Sooty mangabeys, the natural host of simian immunodeficiency virus (SIVsm), generally avoid progressive depletion of CD4+ T cells and opportunistic infections associated with infection of humans (HIV) and macaques (SIVmac). The means by which the SIVsm-infected mangabeys maintain CD4+ T-cell levels despite high rates of viral replication is unknown. One cytokine that has a key role in the regulation of T-cell levels is interleukin-7 (IL-7). Here, the longitudinal assessment of 6 SIVsm-infected mangabeys identified an early increase in plasma IL-7 levels at weeks 1 to 5 after infection. This IL-7 increase correlated with an early decline in CD4+ T-cell levels (decline of 492-1171 cells/μL) accompanying acute viremia. Elevated IL-7 levels were followed by increased T-cell proliferation (Ki67) and maintenance of lower but stable (more than 500 cells/μL) CD4+ T-cell levels in each mangabey through 37 weeks of infection. These data contrast with our earlier studies in SIVmac-infected macaques, in which the IL-7 increase was delayed until 20 to 40 weeks after infection, just before the onset of simian AIDS. Taken together, these data suggest that timely triggering of IL-7 is important for stabilizing healthy T-cell levels in mangabeys and that timely administration of exogenous IL-7 may show benefit during pathogenic SIVmac and HIV infection.
Introduction
The hallmark of HIV infection in humans and simian immunodeficiency virus (SIV) infection in macaques (SIVmac) is the progressive decline in the number of CD4+ T cells. HIV/SIV causes this decline through direct killing of CD4+ T cells (viral cytopathicity) and through activation-induced apoptosis of uninfected bystander cells.1-3 At the same time, dysfunction in the CD4+ T-cell restorative abilities induced by HIV/SIV infection also contributes to progressive decline in CD4+ T cells.4-6 Thus, one important aspect of pathogenic HIV infection in humans and SIV infection in macaques is the loss of T-cell homeostasis, an imbalance between the rate of CD4+ T-cell production and their death.4
Growing evidence indicates that cytokines are involved in the maintenance of T-cell homeostasis in the periphery.7,8 In particular, interleukin-7 (IL-7) and IL-15 have been shown to be critical for the regulation of proper T-cell levels.9,10 IL-15 plays a predominant role in the survival and homeostatic proliferation of CD8+ memory T cells,11,12 but its role in the maintenance of CD4+ T cells appears to be limited.13 In contrast, IL-7 has multiple roles in CD4+ and CD8+ T-cell homeostasis, including increasing the survival of T cells14,15 and the antigen-specific and homeostatic (non–antigen dependent) proliferation of CD4+ and CD8+ memory T cells.10,13,15 Interestingly, IL-7 levels are often elevated in T cell–depleting conditions such as chemotherapy and HIV infection.16 In earlier studies, we observed that a delayed increase in IL-7 levels was unable to restore proper CD4+ T-cell levels in SIVmac-infected macaques that progressed to simian AIDS.17 In contrast, in one macaque that is still alive after 4 years of SIV infection, elevation in IL-7 level was accompanied by increased T-cell proliferation and stabilization of CD4+ T-cell levels.17 After IL-7 immunotherapy in SIV-infected cynomolgus monkeys, a similar increase in the peripheral expansion of T cells was observed.18 Importantly, in both studies, the increased levels of plasma IL-7 were not associated with increased plasma viral loads.17,18 These studies demonstrated that the cytokine IL-7 plays an important role in maintaining T-cell levels during pathogenic HIV and SIVmac infections. However, concerns regarding deleterious effects of high levels of IL-7 during HIV/SIV infection also exist and include a potential increase in viral replication and Fas-mediated apoptosis.19,20
Nonhuman primate models are widely used to study HIV pathogenesis. Sooty mangabeys are a monkey species endemic to West Africa and are infected in the wild, indicating that they are a natural host of the SIVsm virus. Based on strong evidence from sequence homology and geographic localization between SIVsm in mangabeys and HIV-2 (endemic to West Africa), it is likely that the HIV-2 epidemic arose from cross-species transmission of virus from mangabeys to humans.21,22 HIV-2 is related to HIV-1, which also appears to have arisen from cross-species transmission, likely occurring from an SIV-infected chimpanzee.22,23 The SIVsm infection of mangabeys represents a riddle as the virus replicates to high levels (as in HIV+ patients and SIV+ macaques). However, with the exception of 2 reports of AIDS signs in aged SIVsm+ mangabeys,24,25 mangabeys generally do not show clinical signs of simian AIDS, and they have relatively stable CD4+ T-cell numbers.1 The absence of clinical symptoms and the general maintenance of T-cell levels in SIVsm-infected mangabeys provides an opportunity to assess the possible mechanisms involved in the replenishment of T-cell pools during nonpathogenic infection. Our recent studies have shown limited bystander immunopathology in SIVsm-infected mangabeys compared with SIVmac-infected macaques.1 Thus far, mangabey studies have generally compared the naturally (chronically) infected and uninfected animals available at the Yerkes Primate Research Center (Atlanta, GA) colony in a cross-sectional analysis.1,26 Here, to investigate the early immunologic events and their role in stabilizing CD4+ T-cell levels during SIVsm infection, 6 mangabeys were studied longitudinally after intravenous inoculation with SIVsm+ plasma from a naturally infected mangabey. At early times after infection, there was a sharp decline in peripheral blood CD4+ T-cell level in SIVsm-infected mangabeys that was similar to that in SIVmac-infected macaques and HIV-infected humans. This acute-phase decline in peripheral blood CD4+ T-cell levels correlated with a timely homeostatic response (plasma IL-7 levels) that likely enabled CD4+ T-cell counts to stabilize at healthy levels in the peripheral blood of SIVsm-infected mangabeys through 37 weeks of infection. This healthy (timely) homeostatic response may play a key role in the inhibition of progression to simian AIDS observed in SIVsm-infected mangabeys.
Materials and methods
Animals and viral infection
The sooty mangabeys (SMs) used in this study were between 3 and 5 years of age when they were infected with SIV. Three mangabeys—SM1, SM2, and SM3—had intact thymuses, and 3 mangabeys—SM4, SM5, and SM6—had their thymus tissue removed (thymectomy) 7 months before SIVsm infection. The surgery was performed carefully so that all potential thymic tissue was removed. All mangabeys were inoculated intravenously with plasma transferred from mangabey FQi, which had been naturally infected with SIVsm in the Yerkes mangabey colony. Virologic assessment determined that none of the mangabeys contained simian T-cell lymphotropic virus (STLV-1) or simian retrovirus (SRV). In addition, antisera analysis determined that all mangabeys except SM2 were seropositive for cytomegalovirus/simian agent 6 (CMV/SA6), and all except SM2 and SM4 contained antibodies that cross-reacted with herpesvirus B. Mangabeys used in this study were housed at the Yerkes National Primate Center. They were maintained in accordance with National Institutes of Health guidelines, and approvals were obtained from the appropriate Animal Care and Use committees.
Detection of signal joint TRECs in lymphocytes
Peripheral blood mononuclear cells (PBMCs) isolated by Ficoll-Hypaque gradient centrifugation (Amersham Biosciences, Uppsala, Sweden) were first sorted into CD8+ and CD8– T-cell populations using magnetic cell sorting (MACS) columns (Miltenyi Biotec, Bergisch Gladbach, Germany). T-cell receptor excision circle (TREC) levels were then determined in CD8+ and CD8– (to obtain CD4+ TREC levels) populations using real-time polymerase chain reaction (PCR), as previously described.27,28 TREC content in PBMCs represents an indirect measurement of thymic output.
Monoclonal antibodies used for flow cytometry
Antibodies used for immunophenotyping of PBMCs were anti-CD4 (clone SK3), anti-CD8 (clone SK1), anti-CD45RA (clone L48), anti-CD62L (clone SK11) (Becton Dickinson Immunocytometry Systems, Bedford, MA), anti-CD3e (clone SP34) (Pharmingen, San Diego, CA), and anti-Ki67 (clone R0840) (Dako, Carpinteria, CA). Antibodies were conjugated to the following fluorophores: fluorescein isothiocyanate (FITC) (CD45RA, CD3), phycoerythrin (PE) (CD62L, CD4, Ki67), peridin chlorophyll protein (PerCP) (CD8), or allophycocyanin (APC) (CD4).
Immunophenotyping of PBMCs
PBMCs isolated by Ficoll-Hypaque gradient were assessed with 4 fluorometric markers to determine the absolute number of different cell subsets. The percentage of naive/memory T cells was determined using the antibodies CD62L and CD45RA, and the percentage of proliferating T cells was determined by Ki67 staining, as previously described.1,27 Flow cytometric acquisition of samples was undertaken on a FACScalibur flow cytometer using CellQuest software (Becton Dickinson Immunocytometry Systems). Flow cytometry data were analyzed with Paint-a-Gate Pro (Becton Dickinson Immunocytometry Systems) and FlowJo (Tree Star, Ashland, OR) software.
Determination of plasma viral RNA levels
Cytokine levels in plasma
IL-7 and IL-15 levels in the plasma of SIVsm-infected mangabeys were determined using the commercial sandwich enzyme immunoassay kit available for humans (Quantikine kits; R&D Systems, Minneapolis, MN), as described previously.17 The specificity of the IL-7 enzyme-linked immunosorbent assay (ELISA) for mangabey IL-7 was determined by blocking experiments with anti–IL-7 antisera (R&D Systems). The minimum detectable level of IL-7 in plasma was 0.10 pg/mL. The reproducibility of the IL-7 ELISA assay was assessed through incorporation of a control plasma sample with a known IL-7 content in each assay. Variation between assays was 0.1% to 5%.
Statistical analysis
Statistical analysis was performed with the nonparametric Mann-Whitney U test (Prism 3.0; GraphPad Software, San Diego, CA), and results were considered significant if P was less than .05. Pearson correlation coefficient was used to assess the degree of positive and negative association between IL-7 and other host factors.
Results
Viral load and disease outcome in SIVsm-infected mangabeys
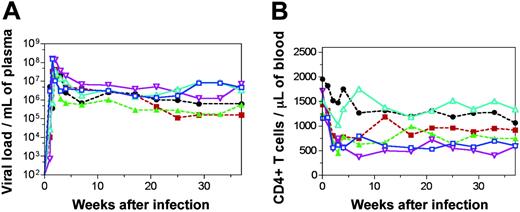
The exact mechanism by which SIVsm-infected mangabeys are able to avoid simian AIDS despite a high rate of viral replication is unknown. In this study, both the impact of early immunologic events and the importance of de novo T-cell production (from the thymus) were assessed in 6 mangabeys (3 underwent thymectomy 7 months before infection; 3 had intact thymuses) inoculated with SIVsm from a naturally infected mangabey. On SIVsm infection, all 6 mangabeys developed high levels of plasma viremia ranging from 107 to 108 copies per milliliter during the first 2 to 3 weeks of infection (Figure 1A). These levels are generally comparable to the viral loads seen in HIV-infected patients and SIVmac-infected macaques during acute infection.17,30 After peak viremia, a decline in viral load by 1 to 2 logs between 3 and 10 weeks of infection was observed. Thereafter, a viral set point was established in the range of 105 to 107 copies per milliliter and remained at that level until 37 weeks after infection (Figure 1A). The viremia in the mangabeys was accompanied by an anti–SIV-specific humoral immune response, as evidenced by the presence of anti-SIV antibodies at 28 weeks after infection (antibody titers ranged between 51 200 and 409 600). Interestingly, none of the 6 SIVsm-infected mangabeys showed symptoms of simian AIDS and generally remained healthy throughout the study period. Thus, the presence or absence of the thymus did not alter viral load or disease outcome in SIVsm-infected mangabeys.
SIVsm infection in mangabeys is accompanied by an early decline in and thereafter maintenance of CD4+ T-cell levels. Plasma viral load (A) and absolute number of CD4+ T cells (B) for 6 SIVsm-inoculated mangabeys. Three had thymuses (continuous lines; SM1, blue, □; SM2, turquoise, ▵; SM3, purple, ▿). Three underwent thymectomy (broken lines; SM4, green, ▴; SM5, black, •; SM6, red, ▪). Data are shown through 37 weeks of infection.
SIVsm infection in mangabeys is accompanied by an early decline in and thereafter maintenance of CD4+ T-cell levels. Plasma viral load (A) and absolute number of CD4+ T cells (B) for 6 SIVsm-inoculated mangabeys. Three had thymuses (continuous lines; SM1, blue, □; SM2, turquoise, ▵; SM3, purple, ▿). Three underwent thymectomy (broken lines; SM4, green, ▴; SM5, black, •; SM6, red, ▪). Data are shown through 37 weeks of infection.
SIVsm infection in mangabeys leads to an early decline in CD4+ T-cell levels
To date, most SIV studies in sooty mangabeys have examined the immune system in chronically infected animals in the Yerkes colony. Because the mangabeys in this current study had been infected intravenously, we were able to assess the viral and immunological changes occurring at the earliest times after infection. Before infection, absolute counts of CD4+ T cells in the peripheral blood of mangabeys typically varied from 1250 to 1955 cells/μL blood (Figure 1B). During acute viremia (Figure 1A) there was an early decline in CD4+ T-cell counts in all 6 mangabeys (decline of 492-1171 CD4+ T cells/μL blood) (Figure 1B). This decline was similar to that observed in the SIVmac-infected macaques in our earlier studies.17 Generally, the decline in peripheral blood CD4+ T cells during primary SIVsm infection in mangabeys was followed by recovery and stabilization at lower but healthy levels (Figure 1B). During recovery, CD4+ T-cell levels in blood increased above the preinfection level in one mangabey (SM2, turquoise line; Figure 1B), whereas in other mangabeys only a moderate increase was observed. A new relatively stable, but lower, CD4+ T-cell set point was observed in all 6 SIVsm+ mangabeys by 16 weeks after infection. Overall, there was an early decline in CD4+ T-cell numbers in blood during primary SIVsm infection in all 6 mangabeys that stabilized at a lower healthy level by 16 weeks after infection.
Early increase in plasma IL-7 levels in SIVsm-infected mangabeys
We next assessed whether normal T-cell homeostatic mechanisms are triggered to regulate T-cell levels in SIVsm-infected mangabeys. IL-15 and IL-7 are 2 of the key cytokines involved in the maintenance of T-cell homeostasis and are important for increasing T-cell levels during immune depletion.10,13,31 An assessment of plasma IL-15 levels in uninfected and SIVsm-infected mangabeys determined that most were below the level of detection for the assay (less than 2 pg/mL plasma). Although there was a trend for an increased level of IL-15 in some SIVsm-infected mangabeys, overall no significant difference between preinfection and postinfection time points were observed (data not shown).
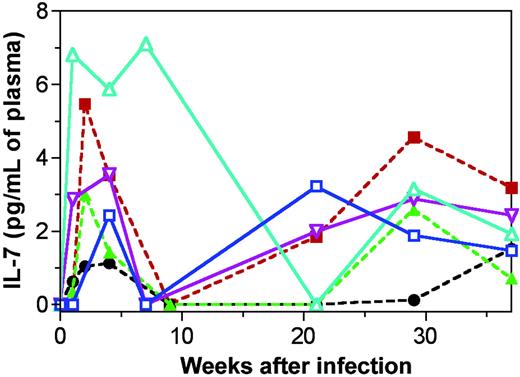
Plasma IL-7 levels are elevated during pathogenic HIV infection in humans and SIVmac infection in macaques.17,32 In our previous cross-sectional analysis performed in naturally SIVsm-infected mangabeys, we observed a trend indicating a slight increase in IL-7 levels, but the increase was not significant.17 Here, a longitudinal assessment of plasma IL-7 levels was undertaken after SIVsm infection in 6 mangabeys. Our ability to begin observing the mangabeys before SIV inoculation increased the chance to observe elevations in IL-7 levels during SIV infection. Before SIVsm infection, plasma IL-7 levels were generally at undetectable levels (less than 0.10 pg/mL) (Figure 2). After infection, IL-7 levels increased to detectable levels in each of the 6 mangabeys and reached peak levels within 1 to 5 weeks after infection (1.1-7.1 pg IL-7/mL plasma) (Figure 2). Elevated IL-7 levels temporally correlated with the early declines in peripheral CD4+ T cell counts seen during primary infection (see Figure 3 for a depiction of this). The increase in IL-7 levels during the acute phase of the infection was generally followed by slightly elevated IL-7 levels from 22 to 37 weeks of infection. Changes in IL-7 levels did not correlate with plasma viral loads, indicating that the viral infection itself was not driving the IL-7 increase. In all, our data indicate that early declines in CD4+ T-cell counts in SIVsm-infected mangabeys were associated with timely increases in plasma IL-7 levels.
SIVsm infection in mangabeys is associated with increased T-cell proliferation
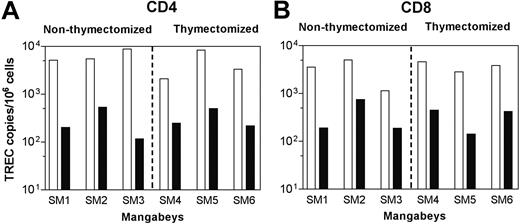
After early declines in CD4+ T-cell levels, we observed relatively stable, healthy T-cell levels in all 6 SIVsm-infected mangabeys through 37 weeks of infection. This was not surprising because cross-sectional analyses of SIVsm+ and uninfected mangabeys had previously identified slightly lower, but healthy, CD4+ T-cell levels in naturally infected mangabeys in the Yerkes colony.1,33 Stabilization of peripheral blood CD4+ T-cell levels in the SIVsm-infected mangabeys could be achieved through the increased generation of new T cells (thymopoiesis)34 and in the expansion of T cells already present in the periphery.35 Thymic output was indirectly measured by quantifying TREC levels and through an assessment of the levels of T cells expressing markers of a naive phenotype. TREC assessment was carried out on CD8+ and CD8– (to obtain CD4+ TREC levels) peripheral blood T-cell populations, as previously described.36 Before infection, all 6 mangabeys (3 that underwent thymectomy, 3 that did not) had approximately 104 TREC copies per million T cells (Figure 4A-B). After SIVsm infection, regardless of thymectomy (37 weeks after infection), TREC levels declined significantly in each mangabey (P > .05) (Figure 4A-B). Similar rates of TREC decline in mangabeys, regardless of thymectomy status, indicated that factors other than thymic output, such as cell death, redistribution, or proliferation, might have influenced T-cell homeostasis in SIVsm-infected mangabeys. Similar levels (range, 22%-56%) of peripheral blood naive CD4+ and CD8+ T-cell populations (CD45RA+/CD62L+) in mangabeys—again, regardless of thymectomy status—throughout the first 37 weeks of infection also indirectly confirmed that the thymus was not a major contributor to T-cell homeostasis in mangabeys during this time period. Most important, despite thymectomy, none of the animals exhibited any signs of simian AIDS, and all 6 mangabeys were able to stabilize CD4+ T cell counts to healthy levels.
SIVsm infection in mangabeys leads to early increases in plasma IL-7 levels. IL-7 levels in the plasma of 6 mangabeys. Three had thymuses (continuous lines; SM1, blue, □; SM2, turquoise, ▵; SM3, purple, ▿). Three underwent thymectomy (broken lines; SM4, green, ▴; SM5, black, •; SM6, red, ▪). Data are shown through 37 weeks of infection. All 6 mangabeys had sharp increases in IL-7 levels during the first 5 weeks of infection.
SIVsm infection in mangabeys leads to early increases in plasma IL-7 levels. IL-7 levels in the plasma of 6 mangabeys. Three had thymuses (continuous lines; SM1, blue, □; SM2, turquoise, ▵; SM3, purple, ▿). Three underwent thymectomy (broken lines; SM4, green, ▴; SM5, black, •; SM6, red, ▪). Data are shown through 37 weeks of infection. All 6 mangabeys had sharp increases in IL-7 levels during the first 5 weeks of infection.
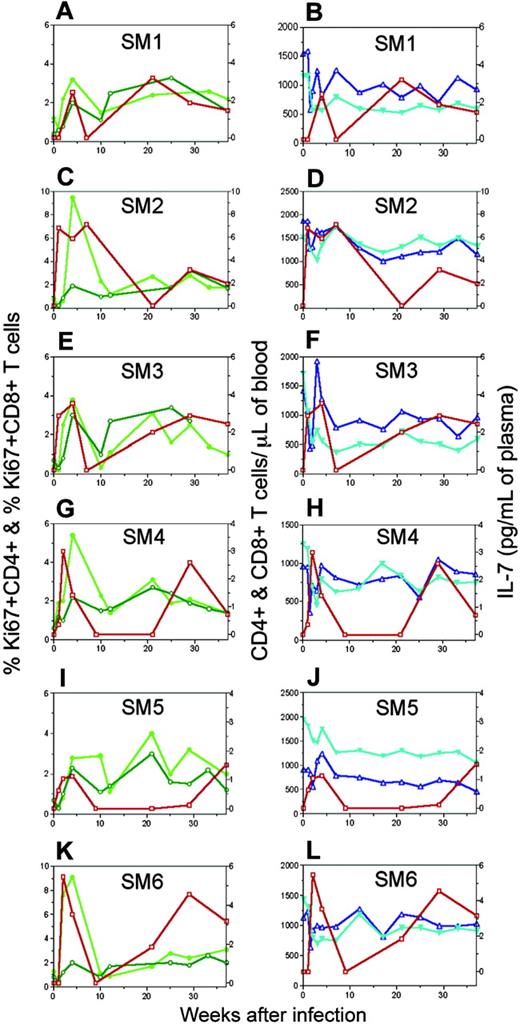
Elevated IL-7 levels are followed by increased T-cell proliferation and maintenance of stable T-cell numbers in SIVsm-infected mangabeys. Longitudinal assessment of the percentage of proliferating (Ki67+) CD4+ (dark green, ○) and CD8+ T cells (light green, •) in peripheral blood (panels A, C, E, G, I, and K) and plasma IL-7 levels (red, □) are plotted for each individual mangabey (SM1, SM2, SM3, SM4, SM5, and SM6) separately. Absolute numbers of CD4+ (turquoise, ▾) and CD8+ T cells (blue, ▵) (panels B, D, F, H, J, and L) are given as markers of T-cell homeostasis.
Elevated IL-7 levels are followed by increased T-cell proliferation and maintenance of stable T-cell numbers in SIVsm-infected mangabeys. Longitudinal assessment of the percentage of proliferating (Ki67+) CD4+ (dark green, ○) and CD8+ T cells (light green, •) in peripheral blood (panels A, C, E, G, I, and K) and plasma IL-7 levels (red, □) are plotted for each individual mangabey (SM1, SM2, SM3, SM4, SM5, and SM6) separately. Absolute numbers of CD4+ (turquoise, ▾) and CD8+ T cells (blue, ▵) (panels B, D, F, H, J, and L) are given as markers of T-cell homeostasis.
SIVsm infection in mangabeys is associated with declines in TREC levels. TREC levels were determined by real-time PCR and presented for CD4 (A) and CD8 (B) T-cell populations in peripheral blood. Preinfection (open bars) and week 37 postinfection (closed bars) data for all 6 mangabeys are depicted. All mangabeys with and without thymuses had similar rates of TREC decline after SIVsm infection.
SIVsm infection in mangabeys is associated with declines in TREC levels. TREC levels were determined by real-time PCR and presented for CD4 (A) and CD8 (B) T-cell populations in peripheral blood. Preinfection (open bars) and week 37 postinfection (closed bars) data for all 6 mangabeys are depicted. All mangabeys with and without thymuses had similar rates of TREC decline after SIVsm infection.
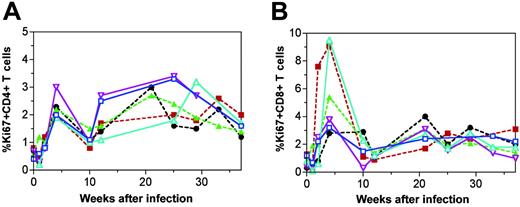
The ability of peripheral T-cell proliferation to potentially increase T-cell populations in SIVsm-infected mangabeys was determined through quantification of the nuclear protein Ki67, which is expressed in replicating cells. We observed that after peak viremia (Figure 1A), there was an early increase in Ki67 expression (2%-3%) in the peripheral blood CD4+ T cells in each of the 6 mangabeys (approximately 4 weeks after SIV infection) (Figure 5A). This response was also observed in the CD8+ T-cell population in which proliferation levels during the acute phase of the infection generally reached even higher levels (3%-9%) (Figure 5B). During the chronic phase of the infection (22-37 weeks), levels of T-cell proliferation generally ranged from 1% to 3% for both T-cell subsets. Overall, there was an increase in T-cell proliferation after SIV infection in mangabeys, and the increase was most evident during the acute stages of infection.
SIVsm infection in mangabeys leads to an early increase in T-cell proliferation. T-cell proliferation determined by percentage of Ki67+ staining is presented for CD4+ (A) and CD8+ (B) T cells. CD4+ and CD8+ T-cell proliferation was sharply increased after SIVsm infection and generally remained elevated throughout 37 weeks of infection. Longitudinal data are shown for 6 mangabeys: 3 with thymuses (continuous lines; SM1, blue, □; SM2, turquoise, ▵; SM3, purple, ▿) and 3 without thymuses (broken lines; SM4, green, ▴; SM5, black, •; SM6, red, ▪).
SIVsm infection in mangabeys leads to an early increase in T-cell proliferation. T-cell proliferation determined by percentage of Ki67+ staining is presented for CD4+ (A) and CD8+ (B) T cells. CD4+ and CD8+ T-cell proliferation was sharply increased after SIVsm infection and generally remained elevated throughout 37 weeks of infection. Longitudinal data are shown for 6 mangabeys: 3 with thymuses (continuous lines; SM1, blue, □; SM2, turquoise, ▵; SM3, purple, ▿) and 3 without thymuses (broken lines; SM4, green, ▴; SM5, black, •; SM6, red, ▪).
Increase in T-cell proliferation correlates with elevated IL-7 levels in SIVsm-infected mangabeys
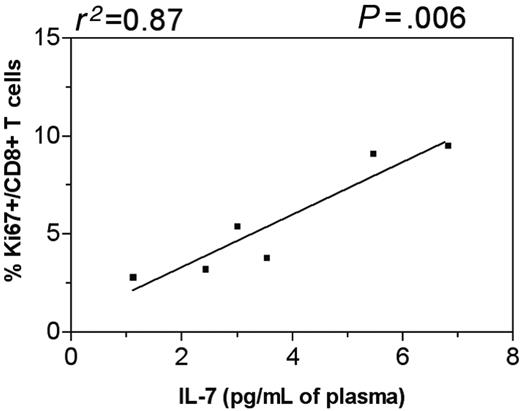
The observed early increase in T-cell proliferation in SIVsm-infected mangabeys might have been caused by antigen-induced expansion or homeostatic proliferation. Unlike the distinction that can be made in mice,13,37 it is difficult to clearly discern which one of these factors contributes to enhanced proliferation in primates. Temporal relationships between factors are helpful in assessing the cause of increased proliferation. One of our goals was to determine whether elevated IL-7 levels were associated with the increased T-cell proliferation seen in SIVsm-infected mangabeys. Overlaying the data for plasma IL-7 and T-cell proliferation for each individual mangabey clearly demonstrated that T-cell proliferation increased immediately after or simultaneously with the IL-7 increase (Figure 3A,C,E,G,I,K). The peak in plasma IL-7 level and Ki67 expression was reached approximately 4 weeks after infection in mangabey SM1 (Figure 3A). For mangabeys SM2 and SM3, increased IL-7 levels were observed as early as 1 week after infection and were followed by increases in CD4+ and CD8+ T-cell proliferation approximately 4 weeks after infection (Figure 3C,E). Similarly, for mangabeys SM4, SM5, and SM6, peak IL-7 levels were reached 2 weeks after infection followed by peak levels of T-cell proliferation 4 weeks after infection (Figure 3G,I,K). Pearson correlation analysis revealed a direct correlation between peak IL-7 levels and corresponding CD8+ T-cell proliferation levels in SIVsm-infected mangabeys (P < .05) (Figure 6). A similar trend between increased plasma IL-7 levels and increased CD4+ T-cell proliferation was observed in 4 of 6 SIVsm-infected mangabeys (Figure 3). In summary, the elevations in plasma IL-7 levels were followed by increases in CD4+ and CD8+ T-cell proliferation in SIVsm-infected mangabeys.
Elevated IL-7 levels are followed by the maintenance of stable CD4+ T-cell numbers in SIVsm-infected mangabeys
One of the reasons for the elevation in IL-7 levels during T-cell depletion is to restore T-cell homeostasis.9 Studies in mouse models have shown that IL-7 is indeed effective in restoring proper T-cell numbers under lymphopenic environments.9 In our previous study, the long-term nonprogressor macaque RM3 exhibited increased plasma IL-7 levels, increased Ki67 expression, and stable CD4 T-cell levels.17 Here, the temporal relationship between IL-7 levels and T-cell levels was determined in each of the SIVsm-infected mangabeys (Figure 3B,D,F,H,J,L). Temporal assessment of early events revealed a correlation between the CD4+ T-cell decline and the increase in plasma IL-7 levels. Further, elevated IL-7 levels were followed by stabilization in CD4+ and CD8+ T-cell counts in each of the SIVsm-infected mangabeys (Figure 3B,D,F,H,J,L). In summary, an increase in plasma IL-7 level is triggered early during primary SIVsm infection in mangabeys and is accompanied by maintenance of lower but healthy CD4+ T-cell levels through 37 weeks of infection.
Elevated IL-7 levels correlated with increased levels of CD8+ T-cell proliferation in SIVsm-infected mangabeys. Direct correlation between peak IL-7 levels and peak percentages of proliferating CD8+ T cells is seen in SIVsm-infected mangabeys. The best-fit line is shown along with Pearson correlation coefficient and relative P value.
Elevated IL-7 levels correlated with increased levels of CD8+ T-cell proliferation in SIVsm-infected mangabeys. Direct correlation between peak IL-7 levels and peak percentages of proliferating CD8+ T cells is seen in SIVsm-infected mangabeys. The best-fit line is shown along with Pearson correlation coefficient and relative P value.
Discussion
The ability of the mangabey to maintain a healthy immune system, including relatively normal levels of CD4+ T cells, is likely to be paramount for inhibiting disease progression after SIVsm infection. Previous studies have determined that the chronic immune activation observed in HIV-infected humans is relatively limited in SIVsm-infected mangabeys.1,33 This report expands on previous studies through an assessment of homeostatic regulation of T-cell levels during SIVsm infection of mangabeys. One of the strengths of this study is that SIVsm-infected mangabeys were followed longitudinally from before infection through 37 weeks after infection. This enabled us to assess the impact of SIVsm infection on the early immune events that were likely critical for determining long-term immune response and disease outcome.38 This study clearly demonstrated that after SIVsm infection, there was an early, sharp decline in the level of CD4+ T cells in the blood (weeks 1-4 after infection) of all 6 mangabeys included in this study. Temporal correlation of the CD4+ T-cell decline with acute viremia suggests that viral cytopathicity may be directly responsible for the elimination of many of these CD4+ T cells. Alternatively, SIVsm infection might have contributed to early CD4+ T-cell loss indirectly through the redistribution of CD4+ T cells as a response to infection.
HIV infection in humans and SIVmac infection in rhesus macaques generally results in progressive decline in the levels of CD4+ T cells as the infections advance to AIDS.17,39 In contrast, SIVsm infection in mangabeys is associated with relatively stable (healthy) levels of CD4+ T cells throughout the course of infection.1,33 Given the early declines in peripheral blood CD4+ T cell levels that accompanied the viral replication in SIVsm-infected mangabeys, we predicted active mechanisms could be in place to stabilize the CD4+ T-cell levels during the course of infection. Indeed, maintaining CD4+ T-cell levels in the SIVsm-infected mangabeys coincided with the timely increase in the levels of IL-7 (1-5 weeks after infection), a key cytokine involved in the maintenance of the size and subset composition of the peripheral T-cell pool.9,13 The temporal correlation between CD4 depletion and increased IL-7 levels during the acute phase of SIVsm infection in mangabeys further suggested that the elevation in IL-7 levels is a homeostatic response to T-cell depletion. Thus, there is a timely and healthy response (IL-7 increase) to early CD4+ T-cell depletion in SIVsm-infected mangabeys. In contrast, in SIVmac-infected macaques, there was a delay of 20 to 40 weeks between CD4+ T-cell decline and the onset of IL-7 increase.17 We hypothesize that the delay in the plasma IL-7 increase until after infection was the result of immune dysregulation in the SIVmac-infected macaques.17 SIV infection thus leads to 2 contrasting scenarios in macaques and mangabeys: (1) In the SIVsm-infected mangabey, a decline in CD4+ T-cell level during primary infection is accompanied by an immediate increase in the level of IL-7, indicating a healthy response. (2) In the SIVmac-infected macaque, an increase in IL-7 level is delayed until the chronic stages of infection, when the immune system is likely to be compromised.
Peripheral T-cell expansion is one of the key components in the establishment of T-cell homeostasis. The level of T-cell proliferation (Ki67) in mangabeys at the Yerkes colony has been assessed previously in 2 studies.1,33 These cross-sectional analyses randomly assessed uninfected and SIVsm-infected mangabeys and were in agreement that Ki67 levels were elevated within the CD8+ T-cell population.1,33 However, only one of these studies identified an increase in the Ki67 levels within the CD4+ T cells.1 Although the Ki67 increases were significant in SIVsm-infected mangabeys, they were modest compared with the higher levels of T-cell proliferation observed in SIVmac-infected macaques.33 Here, we observed an early increase in CD4+ and CD8+ T-cell proliferation during the acute stages of SIVsm infection in mangabeys followed by slightly elevated levels of T-cell proliferation throughout 37 weeks of infection. Ki67 levels at 37 weeks were comparable to the levels observed in the cross-sectional studies undertaken previously.1,33 Overall, these studies suggest that peripheral proliferation may be involved in the stabilization of T-cell numbers in SIVsm-infected mangabeys.
A number of studies have identified a clear role for IL-7 in the homeostatic expansion of T cells in lymphopenic environments.10,14 An increase in T-cell proliferation observed in SIVsm-infected mangabeys in our study accompanied an earlier increase in plasma IL-7 levels. Increased IL-7 levels and T-cell proliferation were followed by the maintenance of lower, but stable and healthy, levels of CD4+ T cells. Interestingly, a similar increase in T-cell proliferation was also observed after increased IL-7 levels in a long-term nonprogressor macaque (RM3) that has remained disease free for the past 4 years.17 These data support a role for IL-7–induced homeostatic proliferation in the stabilization of CD4+ T-cell levels in SIVsm-infected mangabeys. However, antigen-induced proliferation may also play an important role in the T-cell expansion observed in SIVsm-infected mangabeys. In addition, the ability of IL-7 to elicit an antiapoptotic response might also have contributed to the healthy CD4+ T-cell levels in these animals.40,41 Absence or presence of a thymus did not significantly alter the viral replication, anti-SIV antibody response, disease progression, or maintenance of T-cell levels in the SIV+ mangabeys in the 37 weeks studied; further follow-up of these animals is under way. Based on the results obtained thus far, stabilization of CD4+ T cells in SIVsm-infected mangabeys appears to be mainly through peripheral expansion and increased survival of T cells rather than through increased T-cell production from the thymus.
Adjuvant immunotherapy remains one of the viable options for boosting the immune system in patients with HIV who experience immune system recovery with antiretroviral treatment (ART) alone. Currently, a number of potential immunotherapeutic agents, including cytokines such as IL-2, IL-7, and IL-15, are being investigated for their potential to increase T-cell levels in HIV patients.42-47 Of these, IL-7 is promising because of its multiple roles in T-cell homeostasis.16,18,41,48 Our detailed studies in SIVsm-infected mangabeys, SIVmac-infected macaques, and HIV-infected humans confirm the importance of IL-7 in regulating T-cell levels during continued infection.17,49 One of the main issues concerning clinical trials of any therapy is the timing of the treatment to obtain the optimum results. Our longitudinal studies in SIVsm-infected mangabeys suggest that IL-7 administration during acute infection is ideal for recovering T-cell homeostasis. Studies in SIVmac-infected macaques suggest that the delayed IL-7 treatment in the chronic stages of infection may require careful timing of IL-7 administration with respect to antiretroviral treatment.17 In addition to advantages to the peripheral T-cell pool, we have identified correlations between increased plasma IL-7 levels and T-cell proliferation in bone marrow (G. Silvestri, personal communication, December 2004). This demonstrates the potential advantage of IL-7 to have an impact on various lymphoid compartments including bone marrow, but it also demonstrates that increased production of T cells from the thymus might be realized. In addition, IL-7 has been shown to be useful in depleting HIV reservoirs because of its ability to stimulate HIV replication from resting CD4+ T cells.50-52 In summary, these data highlight the potential strengths of IL-7 as immunotherapy for HIV-infected patients if it is provided as a timely addition to effective antiretroviral therapy.
Prepublished online as Blood First Edition Paper, August 16, 2005; DOI 10.1182/blood-2005-01-0394.
Supported by the Elizabeth Glaser Pediatric AIDS Foundation (D.L.S.) and National Institutes of Health grants R01-AI35522 (D.L.S.), R01-AI52755 (G.S.), and R21-54234 (G.S.), and by Yerkes Center for AIDS Research (CFAR) grant AI50409 (S.I.S.).
A.M. and D.Z. analyzed T-cell levels, T-cell proliferation, and cytokine assays. H.M.M. supervised the housing and care of the animals. A.P.B. and M.P. processed and analyzed some of the blood samples at Yerkes Primate Research Center. S.I.S. determined viral load. K.S.C. assayed anti–SIV-specific humoral immune responses. G.S. contributed to the design, analysis, and writing of this manuscript. D.L.S. supervised the design, experimentation, analysis, and writing of this study.
A.M. and D.Z. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Stephanie Ehnert, David Maggio, and Chris Ibegbu for providing expert care to the animals and for their assistance with processing blood samples at the Yerkes Regional Primate Center; Aneta Wozniakowski for excellent technical assistance; and Jeffrey Milush, David Kosub, and Amanda Leone for their helpful comments during the preparation of the manuscript.