Abstract
Background: The recommended imatinib starting dose is 400mg/day for chronic myelogenous leukemia (CML) patients in chronic phase and 600mg/day for patients in accelerated phase or blast crisis. Some studies have reported improved response rates and progression-free survival in chronic phase patients receiving higher imatinib doses. However, little information is available regarding tolerability of higher doses and the actual initial dosing of imatinib dispensed in the clinical setting. To investigate initial imatinib dosing over time, we conducted a retrospective cohort study using the HealthCore Integrated Database. The data used for this analysis captured pharmacy and medical encounters for a base population of approximately 13 million covered lives.
Methods: We identified all patients with an ICD-9 coded diagnosis of CML (205.1) and at least one prescription for imatinib from January 1, 2001 through December 31, 2004. Using the pharmacy database, we identified the first imatinib prescription and calculated the daily dose of that first prescription (based on number of pills dispensed, dose per pill and prescription days supply).
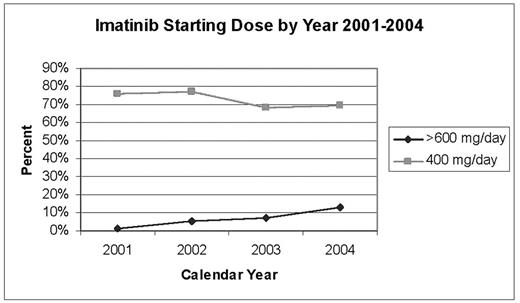
Results: A total of 486 patients were exposed to imatinib (N=40 patients with starting dose <400 mg/day; N=356 patients with starting dose 400 mg/day; N=5 patients with starting dose 500mg/day, N=52 patients with starting dose 600 mg/day; and N=33 patients with starting dose >600 mg/day). Figure 1 presents initial dosing by calendar year for patients starting at 400mg/day and >600 mg/day.
Imatinib Starting Dose by Year 2001–2004
Among patients receiving imatinib >600mg/day, 15% (95% confidence interval (CI) 5.8–30.4%) had a subsequent dose decrease (defined as a decrease of at least 100 mg/day). Among patients starting imatinib 600mg/day, 22.6% had a dose decrease (95% CI 12.9–35.3%), and 5.8% of the 361 patients receiving imatinib 400mg/day had a dose decrease (95% CI 3.7–8.6%).
Conclusions: These data indicate that a greater proportion of CML patients now receive high starting imatinib doses, potentially due to the fact that more aggressive dosing is used in chronic phase patients. Although phase of disease has not been verified at this point, medical record review is underway to determine imatinib indication and reasons for dose changes. Between 15–20% of patients receiving high starting doses of ≥ 600 mg/day underwent dose reduction of at least 100 mg/day.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal