Abstract
Background: Erythropoiesis-stimulating proteins (ESPs) are commonly used to treat anemia in patients with cancer receiving chemotherapy. While it is well established that these agents increase hemoglobin levels and reduce the incidence of red blood cell (RBC) transfusions, the effects of ESPs on overall survival are inconclusive, with reports of both positive and negative impacts on survival. A recent Cochrane meta-analysis of double-blind randomized placebo-controlled trials (Bohlius et al, 2005) determined that the hazard ratio for overall survival associated with recombinant human erythropoietin (rHuEPO) in 19 trials with 2865 patients was 0.81 (95% CI: 0.67 to 0.99) for adjusted data and 0.84 (95% CI: 0.69, 1.02) for unadjusted data, suggesting no negative impact on survival. The objective of the present investigation was to conduct an analysis with identical methodology to that reported by Bohlius et al, but including trials of darbepoetin alfa, which were not part of the Cochrane meta-analysis. Trial inclusion was limited to those that addressed patient populations with chemotherapy-induced anemia (studies by Henke et al and Leyland-Jones et al, which reported decreased survival in patients treated with rHuEPO, were not included in this analysis as they were not conducted in anemic patients).
Methods: Data from 4 randomized, placebo-controlled clinical trials of darbepoetin alfa in chemotherapy-induced anemia were analyzed using meta-analysis methodology as performed in the Cochrane analysis. The primary outcomes included hematological response (hemoglobin increase of ≥ 2 g/dL); change in hemoglobin from baseline; RBC transfusions; and overall survival. Survival was also analyzed by combining the 4 darbepoetin alfa trials with the rHuEPO trials used in the Cochrane analysis.
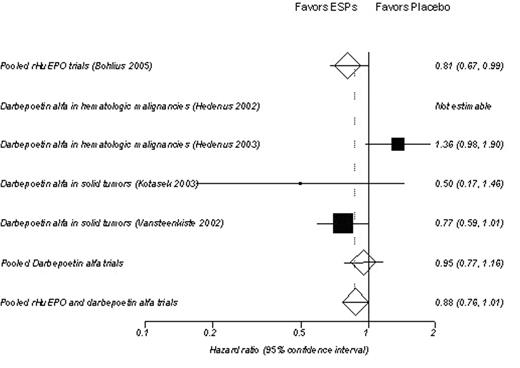
Results: In the meta-analysis of 4 trials (N=759), darbepoetin alfa significantly reduced the risk of receiving a RBC transfusion (RR 0.69; 95% CI 0.59–0.81; p <0.0001). Darbepoetin alfa-treated patients were significantly more likely to have a hematological response (relative risk [RR] 3.30; 95% CI 2.53–4.32; p <0.0001) and a hematopoietic response (RR 2.64; 95% CI 2.17–3.21; p <0.0001). As shown in the figure below, darbepoetin alfa was not likely to be associated with a negative impact on survival in the pooled results of the 4 trials (hazard ratio [HR] 0.95; 95% CI 0.77–1.16; p=0.62). When the meta-analysis was conducted including all of the rHuEPO trials combined with the darbepoetin alfa trials, the overall HR was 0.88 (95% CI: 0.76–1.01).
Conclusions: The results of this meta-analysis of randomized placebo-controlled trials demonstrate that darbepoetin alfa treatment is associated with reduced risk of transfusion and improved hematological and hematopoietic responses and negative effect on survival in patients with cancer receiving chemotherapy is unlikely. These results are consistent with the conclusions of the Cochrane meta-analysis of rHuEPO trials.
Author notes
Corresponding author