Activation and subsequent differentiation of naive CD8+ T cells lead to the development of memory subsets with distinct homing and effector capacities. On nonlymphoid homing subsets, expression of “inflammatory” chemokine receptors (such as CXCR3, CCR5, CX3CR1, and CXCR1) is believed to promote migration into sites of infection/inflammation. Here we show that CXCR1 can be up-regulated to the cell surface within minutes of activating human CD8+ T cells. No concurrent up-regulation of other inflammatory chemokine receptors was observed. Up-regulation of CXCR1 preferentially occurred on central memory CD8+ T cells—that is, cells with a lymph node homing phenotype—and was functionally relevant. Immunofluorescence microscopy showed CXCR1 to be present in intracellular vesicles that do not significantly colocalize with perforin, RANTES (regulated upon activation normal T cell expressed and secreted), or the lysosomal marker CD63. By contrast, partial colocalization with the Golgi marker GM130, the constitutive secretory pathway marker β2-microglobulin, and the early endosome marker EEA1 was observed. Up-regulation of CXCR1 did not occur after T-cell receptor cross-linking. By contrast, supernatants from activated neutrophils, but not from monocytes or dendritic cells, induced its up-regulation. These results suggest that CD8+ T cells can rapidly adapt their homing properties by mobilizing CXCR1 from a distinct intracellular compartment.
Introduction
CD8+ T cells play a key role in host defense against intracellular infection.1 Naive CD8+ T cells are induced to proliferate and differentiate on encounter with cognate antigen presented by dendritic cells in secondary lymphoid structures.2,3 Activation and subsequent proliferation and differentiation leads to the formation of effector and memory CD8+ T-cell subsets.4,5 Effector CD8+ T cells migrate into sites of infection and kill infected cells. Upon clearance of infection, effector CD8+ T cells disappear, while a pool of pathogen-specific memory CD8+ T cells survives. Memory CD8+ T cells persist long-term and allow for a more rapid expansion of effector cells upon re-encounter with the same pathogen.6
Efficient detection of re-infection is key to the function of memory CD8+ T cells. Optimal detection of re-infection is believed to be accomplished by partition of labor between memory CD8+ T-cell subsets: effector memory (EM) and CD45RA re-expressing EM (EMRA, terminally differentiated EM) CD8+ T cells survey nonlymphoid organs in search of cognate antigen and are thought to allow for a recall response in the tissue. In contrast, central memory (CM) CD8+ T cells recirculate between the blood and the lymphoid compartment and are thought to provide a pool of antigen-experienced cells with a high proliferative capacity but a lower activation threshold than naive cells, thus allowing for a more rapid generation of effector cells during a recall response.4,5,7
The chemokine/chemokine receptor system plays a fundamental role in orchestrating trafficking of immune cells. Expression of CCR7 can be used to distinguish CD8+ T-cell subsets.4 Upon encounter with cognate antigen within secondary lymphoid structures, naive and CM CD8+ T cells are thought to down-regulate CCR7 and proliferate and up-regulate inflammatory chemokine receptors such as CXCR3, CCR5, CX3CR1, and CXCR1, endowing them with homing properties that promote migration into sites of infection/inflammation.4,8-12 Little is known on subsequent regulation of inflammatory chemokine receptors expressed by circulating CD8+ T-cell subsets.
Here we describe an intracellular CXCR1-storing CD8+ T-cell compartment that is mobilized rapidly to the cell surface upon exposure to neutrophil-derived inflammatory mediators. Up-regulation was maturation subset selective, with the most pronounced increase in CXCR1 expression observed on memory CD8+ T cells with a lymph node homing phenotype. Acute inflammatory mediators thus seem to have the potential to rapidly change the chemokine receptor expression profile of CD8+ T cells by inducing up-regulation of prestored CXCR1.
Materials and methods
Antibodies
The following antibodies were used: CXCR1 (42705.111), CCR7 (150503), and RANTES (catalog #BAF278) from R&D Systems (Minneapolis, MN), CX3CR1 (2A9-1) from MBL (Woburn, MA), perforin (delta G9) from Ancell Corporation (Bayport, MN), CXCR3 (1C6), CCR5 (3A9), CD45RA (HI100), CD8+ (RPA-T8), CD28 (CD28.2), CD3 (UCHT1), EEA1 (14), GM130 (35) β2-microglobulin (TÜ99), and CD63 (H5C6) from BD Biosciences (San Jose, CA), and CD3 (OKT3) from eBioscience (San Diego, CA). All isotype control antibodies were purchased from R&D Systems.
Isolation of PBMC/CD8+ T cells
Anticoagulated blood was drawn from healthy donors after written informed consent. The study was approved by the board of the Swiss Red Cross blood transfusion service. Peripheral blood mononuclear cells (PBMCs) were prepared by centrifugation through a density gradient on Histopaque-1077 (Sigma-Aldrich, St Louis, MO). Natural killer (NK) cells were depleted and CD8 cells positively selected by means of anti-CD56 and anti-CD8 magnetic beads, respectively (both from Miltenyi Biotec, Auburn, CA). Purity of CD8+ T cells, as assessed by flow cytometry, was usually more than 97% (data not shown).
CD8+ T-cell subset sorting
CD8+ T-cell subset sorting was performed on a Cytomation MoFlo (Dako Cytomation, Fort Collins, CO) cell sorter. The following populations of CD8+ T cells were sorted: CCR7+ CD45RA+, CCR7+ CD45RA–, CCR7– CD45RA–, and CCR7– CD45RA+. For each cell-population, 14 000 to 200 000 cells were sorted. Purity was evaluated by post-sort flow cytometry. Less than 1.5% contamination was observed in all T-cell subsets (data not shown).
Activation and cell surface staining of CD8+ T cells
PBMCs or isolated CD8+ T cells were suspended in medium and incubated with purified phytohaemagglutinin (PHA) (1-20 μg/mL, as indicated) (Phaseolus spp, Remel, Lenexa, KS), or ionomycin (1 μM) (Sigma-Aldrich, St Louis, MO). Subsequently, cells were spun down, resuspended in phosphate-buffered saline (PBS)/1% bovine serum albumin (BSA), and incubated with appropriate antibodies for FACScan-analyses. After incubation for 30 to 45 minutes at 4°C, cells were washed twice in PBS/1% BSA. Alternatively, before activation, PBMCs were incubated for 2 hours on ice with 1 mg/mL cytochalasin D or 5 mg/mL cycloheximide (both from Sigma). Data were acquired with a FACS Calibur flow cytometer (Becton Dickinson, Mountain View, CA) and analyzed using CellQuest software (Becton Dickinson). CD8+ T cells were identified by gating on CD3/CD8 double-positive events within the lymphocyte gate as defined by standard forward scatter (FSC)/side scatter (SSC) criteria. Appropriate isotype-control antibodies were used to define positive events.
Confocal microscopy
Isolated CD8+ T cells were permeabilized for 20 minutes at 4°C in PBS supplemented with 2% BSA, 5 mg/mL sandoglobin (kindly provided by the Red Cross, blood donor center, Bern, Switzerland), and 0.2% saponin (Sigma) (= permeabilization buffer). Subsequently, primary antibodies (anti–perforin–fluorescein isothiocyanate [FITC], anti–EEA1-FITC, anti–GM130-FITC, anti–β2-microglobulin–FITC, anti–CD63-FITC, anti–RANTES (regulated upon activation normal T cell expressed and secreted)–biotin, anti–CXCR1-biotin, or appropriate isotype-control mAb) were added to the buffer and incubated for 20 minutes on ice. After each incubation step, cells were washed 3 times and resuspended in permeabilization buffer. Biotinylated antibodies were detected individually by incubating with streptavidin-alexa-467 (Molecular Probes, Eugene, OR) or streptavidin-FITC (Becton Dickinson) for 20 minutes at 4°C. After extensive washing, the second biotinylated mAb was added and revealed. Following labeling, cells were placed on a 10-chamber slide (chamber diameter 6 mm; Semadeni AG, Ostermundingen, Switzerland), mounted with Vectashield fluorescence mounting medium (Vector Laboratories, Burlingame, CA), and analyzed using the LSM 510 META confocal laser scanning microscopy system (Carl Zeiss, Feldbach, Switzerland). A Zeiss Plan Neofluar 63 ×/1.25 numeric aperture oil (∞/0.17) objective was used, and images were imported into and arranged with Adobe Photoshop software (Adobe Systems, San Jose, CA).
Actin-polymerization assays
Isolated CD8+ T cells were activated with phytohemagglutinin (PHA) (10 μg/mL) for 15 minutes and subsequently incubated with various concentrations of interleukin-8 (IL-8) (as indicated). After 4 minutes, cells were permeabilized with Cytofix/Cytoperm buffer according to the manufacturer's protocol (Becton Dickinson). Permeabilized cells were incubated for 30 minutes at 4°C with OregonGreen-488 coupled phalloidin, washed 3 times in washing buffer supplied with the permeabilization kit, placed on a 10-chamber slide (Semadeni AG), mounted with Vectashield fluorescence mounting medium (Vector Laboratories), and analyzed using an LSM 510 META confocal laser scanning microscopy system (Carl Zeiss).
Chemotaxis assays
Isolated CD8+ T cells were resuspended in RPMI 1640/1% BSA, activated with PHA (10 mg/mL for 15 minutes) as indicated, and loaded in duplicate into 8-μm pore size polycarbonate transwell inserts for 24-well cell culture dishes (Corning, Corning, NY). IL-8 (CXCL8), IP-10 (CXCL10), and MIP-1β (CCL4) (all from PeproTech EC, London, United Kingdom) were added to the lower wells at various concentrations. Cells were spun onto the membrane for 1 minute at 50g. After incubation for 2 hours at 37°C, transmigrated membrane-adherent cells were spun down by centrifugation for 8 minutes at 130g, and total transmigrated cells were counted using a Neubauer counting chamber system (Milian AG, Geneva, Switzerland).
Isolation and activation of neutrophils
Neutrophils were isolated from fresh buffy coats as described previously.13 Briefly, a fresh buffy coat was diluted 1/1 (vol/vol) with PBS-EDTA(ethylenediaminetetraacetic acid) (2 mM), mixed gently with 0.25 volume of 4% dextran T500 (Amersham Pharmacia Biotech, Dübendorf, Switzerland), and left for 30 minutes for erythrocyte sedimentation. Leukocyte-rich supernatant was aspirated and centrifuged for 10 minutes at 200g. The pellet was resuspended in 9 mL of ultrapure water to lyse erythrocytes. Isotonicity was restored by addition of 3 mL of KCl (0.6 M) and 40 mL of NaCl (0.15 M). Cells were then centrifuged 10 minutes at 350g and resuspended in 20 mL of PBS-EDTA (2 mM). This suspension was layered over 20 mL of Ficoll-Hypaque (Sigma) and centrifuged for 30 minutes at 350g. The neutrophil-rich pellet was recovered and washed twice in PBS-EDTA. All manipulations were performed at 4°C, thus minimizing neutrophil activation. For stimulation, neutrophils (107 cells/mL) were diluted 1/1 (vol/vol) in prewarmed (37°C) medium with 1 μg/mL lipopolysaccharide (LPS) (final concentration) (Calbiochem/Behring Diagnostics, La Jolla, CA), and incubated for 20 minutes at 37°C. Neutrophils were removed by centrifugation (4000g at 4°C) and supernatants stored at –80°C until use.
Isolation/activation of monocytes and generation/activation of dendritic cells (DCs)
Monocytes were isolated from PBMC preparations by use of anti-CD14 magnetic beads (Miltenyi Biotec) and activated with 1 μg/mL LPS as indicated. Dendritic cells were generated as described from PBMC preparations.14 Briefly, PBMCs were washed and cultured in complete medium (RPMI 1640, 1% L-glutamine, 1% penicillin/streptomycin, and 10% fetal calf serum [FCS]) for 1 hour. After incubation, nonadherent cells were removed by washing steps. Adherent cells were cultured in complete medium supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 (50 ng/mL each) (both from R&D Systems). On days 2 and 5, medium and supplements were replaced. On day 6, nonadherent DCs were harvested, counted, and plated in 6-well plates (at 1 × 105/mL) in fresh medium containing GM-CSF and IL-4 (50 ng/mL each). DCs were activated as indicated on day 6 by addition of LPS (100 ng/mL).
Incubation of CD8+ T cells with IL-2, IFNγ, TNFα, anti-CD3/CD28 mAb, and neutrophil, monocyte, or DC-conditioned medium
PBMCs or isolated CD8+ T cells were cultured in RPMI 1640 medium with 10% heat-inactivated normal human serum (NHS) with increasing concentrations of IL-2, interferonγ (IFNγ), or tumor necrosis factorα (TNFα) (all from R&D Systems) or in 24-well plates (Nunc, Roskilde, Denmark) that had either been precoated with anti-CD3 mAb (20 μg/mL) plus anti-CD28 mAb (20 μg/mL) or together with soluble anti-CD3 and anti-CD28 mAbs at the same concentration. To test for activity of neutrophil-, monocyte-, or dendritic cell–conditioned medium, isolated CD8+ T cells were resuspended in either supernatant from LPS-activated neutrophils, monocytes, or DCs or medium supplemented with LPS (1 μg/mL, final concentration) (Calbiochem/Behring Diagnostics). After 15 minutes to 3 hours of incubation (as indicated), cells were spun down, resuspended in PBS/1% BSA, and prepared for FACScan analysis.
Determination of MPO activity, IL-8, and TNFα concentrations in supernatants
Myeloperoxidase (MPO) activity was assessed as previously described.13 Briefly, MPO enzymatic activity was measured in a colorimetric assay where 100 μL of substrate buffer (50 mL of citrate-phosphate buffer, pH 5; 20 μL of 30% H2O2; 20 mg of orthophenylenediamine) was added to 20 μL of supernatant of activated neutrophils. Purified MPO (Calbiochem-Novabiochem, La Jolla, CA) was used as standard. The reaction was stopped with H2SO4 and the absorbance measured with a microplate reader (Thermo Max, Molecular Device, Munich, Germany) at 490 nm. Concentrations of IL-8 and TNFα were measured using the BD OptEIA Human ELISA Set (BD Biosciences), according to the manufacturer's instructions.
Statistical analysis
Student t test analyses were performed with JMP Version 3 software (SAS Institute, Cary, NC). P values of .05 or less were considered statistically significant. Data are presented as mean ± standard deviation (SD).
Results
Cell surface expression of inflammatory chemokine receptors on activated CD8+ T cells
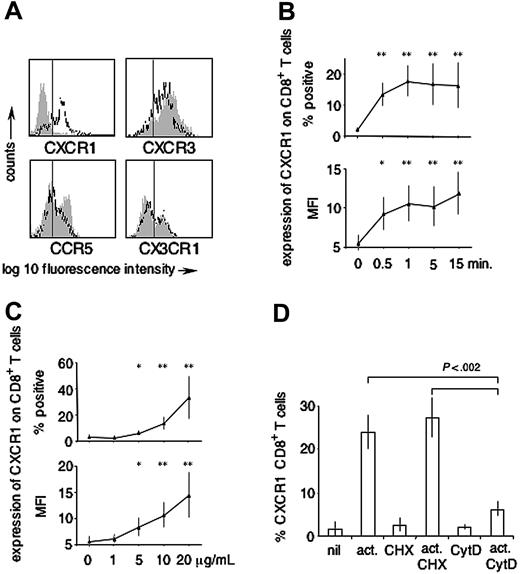
To begin to understand regulation of inflammatory chemokine receptors by short-term activated human CD8+ T cells, CD8+ T-cell surface expression of CXCR3, CCR5, CX3CR1, and CXCR1 was analyzed on PHA-activated versus nonactivated cells. Baseline expression on ex vivo analyzed CD8+ T cells was as follows: CXCR3 82% ± 4%, CCR5 29% ± 9%, CX3CR1 25% ± 7% (n = 3), and CXCR1 6% ± 5% (n = 6). After 15 minutes of activation with PHA (20 μg/mL), the percentage of CD8+ T cells expressing CXCR3, CCR5, or CX3CR1 consistently decreased (CXCR3 75% ± 7%, mean fold decrease 1.1, CCR5 16% ± 7%, mean fold decrease 1.8, CX3CR1 18% ± 6%, mean fold decrease 1.2; n = 3). By contrast, the percentage of CD8+ T cells expressing CXCR1 substantially increased (CXCR1 39% ± 15%, mean fold increase 6.8; n = 6) (Figure 1A). Up-regulation was found to occur within the first minute(s) of stimulation (Figure 1B) in a dose-dependent manner (Figure 1C) and was reflected alike by the percentage of CXCR1+ events according to an isotype control (Figure 1B-C, upper panel), as well as by changes in mean fluorescence intensities (MFIs) (Figure 1B-C, lower panels). This close similarity between the data presented as percentage CXCR1+ events and CXCR1 MFIs held up for all experiments presented in this manuscript (data not shown). Based on the kinetics with which CXCR1 appeared on the cell surface, we assumed up-regulation from a preformed intracellular receptor pool. The assumption was tested for by repeating the activation experiments in presence of (1) cycloheximide (CHX), inhibiting de novo protein synthesis, and (2) cytochalasin D (CytD), inhibiting actin polymerization.15,16 As shown in Figure 1D, inhibition of protein synthesis did not change the expression level of CXCR1 on CD8+ T cells stimulated for 15 minutes with PHA (10 μg/mL) (ex vivo; 1.7% ± 1.9%, ex vivo and CHX 2.88% ± 1.8%, activated; 27.5% ± 4.4%, activated and CHX; 31.4% ± 5.3%; n = 3). By contrast, and further indicative of an intracellular pool of CXCR1, inhibiting actin polymerization largely abolished its up-regulation (ex vivo and CytD; 2.3% ± 0.6%, activated and CytD; 7.1% ± 1.8%, mean inhibition of up-regulation = 74% [range, 68%-83%]; n = 3).
Expression of inflammatory chemokine receptors on activated versus nonactivated CD8+ T cells. (A) Expression of CXCR1, CX3CR1, CXCR3, and CCR5 was assessed on CD8+ T cells ± PHA (15 minutes, 20 μg/mL). Gray shaded histograms represent baseline expression, open closed-line histograms represent expression after activation. The vertical line indicates the cut-off for positivity as determined by isotype-control antibodies (not shown). No PHA-induced up-regulation was observed for CXCR3, CCR5, and CX3CR1 (representative of n = 3). By contrast, substantial PHA-induced up-regulation was observed for CXCR1 (representative of n = 6). (B) Time-course (n = 6; PHA at 10 μg/mL), (C) dose response (n = 6; activation for 15 minutes). *P < .01 as compared with baseline; **P < .001 as compared with baseline. (D) Impact of inhibition of protein synthesis (CHX) and inhibition of actin polymerization (CytD) (n = 3; PHA stimulation at 10 μg/mL for 15 minutes). Up-regulation of CXCR1 after nonspecific CD8+ T-cell stimulation occurred within seconds/minutes, in a dose-dependent manner and independently from protein synthesis, but was largely abolished by inhibiting actin polymerization. Error bars indicate mean ± SD.
Expression of inflammatory chemokine receptors on activated versus nonactivated CD8+ T cells. (A) Expression of CXCR1, CX3CR1, CXCR3, and CCR5 was assessed on CD8+ T cells ± PHA (15 minutes, 20 μg/mL). Gray shaded histograms represent baseline expression, open closed-line histograms represent expression after activation. The vertical line indicates the cut-off for positivity as determined by isotype-control antibodies (not shown). No PHA-induced up-regulation was observed for CXCR3, CCR5, and CX3CR1 (representative of n = 3). By contrast, substantial PHA-induced up-regulation was observed for CXCR1 (representative of n = 6). (B) Time-course (n = 6; PHA at 10 μg/mL), (C) dose response (n = 6; activation for 15 minutes). *P < .01 as compared with baseline; **P < .001 as compared with baseline. (D) Impact of inhibition of protein synthesis (CHX) and inhibition of actin polymerization (CytD) (n = 3; PHA stimulation at 10 μg/mL for 15 minutes). Up-regulation of CXCR1 after nonspecific CD8+ T-cell stimulation occurred within seconds/minutes, in a dose-dependent manner and independently from protein synthesis, but was largely abolished by inhibiting actin polymerization. Error bars indicate mean ± SD.
Subset-specific expression of CXCR1 by CD8+ T cells stimulated with PHA
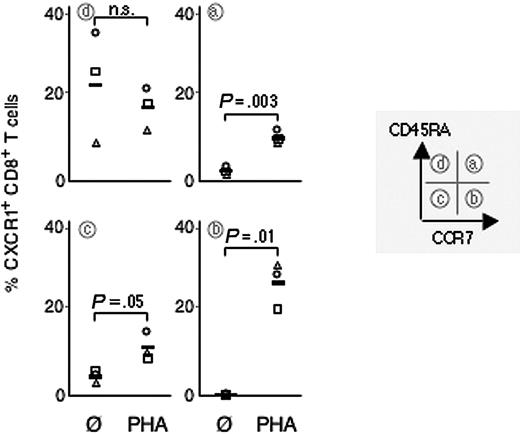
Phenotypic markers can be used to identify subsets of CD8+ T cells with differing functional properties. We analyzed cell surface expression of CXCR1 on PHA-activated and nonactivated naive (CCR7+ CD45RA+), CM (CCR7+ CD45RA–), EM (CCR7– CD45RA–), and EMRA (CCR7–CD45RA+).4,5,7 Expression was assessed by subset gating of bulk CD8+ T cells, as well as on FACSorted CD8+ T-cell subsets. Data obtained using either method were similar, although PHA activation induced minor changes in the proportions of CD8+ T-cell subsets. (Mean relative percentage of CD8+ T-cell subsets before and after PHA activation: naive 34% [range, 22%-55%] vs 39% [range, 22%-62%]; CM 13% [range, 10%-16%] vs 10% [range, 9%-11%]; EM 32% [range, 25%-38%] vs 29% [range, 20%-38%]; EMRA 21% [range, 10%-29%] vs 22% [range, 9%-29%]; n = 3). Of importance, up-regulation was most pronounced on CM CD8+ T cells (mean increase 26% [range, 20%-30%]; n = 3). On naive CD8+ T cells and EM CD8+ T cells only little up-regulation was observed (mean increase 8% [range, 7%-8%] and 7% [range, 3%-10%], respectively; n = 3). On EMRA CD8+ T cells, CXCR1 expression did not significantly change (mean decrease 6% [range, +4% to –14%]; n = 3) (Figure 2). Intriguingly, upon activation, the percentage of CD8+ T cells expressing CXCR1 tended to be higher in the CM subset than in the constitutively CXCR1 expressing EMRA CD8+ T-cell subset (PHA-activated CM CD8+ T cells: mean CXCR1 expression 26% [range, 20%-30%], PHA-activated EMRA CD8+ T cells: mean CXCR1 expression 17% [range, 12%-22%], nonactivated EMRA CD8+ T cells: mean CXCR1 expression 23% [range, 8%-35%]; n = 3) (Figure 2). In summary, regulation of CXCR1 was subset selective and preferentially induced on CD8+ T cells with a CM phenotype.
Subset-selective up-regulation of CXCR1 by CD8+ T cells stimulated with PHA. CD8+ T cells were stimulated with PHA (10 μg/mL for 15 minutes) and expression of CXCR1 assessed on maturation subsets as defined by expression of CCR7 and CD45RA. Up-regulation was most prominent on CD8+ T cells with a central memory phenotype (ie, CCR7+ CD45RA–). Each symbol indicates an individual experiment and donor.
Subset-selective up-regulation of CXCR1 by CD8+ T cells stimulated with PHA. CD8+ T cells were stimulated with PHA (10 μg/mL for 15 minutes) and expression of CXCR1 assessed on maturation subsets as defined by expression of CCR7 and CD45RA. Up-regulation was most prominent on CD8+ T cells with a central memory phenotype (ie, CCR7+ CD45RA–). Each symbol indicates an individual experiment and donor.
Intracellular expression of CXCR1 by CD8+ T cells
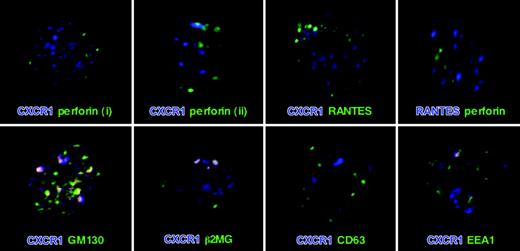
Activation-induced up-regulation of CXCR1 occurred in the presence of inhibitors of protein synthesis, but not after inhibiting actin cytoskeleton rearrangement, together strongly suggesting a regulated intracellular pool of preformed protein. To define the CXCR1-storing compartment with respect to perforin, as well as the recently described RANTES storage granules,16 dual color confocal microscopy analyses were performed. A granular CXCR1 staining pattern throughout the cytoplasm was observed (Figure 3). As expected from the activation experiments, most CD8+ T cells stained positive for intracellular CXCR1 to at least some extent (data not shown). CXCR1 did not colocalize with either perforin or RANTES. Also, as expected from the literature,16 no colocalization of perforin with RANTES was observed (Figure 3, upper panel). CXCR1 thus seems to be stored in a discrete, regulated intracellular compartment that is distinct from the established perforin and RANTES storage granules. To further characterize the intracellular compartment in which CXCR1 is expressed, co-staining was carried out with markers for Golgi (GM130), endosomes (EEA1), and lysosomes (CD63). In addition, we stained intracellular β2-microglobulin as a marker of the constitutive secretory pathway. While no colocalization of CXCR1 with the lysosomal marker CD63 was observed, some discrete colocalization of CXCR1 was detected with GM130, EEA1, and β2-microglobulin, likely indicative of continuous receptor synthesis, expression, and endocytic uptake (Figure 3, lower panel).
Expression of CXCR1 in CD8+ T cells. After permeabilization, CD8+ T cells were double-labeled for CXCR1 and each perforin, RANTES, GM130, β2MG, CD63, and EEA1. In addition, cells were double labeled for perforin and RANTES. Similar to perforin and RANTES, CXCR1 staining was granular, and no colocalization of CXCR1 with either perforin or RANTES was observed. Perforinlow/neg CD8+ T cells tended to express more CXCR1 (i) than did perforinhigh CD8+ T cells (ii). Indicative of ongoing low-level sorting of CXCR1 into the constitutive secretory pathway and its endocytic reuptake, some distinct colocalization of CXCR1 with GM130 (Golgi), β2-microglobulin (constitutive secretory pathway), and EEA1 (early endosomal compartment) was observed in a subset of cells (bottom row). No staining was seen when incubating permeabilized CD8+ T cells with appropriate isotype-control antibodies (data not shown).
Expression of CXCR1 in CD8+ T cells. After permeabilization, CD8+ T cells were double-labeled for CXCR1 and each perforin, RANTES, GM130, β2MG, CD63, and EEA1. In addition, cells were double labeled for perforin and RANTES. Similar to perforin and RANTES, CXCR1 staining was granular, and no colocalization of CXCR1 with either perforin or RANTES was observed. Perforinlow/neg CD8+ T cells tended to express more CXCR1 (i) than did perforinhigh CD8+ T cells (ii). Indicative of ongoing low-level sorting of CXCR1 into the constitutive secretory pathway and its endocytic reuptake, some distinct colocalization of CXCR1 with GM130 (Golgi), β2-microglobulin (constitutive secretory pathway), and EEA1 (early endosomal compartment) was observed in a subset of cells (bottom row). No staining was seen when incubating permeabilized CD8+ T cells with appropriate isotype-control antibodies (data not shown).
Up-regulated CXCR1 is functional
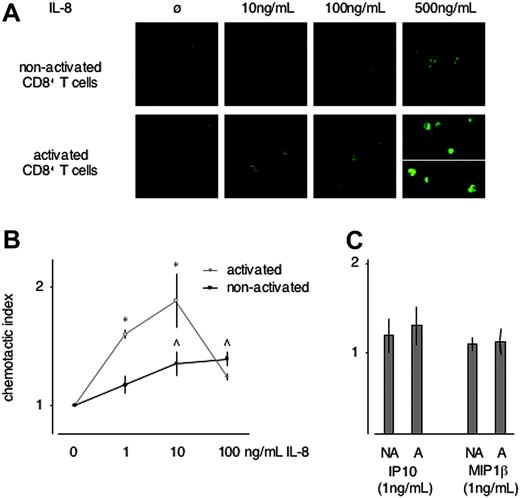
Up-regulation of functional CXCR1 should render cells IL-8 responsive, thereby endowing them with the property to enter sites of innate immune-system activation.11,12,17,18 As an initial test for IL-8 responsiveness of activated versus nonactivated CD8+ T cells, ligand-induced formation of F-actin was assessed. A dose-dependent increase in F-actin formation was seen in activated versus nonactivated CD8+ T cells exposed to IL-8 (CXCL8, CXCR1 ligand) (Figure 4A). By contrast, no difference in F-actin formation was detected in activated versus nonactivated CD8+ T cells exposed to increasing concentrations of IP-10 (CXCL10, CXCR3 ligand) and MIP-1α (CCL4, CCR5 ligand) (internal controls, data not shown).
We next assessed the chemotactic response of activated versus nonactivated CD8+ T cells in gradients of IL-8, IP-10, and MIP-1β, using a trans-well chemotaxis assay. An increase in chemotactic activity of activated versus nonactivated CD8+ T cells was observed in a gradient of IL-8 (Figure 4B). Activation, and thus up-regulation of CXCR1, induced IL-8 responsiveness at IL-8 concentrations as low as 1 ng/mL. By contrast, only little chemotactic activity of either activated or nonactivated CD8+ T cells was observed in gradients toward 1 ng/mL of IP-10 or MIP-1β (Figure 4C). At higher concentrations of either chemokine, chemotaxis was similarly increased in activated versus nonactivated CD8+ T cells (data not shown). Thus, upon up-regulation of CXCR1, activated CD8+ T cells are rendered exclusively and selectively IL-8 responsive, as demonstrated by increased IL-8 selective chemotactic activity at already low chemokine concentrations and by increased IL-8–induced F-actin formation.
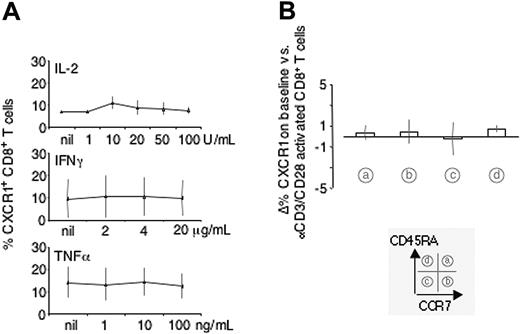
Impact of IL-2, IFNγ, TNFα, and T-cell receptor (TcR) cross-linking on expression of CXCR1 on CD8+ T-cell subsets
Activation of CD8+ T cells can be grouped into antigen dependent (ie, TcR-dependent) versus antigen independent. As shown in Figure 5A, activation with IL-2, IFNγ, and TNFα did not change expression of CXCR1 to any significant extent. Also, activation of CD8+ T cells with αCD3 and αCD28 mAbs did not change expression of CXCR1 on CD8+ T-cell subsets as defined by CCR7 and CD45RA (Figure 5B), while up-regulation of the activation marker CD69 was readily observed within 2 to 3 hours of activation under the same conditions (data not shown).
IL-8 responsiveness of nonactivated versus activated CD8+ T cells. (A) F-actin was visualized in unstimulated and PHA-stimulated CD8+ T cells exposed to increasing concentrations of IL-8. In absence of IL-8, only cortical F-actin was visualized. An increasingly dense meshwork of F-actin throughout the cytoplasm was observed in most activated CD8+ T cells exposed to 10, 100, and 500 ng/mL IL-8 (bottom row). By contrast, in nonactivated CD8+ T cells a similar though less dense meshwork was formed only at 500 ng/mL IL-8 and only in some of the cells (top row) (representative of n = 3). (B) Chemotactic activity toward IL-8 was assessed in PHA-activated versus unstimulated CD8+ T cells. PHA activation (and hence up-regulation of CXCR1) sensitized CD8+ T cells for chemotactic activity toward IL-8 (representative of n = 4). *P < .05 between the chemotactic index of activated versus nonactivated CD8+ T cells; ^ P < .05 between background migration versus chemotaxis of nonactivated CD8+ T cells. Note that in activated CD8+ T cells significant chemotactic activity was observed already toward 1 ng/mL of IL-8. (C) In contrast to IL-8, only minimal chemotactic activity of nonactivated (NA) as well as activated (A) CD8+ T cells was observed in a gradient toward 1 ng/mL of IP-10 or MIP-1β (representative of n = 3). Error bars indicate mean ± SD.
IL-8 responsiveness of nonactivated versus activated CD8+ T cells. (A) F-actin was visualized in unstimulated and PHA-stimulated CD8+ T cells exposed to increasing concentrations of IL-8. In absence of IL-8, only cortical F-actin was visualized. An increasingly dense meshwork of F-actin throughout the cytoplasm was observed in most activated CD8+ T cells exposed to 10, 100, and 500 ng/mL IL-8 (bottom row). By contrast, in nonactivated CD8+ T cells a similar though less dense meshwork was formed only at 500 ng/mL IL-8 and only in some of the cells (top row) (representative of n = 3). (B) Chemotactic activity toward IL-8 was assessed in PHA-activated versus unstimulated CD8+ T cells. PHA activation (and hence up-regulation of CXCR1) sensitized CD8+ T cells for chemotactic activity toward IL-8 (representative of n = 4). *P < .05 between the chemotactic index of activated versus nonactivated CD8+ T cells; ^ P < .05 between background migration versus chemotaxis of nonactivated CD8+ T cells. Note that in activated CD8+ T cells significant chemotactic activity was observed already toward 1 ng/mL of IL-8. (C) In contrast to IL-8, only minimal chemotactic activity of nonactivated (NA) as well as activated (A) CD8+ T cells was observed in a gradient toward 1 ng/mL of IP-10 or MIP-1β (representative of n = 3). Error bars indicate mean ± SD.
Impact of neutrophil-, monocyte-, and DC-conditioned medium on expression of CXCR1 on CD8+ T cells
Neutrophils are among the first cells to arrive at a site of pathogen-induced injury, and early during an innate immune response IL-8 is one of the most abundantly produced chemokines.17,18 We therefore hypothesized that one or several neutrophil-derived factor(s) may provide a TcR-independent signal inducing up-regulation of CXCR1 in CD8+ T cells. As shown in Figure 6A-B, supernatant from LPS-activated neutrophils induced up-regulation of CXCR1 on CD8+ T-cell subsets with similar subset selectivity for the CM compartment and with similar kinetics, as did activation with PHA: naive CD8+ T cells; mean increase, 7.5% (range, –5.3%-13.0%), CM CD8+ T cells; mean increase, 14.0% (range, 7.3%-24.0%), EM CD8+ T cells; mean increase, 8.9% (range, 6.1%-17.9%), EMRA CD8+ T cells; mean increase, 9.3% (range, 3.7%-18.2%) (n = 15). While variability in the observed up-regulation of CXCR1 was substantial, it was similar when comparing autologous versus allogeneic supernatants (Figure 6A). LPS itself reduced expression of CXCR1 on CD8+ T cells by a mean of 4.4% (range, –9.7%-0.32%) (n = 15). Not surprisingly, after only 20 minutes of activation, key inflammatory mediators such as MPO, IL-8, or TNFα were not detected in the supernatant from neutrophils (data not shown).
Monocytes and DCs play a crucial role at the interface between innate and adaptive immunity and, with some temporal delay compared to neutrophils, accumulate at sites of infection/inflammation. However, incubating CD8+ T cells with either supernatant from LPS-activated monocytes or DCs (their respective phenotype is shown in Figure 6C), down-regulation rather than up-regulation of CXCR1 was observed (Figure 6D). Similar to neutrophil-derived supernatants, no IL-8 or TNFα was detected after 20 minutes of activating monocytes and DCs with LPS, while both cytokines were readily detected after 4 and 24 hours of activation (data not shown). Induction of CXCR1 expression thus was non-TcR dependent and seems to be regulated by one or several factor(s) released early upon activation of neutrophils.
Discussion
The chemokine/chemokine receptor system orchestrates T-cell trafficking, and expression of chemokine receptors defines T-cell maturation subsets with distinct functional properties and tropism for particular tissues and/or microenvironments.19-23 Little is known on how acute inflammatory conditions impact on homing properties of T-cell maturation subsets.
To begin to understand the dynamics with which inflammatory chemokine receptor expression is regulated, we compared cell-surface expression of CXCR3, CCR5, CX3CR1, and CXCR1 on CD8+ T cells directly ex vivo and after short-term stimulation (maximum 30 minutes) with PHA (or ionomycin; data not shown). Using either stimulus, a selective increase in cell surface expression of CXCR1 was observed within seconds/minutes of activation. While inhibition of new protein synthesis had no effect on this rapid up-regulation, inhibiting F-actin formation largely abolished it. Confocal fluorescence microscopy of permeabilized CD8+ T cells revealed a granular CXCR1 staining pattern that did not colocalize with either perforin, RANTES, or CD63 (lysosomes), while some cells showed discrete colocalization with GM130 (Golgi), β2-microglobulin (constitutive secretory pathway), and EEA1 (early endosomes). These data are evidence (1) for CXCR1 to be stored in distinct intracellular granules that form part of the regulated secretory pathway, and (2) for continuous low-level receptor synthesis, expression, and endocytic uptake in some of the cells.11 The regulated secretory pathway is characterized by mobilization of intracellular granules, and hence their exocytosis, upon engagement of specific cell surface receptors.24 In lymphocytes, the regulated release of preformed proteins has been associated with (1) cytotoxic function, as exocytosis of lysosomal granules containing perforin and granzymes is a major effector pathway of cytotoxicity both in vitro and in vivo,25 and (2) secretion of inflammatory chemokines, such as MIP-1α (CCL3), MIP-1β (CCL4), and RANTES (CCL5).16,26 As for RANTES, a distinct intracellular compartment, termed RANTES secretory vesicles, recently has been identified both directly ex vivo and in blasting CD8+ T cells.16 In their original report, Catalfamo et al describe preformed RANTES to become detectable in the supernatant of CD8+ T cells after 30 minutes of stimulation with phorbol myristate acetate (PMA) and ionomycin.16 By contrast, under similar experimental conditions, up-regulation of CXCR1 occurred within seconds/minutes, and at 30 minutes re-internalization started to become evident in most experiments. Differing kinetics of up-regulation versus release are in agreement with our observed lack of colocalization of CXCR1 with either perforin or RANTES, indicating CXCR1 to be stored in a distinct and highly mobilizable population of granules. Of importance, mobilization of CXCR1-containing granules rendered CD8+ T cells as sensitive as neutrophils (regarded widely as the prototypic IL-8–sensitive cell type) for IL-8–induced chemotaxis.27-30
Impact of IL-2, IFNγ, TNFα, and TcR cross-linking on expression of CXCR1 on CD8+ T cells. CD8+ T cells were incubated with increasing concentrations of IL-2, IFNγ, or TNFα (A) or with activating αCD3/αCD28 mAbs (B) and cell surface expression of CXCR1 assessed by FACScan analysis. None of the tested cytokines, nor αCD3/αCD28 mAb activation, induced significant changes in expression of CXCR1.
Impact of IL-2, IFNγ, TNFα, and TcR cross-linking on expression of CXCR1 on CD8+ T cells. CD8+ T cells were incubated with increasing concentrations of IL-2, IFNγ, or TNFα (A) or with activating αCD3/αCD28 mAbs (B) and cell surface expression of CXCR1 assessed by FACScan analysis. None of the tested cytokines, nor αCD3/αCD28 mAb activation, induced significant changes in expression of CXCR1.
Impact of neutrophil-, monocyte-, and DC-conditioned medium on expression of CXCR1 on CD8+ T cells. (A) LPS itself reduced expression of CXCR1 on CD8+ T cells by a mean of 4.4% (range, –9.7% to 0.32%) (n = 15). By contrast, supernatant from LPS-stimulated neutrophils induced its subset-selective up-regulation (n = 15). Filled symbols indicate data obtained after incubation with autologous supernatant; open symbols, data obtained after incubation with allogeneic supernatant. (B) Time course of neutrophil supernatant-induced CXCR1 up-regulation. Peak up-regulation was observed after 15 minutes of incubation; thereafter, receptor re-internalization occurred (representative of n = 2). (C) Phenotypic characterization of monocytes (top panel) and immature versus mature DCs (bottom panel) assayed for their release of mediators impacting on expression of CXCR1 by CD8+ T cells. (D) Neither monocyte- nor DC-derived supernatants induced up-regulation of CXCR1 on CD8+ T cells (n = 3, each tested with supernatant derived from 2 individual donors).
Impact of neutrophil-, monocyte-, and DC-conditioned medium on expression of CXCR1 on CD8+ T cells. (A) LPS itself reduced expression of CXCR1 on CD8+ T cells by a mean of 4.4% (range, –9.7% to 0.32%) (n = 15). By contrast, supernatant from LPS-stimulated neutrophils induced its subset-selective up-regulation (n = 15). Filled symbols indicate data obtained after incubation with autologous supernatant; open symbols, data obtained after incubation with allogeneic supernatant. (B) Time course of neutrophil supernatant-induced CXCR1 up-regulation. Peak up-regulation was observed after 15 minutes of incubation; thereafter, receptor re-internalization occurred (representative of n = 2). (C) Phenotypic characterization of monocytes (top panel) and immature versus mature DCs (bottom panel) assayed for their release of mediators impacting on expression of CXCR1 by CD8+ T cells. (D) Neither monocyte- nor DC-derived supernatants induced up-regulation of CXCR1 on CD8+ T cells (n = 3, each tested with supernatant derived from 2 individual donors).
TcR engagement leads to measurable granule exocytosis of both perforin storage granules as well as the RANTES secretory vesicles.16,26 By contrast, TcR engagement had no significant effect on up-regulation of CXCR1 in purified CD8+ T cells stimulated with anti-CD3 and anti-CD28 mAbs. The fact that PHA readily induced up-regulation of CXCR1, while αCD3/αCD28 mAb activation did not, is likely reflective of both qualitative and quantitative differences between these 2 activation modalities.31,32 Clearly, PHA has powerful, non–TcR-dependent activatory properties, as perhaps illustrated best by the fact that PHA at high concentrations can induce activation-induced cell death in a CD3-defective T-cell line.32 The CXCR1-storing subcellular compartment thus seems to be physically distinct and regulated independently from perforin granules and RANTES secretory vesicles.
The rapid, yet TcR-independent, up-regulation of CXCR1 by stimulated CD8+ T cells directed us to explore non–TcR-dependent pathways inducing mobilization of CXCR1-containing granules. Soluble factors such as IL-2, IFNγ, or TNFα had no effect on the expression level of CXCR1. We therefore hypothesized that inflammatory mediators released even before the production of the factors just mentioned, that is, within minutes of activating the innate arm of the immune system, may influence homing properties of CD8+ T cells by mobilizing CXCR1 to the cell surface. Given that neutrophils are among the first cells to migrate into sites of pathogen-induced injury, where they are activated and degranulate, we exposed CD8+ T cells to supernatant from neutrophils activated with the TLR4 agonist LPS for 20 minutes. In parallel, we also exposed CD8+ T cells to supernatant from LPS-activated monocytes and DCs. These experiments revealed the presence of one or several factor(s) released immediately after activation of neutrophils (but not monocytes or DCs) that signal(s) up-regulation of preformed CXCR1 in CD8+ T cells. Of interest, several in vivo models underscore the importance of a tightly regulated neutrophil–T-cell cooperation, and numerous reports, some describing viral infection models, provide evidence for in vivo IL-8 concentrations in humans similar to those found to induce migration of PHA-activated CD8+ T cells.33-35
Surprisingly, when analyzing maturation subsets of CD8+ T cells, CXCR1 up-regulation was most pronounced on the CM subset (CD45RA– CCR7+). Within the CD8+ T-cell subset enriched for naive cells (CD45RA+ CCR7+), up-regulation on only a small percentage (∼8%) of cells was observed, perhaps representing antigen-experienced cells reverting to that phenotype.36 In effector-memory type CD8+ T cells (CCR7–), up-regulation, although to a lesser extent than on CM cells, also was observed. Intriguingly, while the EMRA subset is enriched for CXCR1 in directly ex vivo analyzed CD8+ T cells,11,12 the CXCR1 expression level on activated CM CD8+ T cells was often similar or even higher. A similar pattern of subset-selective up-regulation was seen both in PHA-stimulated (or ionomycin-stimulated [data not shown]) CD8+ T cells, as well as in CD8+ T cells exposed to supernatant from activated neutrophils. When exposed to supernatant from LPS-activated neutrophils, up-regulation was transient, with CXCR1 expression levels returning nearly to baseline within 30 minutes of exposure. This indicates that neutrophils have the potential to rapidly induce transient IL-8 responsiveness in selected CD8+ T-cell subsets, with preferential induction of cell surface CXCR1 expression in CM cells. IL-8/CXCR1–mediated CD8+ T-cell homing thus seems to have (1) a constitutive and (2) a dynamic component to it: while expression of CXCR1 directly ex vivo identifies a subset of highly cytotoxic CD8+ T cells,11,12 activation-induced up-regulation preferentially targets CM CD8+ T cells with little immediate cytotoxic potential but a high proliferative capacity.4 Together, these data provide evidence that acute inflammatory conditions may not only rapidly attract highly cytotoxic CXCR1+ CD8+ T cells but also significantly skew steady state homing properties of CM CD8+ T cells. The capability of CD8+ T cells to rapidly redirect homing toward sites of IL-8 production, that is, sites of innate immune system activation, possibly increases their odds of interacting with cognate antigen.11,17,18
In summary, our results indicate that up-regulation of a preformed pool of highly mobilizable CXCR1 allows CD8+ T cells and, in particular, CM CD8+ T cells, to respond to IL-8 with high sensitivity. By closely integrating innate and acquired immunity, such flexibility of the migratory properties of CD8+ T cells may significantly impact on immune responses in vivo.
Prepublished online as Blood First Edition Paper, August 4, 2005; DOI 10.1182/blood-2005-04-1366.
Supported by a Swiss Clinicians Opting for Research (SCORE) grant (3200B0/103253/1 and 3200B0/103254/1) from the Swiss National Science Foundation (O.G., C.H., A.M., C.E.), the Nora van Meeuwen-Haefliger Foundation (C.H.), and the Altana Foundation (C.H.).
O.G. designed and performed most experiments, analyzed data, and helped write the report; A.M. designed and performed experiments and analyzed data; C.E. designed and performed experiments and analyzed data; and C.H. initiated the study, designed and supervised the research, analyzed data, and wrote the report.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Andrew D. Luster, Juerg A. Schifferli, and Lukas Hunziker for advice and critical review of the manuscript.