Abstract
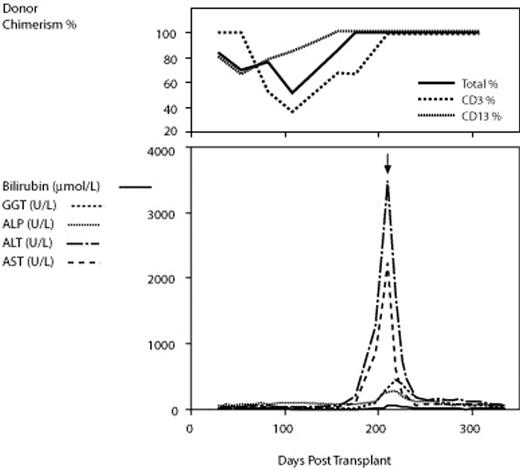
Chronic graft-versus-host disease (cGVHD) of the liver mimicking autoimmune hepatitis is an increasingly well recognized complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT). The target antigens and pathogenesis of this complication are poorly understood. We describe a case of hepatitic cGVHD in which cytochrome P450 1A2 (CYP1A2) was identified as a target antigen. A 47 year old male received an HLA matched sibling transplant for acute myeloid leukemia with normal cytogenetics following 3 cycles of idarubicin, cytarabine and etoposide remission induction therapy. Conditioning was with fludarabine 50mg/m2 and cyclophosphamide 120 mg/m2. Both donor and recipient were CMV IgG positive. Standard cyclosporin and short course methotrexate GVHD prophylaxis was used. Due to mixed lymphoid chimerism, cyclosporin was tapered from D94, and a donor leukocyte infusion (DLI) given at D124. One month following DLI, concomitant with attainment of 100% donor lymphoid chimerism, the patient developed florid oral changes of cGVHD and striking elevation of hepatic transaminases (ALT and AST peaked at 3604 (R 0–55 IU/L) amd 2241 (R 0–45 IU/L) respectively) with relatively minor elevation in hepatic ductal markers (GGT 327, R 0–60 IU/L). Liver biopsy was consistent with autoimmune hepatitis. Multiple recipient serum samples at this time demonstrated the presence of a high titre anti-liver microsomal (LM) antibody (titre 1:1280 by indirect immunofluorescence) reminiscent of hydralazine-induced hepatitis and immunofixed an antigen of approximately 51 kDa by immunoblotting using human liver homogenate. Neither pre-transplant recipient nor donor sera were reactive. Immunoblotting for a panel of 13 CYP450 antigens demonstrated that the antibody was specific for CYP1A2. This specificity was confirmed with blocking and inhibition experiments. Sequencing of the entire coding region of the CYP1A2 gene did not identify any coding variants present in the recipient but not the donor. Oral prednisolone resulted in brisk improvement in hepatitis and oral cGVHD, and was ceased at 12 months post transplant. The patient remains well with no further GVHD over 2 years post transplant. This is the first description of a target antigen in hepatitic cGVHD. The mechanism is unclear, but does not appear to be a CYP1A2 polymorphism acting as a minor histocompatibility antigen. Induction of hepatic autommune-like responses have been described in liver transplant recipients receiving calcineurin inhibitors, and it is possible that cyclosporin facilitated an autoimmune-type response in this case. Characterisation of autoantibody responses in additional cases of hepatitic cGVHD will be of great interest.
Author notes
Corresponding author