMA (100 ng/mL) for 15 minutes and incubated with coated proteins as in panel A. (C) Static adhesion of peritoneal neutrophils to coated mindin. Peritoneal neutrophils (5 × 104/well) from thioglycollate-induced cells were incubated and counted as in panel A. (D) Mindin-mediated neutrophil migration. BM neutrophils were induced to migrate by fMLP with or without coated protein matrix on transwell membranes. Shown are the numbers of neutrophils migrated to the lower chambers. All data are mean ± SD from triplicate samples and representative of 3 experiments. *P < .001; **P < .05.
Mindin mediates neutrophil migration in vitro
Mindin, as an ECM protein, may directly interact with receptors expressed on neutrophils and macrophages and provide adhesion sites for leukocyte migration. To determine whether neutrophils can directly adhere to immobilized mindin, we incubated purified neutrophils from wild-type mice with coated mindin. Two other ECM proteins, laminin and fibronectin, were used as positive controls. BSA was used as a control for baseline adhesion. BM neutrophils displayed slightly increased binding to immobilized mindin and laminin over BSA but strong adhesion to fibronectin (Figure 4A). The weak neutrophil adhesion to mindin may be related to their nonactivated status. We then activated BM neutrophils with PMA and tested their binding to mindin matrix. As shown in Figure 4B, activated BM neutrophils displayed enhanced adhesion to mindin and laminin matrix. We also examined the adhesion of activated neutrophils from inflamed peritoneum. Neutrophils from thioglycollate-induced peritoneal cells displayed a 3- to 4-fold enhanced binding to coated mindin as well as laminin, similar to that exhibited by fibronectin (Figure 4C). These results demonstrate that activated neutrophils adhere to immobilized mindin.
We next assessed whether mindin can promote neutrophil migration in a transwell-migration assay. Mindin and fibronectin were coated on the membranes of transwells. Neutrophils were induced to migrate through the membranes with fMLP. Both mindin and fibronectin enhanced fMLP-induced neutrophil migration by 2- to 3-fold, whereas BSA had no such effect (Figure 4D). Moreover, neutrophils purified from mindin-/- mice behaved similarly to those from wild-type mice in the binding and migration assays (Figure 5A; data not shown). These results suggest that mindin promotes neutrophil migration through adhesion rather than regulating neutrophil cellular function.
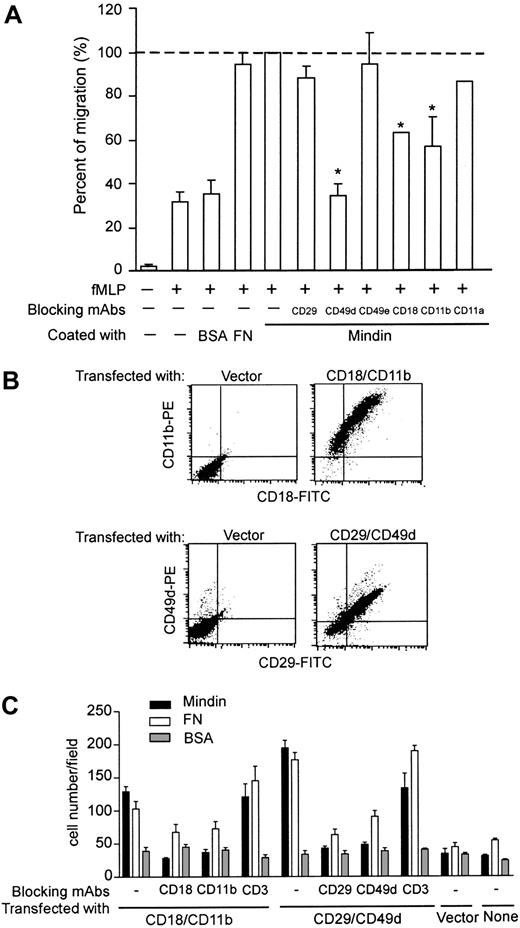
Mindin interacts with integrins. (A) Inhibition of mindin-mediated neutrophil migration by anti-integrin antibodies. BM neutrophils from mindin-/- mice were incubated with indicated anti-integrin mAbs and tested in mindin-mediated migration as in Figure 4D. Shown are the percent of migration in the presence of antibodies with mindin-mediated neutrophil migration without any blocking antibody as 100%. *P < .001. Error bars indicate mean ± SD. (B) Expression of integrins on HEK-293 cells. HEK-293 cells were transiently transfected with plasmids encoding integrins αM plus β2, α4 plus β1, or vector alone. Forty-eight hours later, integrin expression was examined by FACS. (C) Cell adhesion to mindin matrix. HEK-293 cells expressing integrins as indicated were tested for their binding to coated proteins with or without blocking antibodies. Shown are the numbers of adherent cells in each field (mean ± SD). Data are representative of 2 to 3 experiments.
Mindin interacts with integrins. (A) Inhibition of mindin-mediated neutrophil migration by anti-integrin antibodies. BM neutrophils from mindin-/- mice were incubated with indicated anti-integrin mAbs and tested in mindin-mediated migration as in Figure 4D. Shown are the percent of migration in the presence of antibodies with mindin-mediated neutrophil migration without any blocking antibody as 100%. *P < .001. Error bars indicate mean ± SD. (B) Expression of integrins on HEK-293 cells. HEK-293 cells were transiently transfected with plasmids encoding integrins αM plus β2, α4 plus β1, or vector alone. Forty-eight hours later, integrin expression was examined by FACS. (C) Cell adhesion to mindin matrix. HEK-293 cells expressing integrins as indicated were tested for their binding to coated proteins with or without blocking antibodies. Shown are the numbers of adherent cells in each field (mean ± SD). Data are representative of 2 to 3 experiments.
Neutrophils interact with mindin through integrins
Like many other ECM proteins, mindin may mediate adhesion of neutrophils through integrins. To test this, we examined the effect of several anti-integrin mAbs on fMLP-induced neutrophil migration through mindin-coated membranes. Among the 6 antibodies tested, anti-β1 (CD29), -α5 (CD49e), and -αL (CD11a) integrin mAbs did not have a significant effect on mindin-mediated neutrophil migration (Figure 5A). In contrast, anti-α4 (CD49d), -αM (CD11b), and -β2 (CD18) integrin mAbs inhibited neutrophil migration in this assay (Figure 5A). Anti-α4 integrin mAb blocked mindin-mediated neutrophil migration to the baseline level mediated by BSA, whereas anti-αM and -β2 mAb treatment decreased mindin-specific neutrophil migration by 50% to 60% (Figure 5A). These results suggest that mindin may serve as a ligand for integrins α4, αM, and β2 in neutrophil recruitment.
To further assess integrin-mindin interaction, we transiently cotransfected HEK-293 cells with expression plasmids encoding αM and β2 or α4 and β1 integrins to achieve cell surface integrin expression. FACS analysis revealed that 60% to 70% of transfected HEK-293 cells expressed the appropriate integrins (Figure 5B). We first determined the adhesion of unstimulated HEK-293 cells transfected with αMβ2 or α4β1 integrins to immobilized mindin and BSA. We did not observe significant increase in cell binding to mindin over BSA (data not shown). We then activated the HEK-293 cells with PMA and tested for their adhesion. Expression of αMβ2 or α4β1 integrins enhanced HEK-293 cell binding to mindin by 3- to 4-fold (Figure 5C). In contrast, vector-transfected and parental HEK-293 cells did not display any increased binding to mindin after PMA activation. Interestingly, the enhanced adhesion of HEK-293 cells expressing αMβ2 or α4β1 integrins to mindin was blocked by all 4 anti-integrin mAbs, including anti-β1 (Figure 5C). In contrast, anti-CD3 mAb serving as a negative control did not affect the binding of HEK-293 cells expressing αMβ2 or α4β1 integrins to mindin (Figure 5C). As expected, the anti-integrin mAbs blocked the binding of the transfected HEK-293 cells to fibronectin (Figure 5C). Taken together, these results demonstrate that mindin specifically interacts with α4, αM, β1, and β2 integrins.
Discussion
In this report, we demonstrate that the ECM protein mindin has a critical role in inflammatory cell recruitment in vivo and functions as a ligand for integrins in vitro. Leukocyte recruitment to inflamed sites depends on the interaction of various integrins expressed on leukocytes and their ligands expressed on endothelial cells or in the interstitium. The roles of integrins in macrophage and neutrophil extravasation during inflammation have been extensively investigated.5-7,22-26 Numerous studies have also demonstrated that ECM proteins, such as laminin, thrombospondin, and fibronectin, contribute to neutrophil and macrophage adhesion and migration in vitro and in vivo by functioning as integrin ligands.9-14,27,28 Our data have added another ECM molecule into the list of an increasing number of proteins with an important function in inflammatory cell recruitment.
The defective inflammatory cell recruitment in mindin-deficient mice likely reflects a combined effect of an impaired in vivo interaction between mindin in the interstitium and integrin αM, β2, and α4 expressed on neutrophils and macrophages as well as an impaired activation of innate immune cells. Although our previous data demonstrate that mindin functions as a pattern-recognition molecule for microbial pathogens and that mindin-deficient macrophages and mast cells have defective activation by microbial pathogens,19 the impaired inflammatory cell recruitment in mindin-deficient mice is not solely due to a secondary effect of impaired innate cell activation. Several lines of evidence support the notion that mindin has a direct role in leukocyte migration in vivo by functioning as a ligand for integrins during inflammatory cell recruitment. First, neutrophils directly adhere to mindin matrix, and mindin can mediate neutrophil migration in transwell migration assays. Second, mindin-mediated neutrophil migration can be blocked by anti-α4, -αM, and -β2 mAbs. Third, HEK-293 cells expressing these integrins exhibit specific adhesion to mindin matrix. Fourth, importantly, mindin-deficient mice exhibit defective neutrophil and macrophage cell recruitment during inflammations induced by nonpathogenic stimuli.
The roles of integrin α4 and αMβ2 in inflammatory cell recruitment have been firmly established.22,23,25,26,29-33 The α4 and αMβ2 integrins play different roles in leukocyte recruitment, with αMβ2 mediating adhesion of leukocytes to endothelium and α4 interacting with ECM. Nevertheless, it appears that mice deficient for β2 integrin exhibit only a partial defect in neutrophil recruitment,22,23,25,29 suggesting a β2 integrin–independent pathway for neutrophil recruitment in which α4 integrin may play a major role.5 Therefore, impaired inflammatory cell recruitment in mindin-deficient mice may result from a lack of interactions between these several integrins and mindin in the ECM. In addition, mindin may also serve as a ligand for other integrins that also play important roles in inflammatory cell recruitment. It is interesting to note that while the binding of neutrophils to mindin was not blocked by anti–β1 integrin mAb, the binding of β1 integrin–transfected HEK-293 cells to mindin was blocked by the same mAb. This differential effect by anti-β1 mAb suggests that β1 integrin may assume different configurations on neutrophils and HEK-293 cells.
Why do other ECM ligands for α4β1 and αMβ2 integrins, such as fibronectin, fail to compensate for mindin deficiency in vivo? This may be related to the binding properties of these integrins. Integrins including α4 and αMβ2 display low-affinity and high-affinity binding depending on their state of activation.4,34 These integrins exhibit low-affinity binding in their default conformation in resting leukocytes. When activated by so-called inside-out signaling, integrins display high-affinity binding via conformational changes rather than an increase of their expression levels. We observed that the binding to mindin by neutrophils and transfected HEK-293 cells was at a minimum level without activation. However, activation of these cells with PMA markedly increased their binding to mindin. In contrast, consistent with previous data, PMA activation of neutrophils did not enhance their binding to fibronectin.28,35 These results suggest that the adhesive binding of neutrophils in vivo may require their interaction with multiple ECM proteins including fibronectin and mindin. Neutrophils only interact with mindin in a high-affinity state after activation during an inflammation. This type of interaction is unique for mindin and cannot be compensated by other ECM ligands. Alternatively, mindin may serve as a ligand for other unidentified adhesion receptors.
Our results raise an interesting question whether α4, αM, β1, and β2 integrins serve as coreceptors for recognition of microbial pathogens in macrophages and mast cells. Mindin-deficient macrophages have defective responses to a broad spectrum of microbial stimuli.19 Microbial pathogens may be recognized on one hand by Toll-like receptors (TLRs) and on the other hand interact with integrins through mindin. Both types of interactions may be required to efficiently activate innate immune cells. This notion is supported by recent works showing that integrin αMβ2 forms clusters with TLRs and other pattern recognition receptors in lipid rafts of activated cells.36 Furthermore, in cooperation with CD14 and TLR2, integrin αMβ2 participates in the recognition of bacterial fimbriae.37 Future studies on the roles of integrins in pathogen recognition will shed novel insights on innate immune mechanisms.
Prepublished online as Blood First Edition Paper, August 16, 2005; DOI 10.1182/blood-2005-04-1658.
Supported by National Institutes of Health (NIH) grant AI054658 (Y.-W.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Michael Dee Gunn for help in migration assay and Drs Guglielmo Venturi, Jonathan Poe, and Heather Hartig for critical review of this manuscript.