Abstract
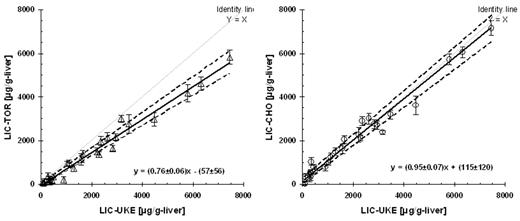
Assessing iron chelator efficacy in clinical trials requires standardization of liver iron concentration measurements. In a blinded round-robin test, liver iron concentration (LIC) was measured within 1 month in 18 patients with thalassemia or sickle cell disease and in 10 normal subjects at the 3 SQUID biosusceptometer systems located in Hamburg (UKE), Torino (TOR), and Oakland (CHO). Mean LIC values (range: normal up to 8000 μg/g-liver) were determined from 5 separate vertical scans. The observed intrasite precision (SD of Altman-Bland differences from duplicate measurements with repositioning) was found in the expected range of ±130 (UKE), ±200 (TOR), and 220 μg/g-liver (with 3 operators involved at CHO). Prediction of LIC at TOR and CHO in comparison with UKE was very good, with coefficients of determination between sites of R2 = 0.97, resulting in intersite standard deviations (SD) of 247 and 326 μg/g-liver, respectively. Differences of 24, 20, and 5% were noted for the comparisons UKE-TOR, CHO-TOR, and UKE-CHO, respectively (see Fig. 1). This suggests the need for further standardization of analysis methods. In conclusion, we found that intra-site precision was within an acceptable range for repeat measurements in the majority of iron overloaded subjects. Prediction of liver iron concentration at the three centers was highly correlated.
Agreement (Passing-Bablok regression) between liver iron measurements (mean LIC from 2 positions) by the SQUID biosusceptometer system in Hamburg (LIC-UKE) and the systems in Torino (LIC-TOR) and Oakland (LIC-CHO).
Agreement (Passing-Bablok regression) between liver iron measurements (mean LIC from 2 positions) by the SQUID biosusceptometer system in Hamburg (LIC-UKE) and the systems in Torino (LIC-TOR) and Oakland (LIC-CHO).
Author notes
Corresponding author