Abstract
Inflammatory responses are controlled by T helper 1 (Th1) lymphocytes. An important function of this polarity is the ability of T cells to traffick appropriately in vivo. This differential trafficking is dependent upon the binding of P-selectin glycoprotein ligand-1 to P- and E-selectin on inflamed endothelium as well as the expression of specific chemokine receptors. Here we show that in the absence of T-box expressed in T cells (T-bet), selective migration of T cells in vivo is completely abrogated and that T-bet regulates the binding of CD4+ T cells to P-selectin. T-bet is also required for the expression of the chemokine receptor CXCR3. Thus, T-bet controls Th1-cell migration to inflammatory sites, which has fundamental consequences for the control of immunologic disease.
Introduction
CD4+ T-cell phenotypes can be defined according to the pattern of cytokines secreted.1 Type 1 immunity relies on the generation of T helper 1 (Th1) cells, whose hallmark cytokine is interferon-gamma (IFN-γ).2 Th2 cells produce a different spectrum of cytokines including interleukin-4 (IL-4) and interleukin-5 (IL-5) and are important in the generation of type 2 immunity.1 T-box expressed in T cells (T-bet) is a T-box transcription factor essential to Th1-cell generation and effector function.3 Recently, the expression of T-bet has also been described in natural killer (NK) cells, dendritic cells, and CD8+ cells.4-6 T-bet directly transactivates IFNG in CD4+ T cells and increases the expression of the IL-12 receptor β chain on activated cells. Indeed, a positive feedback loop is observed, since signal transducer and activator of transcription 1 (STAT1) downstream of the IFN-γ receptor activates T-bet expression, which further serves to increase IFN-γ secretion.7,8 The strong transactivation of IFN-γ by T-bet makes it difficult to dissect which genes are targets of T-bet and which lie downstream of IFN-γ, as this cytokine is known to induce the expression of many hundreds of genes. When overexpressed in fully polarized Th2 cells, T-bet can reverse their lineage commitment and induce Th1-specific genes, particularly IFN-γ and its known targets.3,4,9 Animals deficient in T-bet demonstrate a marked reduction in severity to a number of inflammatory diseases, including systemic lupus erythematosus (SLE), colitis, diabetes, hepatitis, and arthritis, with a number of different abnormalities in effector function described in CD4+ and CD8+ cells.10-14 However, it has been difficult to dissect the precise mechanisms of this protection, as many cell types in the immune system express T-bet. Furthermore, given its function as a master regulator of T-cell lineage commitment, it is likely to direct the transcription of many genes involved in both cytokine production and other effector pathways.
Effector Th1 and Th2 cells differ profoundly in their migratory properties.15-18 Th1 cells migrate to sites of inflammatory immune responses, whereas Th2 cells migrate predominantly to mucosal sites in the settings of allergy or helminth infection. E- and P-selectin ligands are expressed mainly on Th1 cells, being absent on naive T cells and greatly reduced on Th2 cells when polarized in vitro.19 However a recent report has shown binding of selectin–immunoglobulin (Ig) fusion proteins in vivo to cells that express cytokines other than IFN-γ,20 suggesting additional complexity. The initial step in Th1-cell recruitment is binding to P- and E-selectin expressed on activated vascular endothelium, interactions mediated mostly by the leukocyte ligand P-selectin glycoprotein ligand-1 (PSGL-1).21,22 Of interest, PSGL-1 undergoes further enzymatic posttranslational modification including core-2 glycosylation, fucosylation, and tyrosine sulfation, all of which are required to produce a functional selectin ligand.23 Up-regulation of these selectin ligands has thus far been ascribed to the actions of IL-12 on activated lymphocytes, largely in a STAT4-dependent manner.19,24 Chemokines also play critical roles in T-cell recruitment by mediating both the transition from selectin-dependent rolling to integrin-mediated firm adhesion,25 as well as the subsequent locomotion and transendothelial migration of T cells.26 Differential expression of chemokine receptors plays a key part in the process of T-cell migration to inflammatory sites. The chemokine receptors CXCR3 and CCR5 are preferentially expressed on Th1 rather than Th2 T cells and, in addition to P- and E-selectin, are thought to be responsible for the specific recruitment of Th1 cells to inflammatory sites.27,28 Other chemokine receptors (eg, CCR4, CCR10, and CCR9) have also been described to mediate tissue-specific homing, although not necessarily in a Th1-specific manner.29 In contrast, other lymphocyte adhesion molecules implicated in the adhesion cascade, in particular the β1 and β2 integrins, leukocyte function antigen 1 (LFA-1), and very late antigen 4 (VLA-4), have not been implicated in the specific recruitment of Th1 cells, rather of activated lymphocytes in general. The regulation of cellular trafficking is therefore crucial to an effective immune response.
The resistance of T-bet–deficient (T-bet–/–) mice to inflammatory diseases is characterized by a lack of T-cell infiltration at pathologic sites.11,12,30 For example, adoptively transferred T-bet–/– CD4+ T cells do not migrate to the intestine in severe combined immunodeficient (SCID) mice.11 However, most of the disease models studied in this context have relied critically upon intact effector function for expression of disease and as such it has been impossible to study T-cell trafficking in isolation. These observations led us to investigate the hypothesis that, in addition to cytokine secretion, T-bet directly controls a specific T-cell migratory program.
Materials and methods
Mice
T-bet–/– mice10 were backcrossed onto the BALB/c strain for more than 10 generations and crossed onto the DO11.10 T-cell receptor (TCR)–transgenic (Tg) strain. All DO11.10 mice and DO11.10 × T-bet–/– mice were heterozygous for the TCR transgene. T-bet CD2-Tg mice were generated as described and maintained on the BALB/c background.31 BALB/c IFN-γ–/– mice (Jackson Laboratories, Bar Harbor, ME) were crossed with the T-bet–/– strain to generate the T-bet–/– × IFN-γ–/– strain. Mice were housed in a specific pathogen-free barrier unit at the Harvard School of Public Health. Handling of mice and experimental procedures were in accordance with institutional requirements for animal care and use. Mice were used at 4 to 6 weeks of age.
Cell preparation
Primary lymph node (LN) CD4+ T cells were prepared by positive selection with magnetic beads (Miltenyi, Auburn, CA) as previously described.4 T cells (> 95% pure as assessed by flow cytometry) were stimulated with plate-bound anti-CD3 and anti-CD28 antibodies (2 μg/mL; Pharmingen, San Diego, CA) or with ovalbumin (OVA) peptide and syngeneic splenocytes in experiments using cells from DO11.10 animals. Th0-, Th1-, and Th2-polarizing conditions were as previously described.4 T cells were used at days 4 to 5 following primary stimulation.
Flow cytometry
Antibodies used were from Pharmingen (BD Biosciences, San Diego, CA) except the anti-CXCR3 antibody, which was from R&D Systems (Minneapolis, MN). Stained cells were acquired on a FACS Calibur (BD Biosciences, San Diego, CA) and analyzed with CellQuest software.
Adoptive transfer
Adoptive transfers were performed as described.16 Briefly, DO11.10 and DO11.10 × T-bet–/– CD4+ T cells were activated with OVA peptide (Sigma-Genosys, St Louis, MO) and BALB/c splenocytes under Th1- or Th2-polarizing conditions. Five days after activation, 20 × 106 T cells were transferred intravenously into unirradiated BALB/c mice. Twenty-four hours following transfer, recipient mice were injected intraperitoneally with OVA in incomplete Freund adjuvant (IFA) or phosphate-buffered saline (PBS) in IFA. Forty-eight hours later, spleen, mesenteric, and inguinal LNs were harvested and peritoneal lavage was performed. Analysis of in vivo trafficking was performed by staining cells with anti–KJ1-26 (anticlonotypic) and anti-CD4 antibodies and by determining the number and percentage of cells that were double positive for these markers at the various anatomic locations.
Flow-chamber assays
The interactions of CD4+ T cells with immobilized P- and E-selectin were examined under conditions of fluid shear in a parallel plate flow chamber as previously described.19 For assessment of chemokine-triggered adhesion to endothelial cells (ECs), cardiac ECs were isolated and used as previously described.32 All P-selectin interactions were blocked by the addition of an anti–PSGL-1 polyclonal antibody to the T cells (not shown).
Sulfation assays
Assays for tyrosine sulfation were performed as described.46 Briefly, CD4+ T cells from wild-type (WT) and T-bet–/– mice were activated under Th1-polarizing conditions and transferred to sulfate-free medium at day 3 and grown in the presence of [35S]-sulfate. Twenty-four hours later, the cells were harvested, and PSGL-1 was immunoprecipitated with an anti–PSGL-1 antibody (P2, from Dr Bruce Furie, Beth Israel Deaconess Medical Center, Boston, MA). The cells were then divided into aliquots that were either untreated or treated with the following enzymes: N-glycosidaseF (to remove N-glycans) or neuroaminidase and O-glycosidase (to remove O-glycans). PSGL-1 was then resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and sulfation was visualized by autoradiography.
Real-time polymerase chain reaction (PCR)
Total RNA was extracted from CD4+ T cells with TRIzol solution (Invitrogen, Carlsbad, CA) and reverse transcription carried out with the iScript cDNA synthesis kit (Biorad, Hercules CA). The amount of amplicon generated was monitored with an Applied Biosystems 7700 (Applied Biosystems, Foster City, CA) apparatus. A specific probe labeled with both a reporter and a quencher dye was added into the Taqman PCR mix (Applied Biosystems) at the beginning of the reaction. The sequences of the primers and Taqman probes used in this study are available on request. Cycle number was normalized to β-actin.
Retroviral transduction
T-bet and control retroviruses were produced and titred as described.3 Briefly, CD4+ T cells from WT, T-bet–/–, and T-bet–/– × IFN-γ–/– mice were activated with anti-CD3 and anti-CD28 and spin infected with retrovirus after 36 hours in the presence of 4 μg/mL polybrene. Fresh medium was added 24 hours later and the cells were sorted by green fluorescence protein (GFP) expression and/or used for flow cytometric and transwell analysis at 5 days. The transduction efficiencies were typically 40% to 60% as assessed by GFP expression, and this level of expression was the same between empty and T-bet–expressing retrovirus.
Transwell assays
Transwell assays were performed in 24-well plates with 5-μm transwells that had been blocked with fibronectin (Corning, Corning, NY). CD4+ T cells (3 × 105) were placed in the upper chamber, and a dilution of chemokine (Peprotech, Rocky Hill, NJ) was placed in the lower chamber. The cells were placed at 37°C for 4 hours and transmigration was enumerated by flow cytometry. For the transwell experiments with the retrovirally transduced cells, transmigrated cells were counted by gating on live cells that were also positive for GFP.
Results
Reduced in vivo migration of T cells from T-bet–/– mice
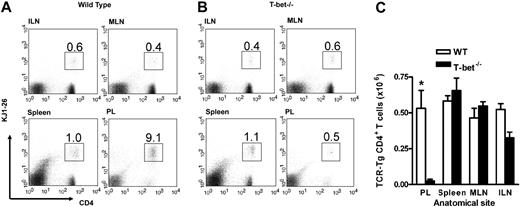
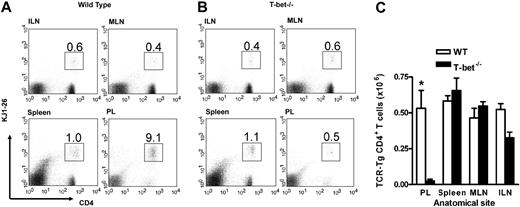
In order to establish whether T-cell migration was impaired in these mice, we assessed the recruitment of adoptively transferred CD4+ T cells to a peripheral inflammatory site in vivo. First, antigen-specific TCR-Tg T cells (DO11.10 and DO11.10 × T-bet–/–) were generated in vitro and assessed for the expression of certain well-described adhesion molecules. T-bet–/– Th1 and Th2 cells demonstrated similar levels of expression of PSGL-1, CD43a, CD43c, and L-selectin when compared with WT cells (Supplemental Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Cells generated in this way were injected into unirradiated syngeneic WT mice followed by induction of peritoneal inflammation with or without their specific T-cell antigen (ovalbumin).16 This model allows assessment of trafficking of the transferred cells independent of any defect in effector function that may be present in the T-bet–/– T cells and has been shown to be dependent upon both selectin16 and chemokine-mediated interactions.34 Transfer of DO11.10 and DO11.10 × T-bet–/– CD4+ T cells activated under either Th1- or Th2-polarizing conditions revealed unimpaired trafficking of these cells to secondary lymphoid organs in the absence of T-bet since the percentage of transferred T cells in spleen and lymph nodes was similar (Figures 1 and S1). In fact, the numbers of adoptively transferred cells were marginally higher in the spleen in the absence of T-bet (Figure 1A,C). Consistent with our previously published data,16 there was substantial trafficking of transferred DO11.10 CD4+ T cells activated under Th1 conditions to the peritoneum (Figure 1B-C), whereas this migration was abrogated in DO11.10 × T-bet–/– CD4+ T cells (Figure 1C), demonstrating a profound defect in proinflammatory T-cell trafficking. This defect was similar in the presence or absence of antigen in the peritoneum, as we have previously demonstrated.16
T-bet–/– T cells exhibit impaired binding to P-selectin but not E-selectin
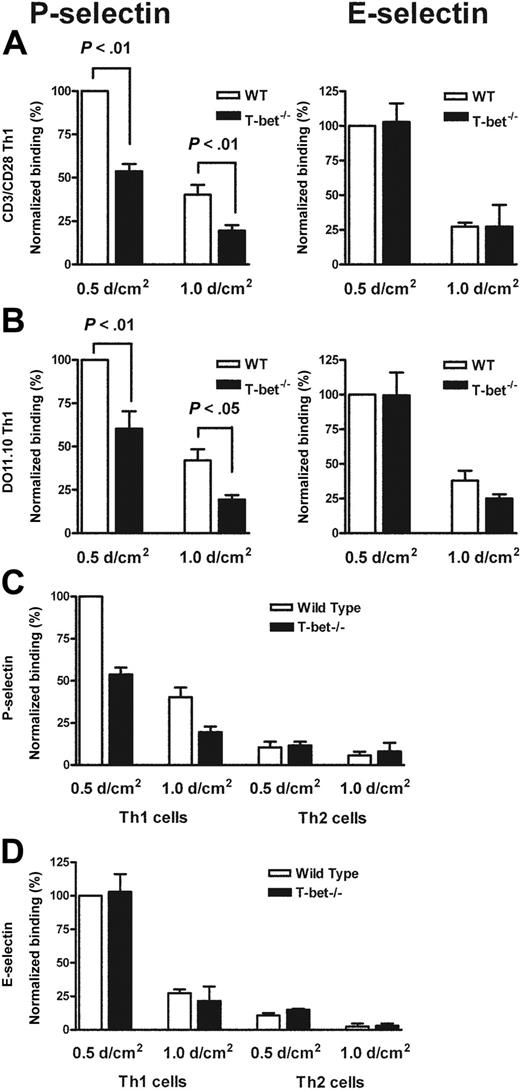
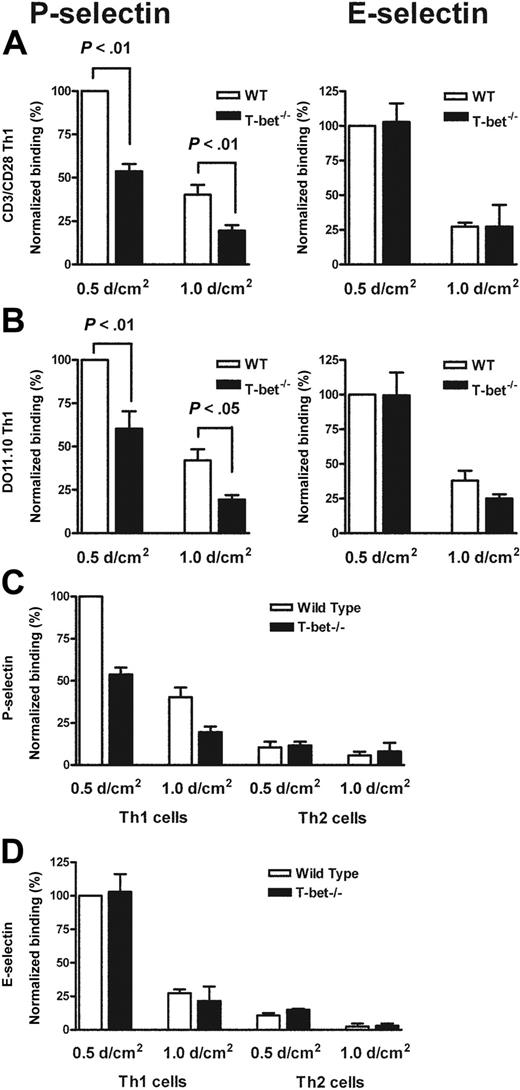
Having observed a defect in migration of T cells to a peripheral inflammatory site in vivo, we postulated that T-bet–/– T cells might exhibit reduced interactions either with E- and/or P-selectin, or with specific Th1 chemokines. In order to assess this further, we assessed the binding of primary WT and T-bet–/– CD4+ T cells that had been activated by ligation of TCR and CD28 under Th1- or Th2-polarizing conditions to immobilized recombinant P- and E-selectin under conditions of physiologic laminar shear flow. In the absence of T-bet, Th1 cells displayed markedly reduced binding to P-selectin but normal binding to E-selectin (Figure 2A). Th2 cells showed minimal binding to either selectin regardless of the presence or absence of T-bet (Figure 2C-D). These findings were recapitulated when CD4+ T cells from TCR-Tg (DO11.10 vs DO11.10 × T-bet–/–) mice were activated by antigen and antigen-presenting cells (Figure 2B).
In vivo trafficking of adoptively transferred WT and T-bet–/– antigen-specific T cells activated under Th1-polarizing conditions. (A-B) Flow cytometric analysis of WT (DO11.10) and T-bet–/– (DO11.10 × T-bet–/–) CD4+ T cells. Percentages of cells positive for CD4 and the clonotypic antibody KJ1-26 are indicated in various secondary lymphoid organs and in inflamed peritoneum (ILN indicates inguinal lymph nodes; MLN, mesenteric lymph nodes; and PL, peritoneal lavage). (C) Cell counts of secondary lymphoid organs and peritoneal lavage from BALB/c mice adoptively transferred with DO11.10 (WT) and DO11.10 × T-bet–/– (T-bet–/–) CD4+ T cells (mean ± SEM, *P < .001) activated with OVA peptide and mitomycin C–treated syngeneic splenocytes. □ indicates WT; ▪, T-bet–/–.
In vivo trafficking of adoptively transferred WT and T-bet–/– antigen-specific T cells activated under Th1-polarizing conditions. (A-B) Flow cytometric analysis of WT (DO11.10) and T-bet–/– (DO11.10 × T-bet–/–) CD4+ T cells. Percentages of cells positive for CD4 and the clonotypic antibody KJ1-26 are indicated in various secondary lymphoid organs and in inflamed peritoneum (ILN indicates inguinal lymph nodes; MLN, mesenteric lymph nodes; and PL, peritoneal lavage). (C) Cell counts of secondary lymphoid organs and peritoneal lavage from BALB/c mice adoptively transferred with DO11.10 (WT) and DO11.10 × T-bet–/– (T-bet–/–) CD4+ T cells (mean ± SEM, *P < .001) activated with OVA peptide and mitomycin C–treated syngeneic splenocytes. □ indicates WT; ▪, T-bet–/–.
Mechanistic analysis of the selectin binding properties of WT, T-bet–/–, DO11.10, and DO11.10 × T-bet–/– primary CD4+ T cells under conditions of shear flow. (A-B) Interaction of primary CD4+ T cells of different genotypes with immobilized P-selectin (left columns) and E-selectin (right columns) under conditions of laminar shear stress as indicated. (A) Wild-type (WT, □) and T-bet–/– (▪) CD4+ Th1 cells activated by polyclonal stimulation with CD3 and CD28 antibodies. (B) CD4+ T cells generated from DO11.10 and DO11.10 × T-bet–/– TCR-Tg animals and activated in an antigen-specific manner. (C-D) Interaction of WT (□) and T-bet–/– (▪) Th1 and Th2 cells, activated with plate-bound CD3 and CD28 antibodies, with (C) immobilized P-selectin and (D) E-selectin under conditions of laminar shear stress as indicated. All data are expressed as mean ± SEM.
Mechanistic analysis of the selectin binding properties of WT, T-bet–/–, DO11.10, and DO11.10 × T-bet–/– primary CD4+ T cells under conditions of shear flow. (A-B) Interaction of primary CD4+ T cells of different genotypes with immobilized P-selectin (left columns) and E-selectin (right columns) under conditions of laminar shear stress as indicated. (A) Wild-type (WT, □) and T-bet–/– (▪) CD4+ Th1 cells activated by polyclonal stimulation with CD3 and CD28 antibodies. (B) CD4+ T cells generated from DO11.10 and DO11.10 × T-bet–/– TCR-Tg animals and activated in an antigen-specific manner. (C-D) Interaction of WT (□) and T-bet–/– (▪) Th1 and Th2 cells, activated with plate-bound CD3 and CD28 antibodies, with (C) immobilized P-selectin and (D) E-selectin under conditions of laminar shear stress as indicated. All data are expressed as mean ± SEM.
T-bet CD2-Tg mice exhibit increased selectin binding under Th2 polarization conditions
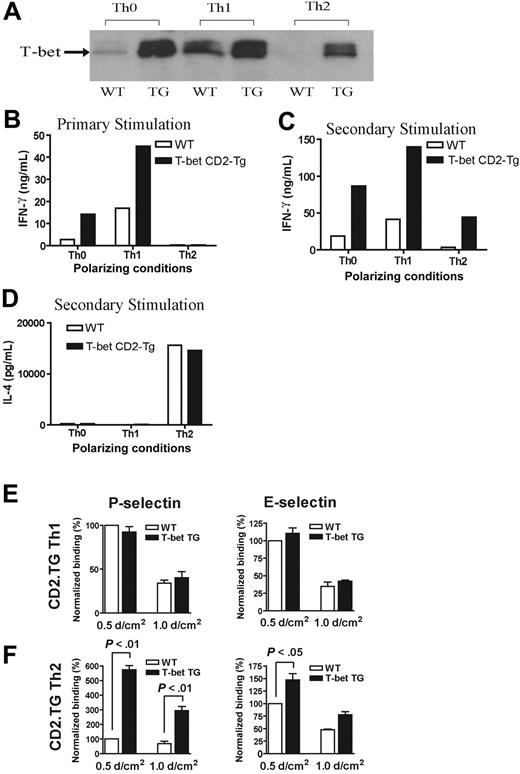
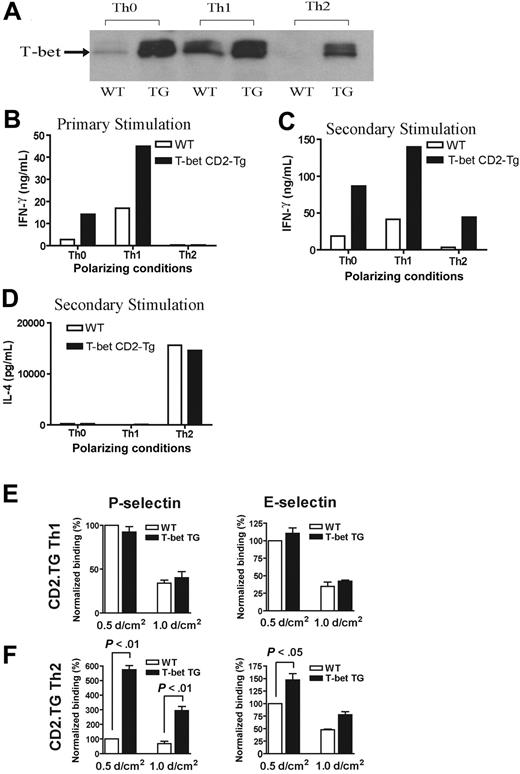
In order to explore further the relationship between alterations in T-bet expression and T-cell selectin binding, we analyzed CD4+ T cells purified from transgenic mice engineered to express T-bet under the control of the human CD2 promoter. The mice were produced by standard techniques and were bred onto the same background as all of the other transgenic strains used in this study (BALB/c). These mice (T-bet CD2-Tg) expressed T-bet in a manner independent of their cytokine phenotype. Thus, CD2-Tg CD4+ T cells activated under Th2-polarizing conditions expressed significant levels of T-bet unlike their WT littermate controls (Figure 3A). As would be predicted, CD2-Tg T cells produce more IFN-γ under unskewed (Th0) or Th1 conditions during primary activation, but ectopic T-bet expression was unable to drive IFN-γ production under Th2-polarizing conditions (Figure 3B) during primary stimulation. However, T-bet expression did induce significant IFN-γ production under Th2 conditions after secondary stimulation (Figure 3C). There was no difference in IL-4 secretion between the T-bet CD2-Tg T cells and WT T cells after secondary stimulation (Figure 3D). Of interest, whereas Th1-cell binding to P- or E-selectin was unaffected by the presence of the T-bet transgene (Figure 3E), Th2 cells from T-bet CD2-Tg mice showed a marked increase in P-selectin binding (Figure 3F), suggesting that T-bet directly induces selectin ligand expression. A small increase in binding of Th2 cells to E-selectin was also noted at low shear, although this was considerably less marked than that observed with P-selectin. There was no significant difference observed in the binding of T-bet CD2-Tg T cells to either selectin activated under unskewed conditions (not shown).
Impairment of P-selectin binding is due to a reduction in tyrosine sulfation of PSGL-1
As binding to E-selectin was normal (a process critically dependent on fucosylation35 ), it was unlikely that carbohydrate modification of PSGL-1 was responsible for the deficit observed in P-selectin binding. The alterations in binding to P-selectin occurred in the context of normal surface expression of PSGL-1 and the core-2–dependent epitope CD43a, as assessed by flow cytometry (Figure 4A).24,36 Alterations in P-selectin binding of T cells from the T-bet CD2-Tg mice were also not likely due to changes in the cell-surface markers CD43a, CD43c, or PSGL-1 (Table 1). Expression of FucTVII mRNA was also similar for WT and T-bet–/– T cells, as assessed by real-time reverse-transcriptase (RT)–PCR, thus making a defect in either of these enzymes less likely (Figure 4B). We therefore tested whether defects in tyrosine sulfation of PSGL-1 were present in T-bet–/– cells to account for the specific reduction in binding to P-selectin.37 Expression of tyrosyl protein sulfotransferase-1 (TPST-1) mRNA was the same in both WT and T-bet–/– Th1 cells, whereas a 50% reduction in the level of TPST-2 mRNA was observed in T-bet–/– Th1 cells (Figure 4C). There was no detectable increase in the mRNA levels of FucTVII in CD4+ T cells from the T-bet CD2-Tg mice (Figure S2), although there was an increase of TPST-1 and TPST-2 mRNA (Figure S3). Furthermore, we found that T-bet–/– CD4+ Th1 cells showed a substantial reduction of PSGL-1 tyrosine sulfation (Figure 4D). This reduction was independent of sulfation of glycosyl residues, because the difference in 35S incorporation persisted even after removal of both O- and N-linked glycans (Figure 4F). These differences in tyrosine sulfation occurred in the context of similar levels of PSGL-1 between WT and T-bet–/– Th1 cells (Figure 4A,E). Furthermore, densitometry showed some correlation between the reduction in sulfation and the levels of mRNA of TPST-2 (Figure 4C-D). Chromatin immunoprecipitation (IP) experiments failed to detect direct binding of T-bet to the promoter of either TPST-1 or TPST-2 when the analysis was performed with primers spanning the first kilobase of genomic DNA around the putative transcription start site (G.M.L. and L.H.G., unpublished observations, January 2005).
Characterization of a transgenic mouse expressing T-bet under the human CD2 promoter. (A) Western blot of T-bet expression by CD4+ T cells from either WT BALB/c (WT) or T-bet CD2-transgenic (TG) mice (also on the BALB/c background) activated with anti-CD3 and anti-CD28 antibodies under different polarizing conditions. (B-D) Cytokine profiles measured by enzyme-linked immunosorbent assay (ELISA) secreted by CD4+ T cells activated under different polarizing conditions. (B) IFN-γ production during primary stimulation from WT (□) and TG (▪) T cells. (C) IFN-γ production during secondary stimulation from WT and TG T cells. (D) IL-4 production during secondary stimulation from WT and TG T cells. (E-F) Interaction of WT and TG CD4+ T cells with immobilized P-selectin (left columns) and E-selectin (right columns) under conditions of laminar shear stress as indicated. Cells were by stimulated with plate-bound CD3 and CD28 antibodies in the presence of appropriate skewing cytokines. (E) CD4+ Th1-cell interactions. (F) Th2-cell interactions. All data are expressed as mean ± SEM.
Characterization of a transgenic mouse expressing T-bet under the human CD2 promoter. (A) Western blot of T-bet expression by CD4+ T cells from either WT BALB/c (WT) or T-bet CD2-transgenic (TG) mice (also on the BALB/c background) activated with anti-CD3 and anti-CD28 antibodies under different polarizing conditions. (B-D) Cytokine profiles measured by enzyme-linked immunosorbent assay (ELISA) secreted by CD4+ T cells activated under different polarizing conditions. (B) IFN-γ production during primary stimulation from WT (□) and TG (▪) T cells. (C) IFN-γ production during secondary stimulation from WT and TG T cells. (D) IL-4 production during secondary stimulation from WT and TG T cells. (E-F) Interaction of WT and TG CD4+ T cells with immobilized P-selectin (left columns) and E-selectin (right columns) under conditions of laminar shear stress as indicated. Cells were by stimulated with plate-bound CD3 and CD28 antibodies in the presence of appropriate skewing cytokines. (E) CD4+ Th1-cell interactions. (F) Th2-cell interactions. All data are expressed as mean ± SEM.
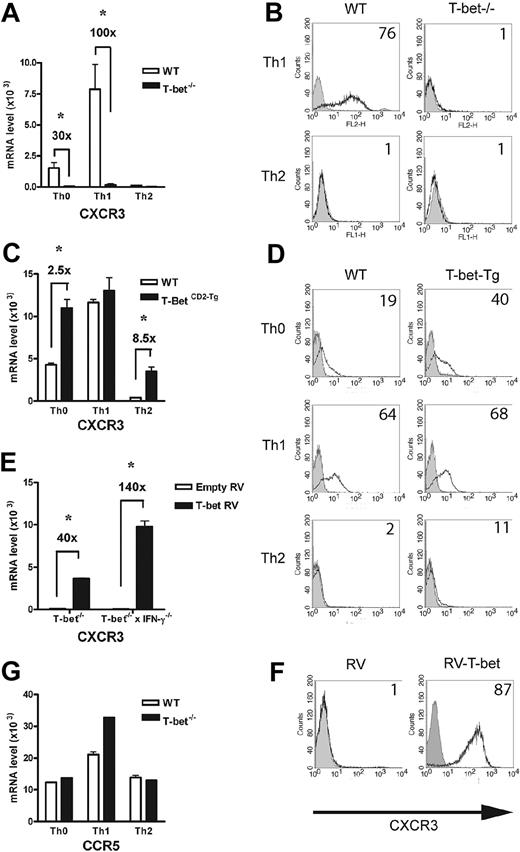
Expression and function of CXCR3, but not CCR5, are impaired in T-bet–/– T cells
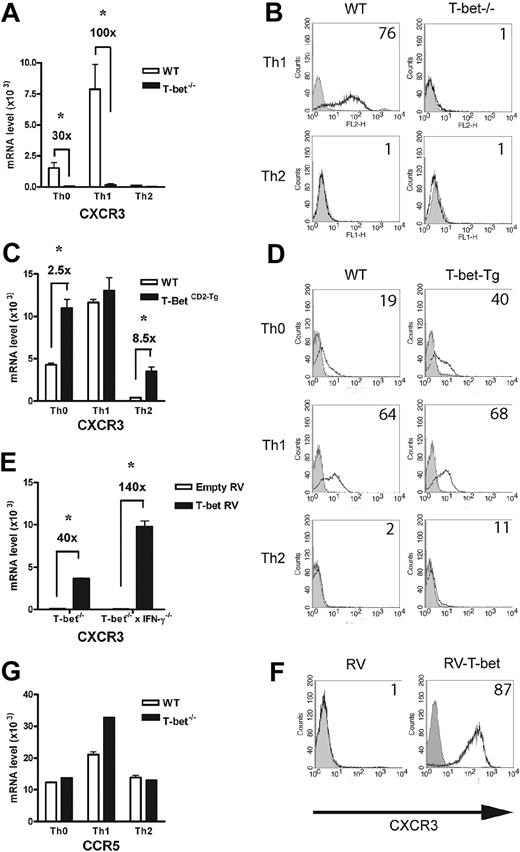
In addition to selectin ligand expression, CD4+ Th1-cell migration is dependent on chemokine receptor expression, notably CCR5 and CXCR3. In the absence of T-bet, levels of CXCR3 transcripts were minimal compared with WT T cells activated under Th1-polarizing conditions (Figure 5A). These changes were mirrored by surface expression of CXCR3 (Figure 5B), with no detectable receptor present on the T-bet–/– T cells. In contrast, T-bet CD2-Tg T cells expressed significantly more CXCR3 mRNA than WT T cells when activated under nonpolarizing or Th2 conditions (Figure 5C). Again, the transgenic overexpression of T-bet led to greater surface expression of CXCR3 in these cells (Figure 5D). Although these results suggest T-bet may directly regulate expression of CXCR3, its expression has also been reported to be regulated by IFN-γ,38 a cytokine directly controlled by T-bet. To determine whether the impaired expression of CXCR3 was secondary to the reduced levels of IFN-γ produced by T-bet–/– T cells, T-bet was retrovirally transduced into T-bet–/– and T-bet–/– × IFN-γ–/– CD4+ T cells. T-bet transduction into T-bet–/– T cells induced a striking up-regulation of CXCR3 mRNA. This induction was entirely independent of IFN-γ production, as marked induction of CXCR3 was seen when T-bet was transduced into T-bet–/– × IFN-γ–/– CD4+ T cells (Figure 5E). CXCR3 was highly induced on the surface of T-bet–/– × IFN-γ–/– CD4+ T cells transduced with T-bet (Figure 5F). In marked contrast, levels of CCR5, the other Th1 selective chemokine receptor, were unaffected by the absence of T-bet (Figure 5G), indicating selective regulation of a Th1-specific chemokine receptor by T-bet. The surface expression of CCR5 was similar on primary CD4+ T cells activated under Th1-polarizing conditions on WT, T-bet–/–, and T-bet–/– × IFN-γ–/– CD4+ T cells, with no detectable CCR5 on T cells of any genotype activated under Th2-polarizing conditions (Table S2). Additionally, CCR5 mRNA levels were unchanged in T-bet CD2-Tg T cells (Figure S4). The expression of other chemokine receptors (eg, CCR4, CXCR4, and CCR7) was also unchanged in the absence of T-bet.
Posttranslational modification of selectin ligands. (A) Flow cytometric surface staining of CD43a (top), CD43c (middle), and PSGL-1 (bottom) on WT and T-bet–/– T cells activated under Th1- or Th2-polarizing conditions (gated on live CD4+ cells; black = isotype, red = WT, and green = T-bet–/–). (B) Real-time PCR analysis of mRNA levels of FucTVII expressed in CD4+ T cells under Th1- and Th2-polarizing conditions (normalized to β-actin). (C) Real-time PCR analysis of mRNA levels of TPST-1 and TPST-2 expressed in CD4+ T cells under Th1-polarizing conditions (normalized to β-actin). □ indicates WT; ▪, T-bet–/–. (D) Autoradiograph of 35S incorporation into PSGL-1, with or without removal of O- and N-linked glycans. (E) Western blot for PSGL-1 in WT and T-bet–/– (knock out [KO]) CD4+ T cells activated under Th1-polarizing conditions (top panel = PSGL-1 dimer; bottom panel = PSGL-1 monomer). (F) Quantification of protein expression of PSGL-1 either with or without removal of O- and N-linked glycans. Results are expressed as mean ± SEM. WB indicates Western blot.
Posttranslational modification of selectin ligands. (A) Flow cytometric surface staining of CD43a (top), CD43c (middle), and PSGL-1 (bottom) on WT and T-bet–/– T cells activated under Th1- or Th2-polarizing conditions (gated on live CD4+ cells; black = isotype, red = WT, and green = T-bet–/–). (B) Real-time PCR analysis of mRNA levels of FucTVII expressed in CD4+ T cells under Th1- and Th2-polarizing conditions (normalized to β-actin). (C) Real-time PCR analysis of mRNA levels of TPST-1 and TPST-2 expressed in CD4+ T cells under Th1-polarizing conditions (normalized to β-actin). □ indicates WT; ▪, T-bet–/–. (D) Autoradiograph of 35S incorporation into PSGL-1, with or without removal of O- and N-linked glycans. (E) Western blot for PSGL-1 in WT and T-bet–/– (knock out [KO]) CD4+ T cells activated under Th1-polarizing conditions (top panel = PSGL-1 dimer; bottom panel = PSGL-1 monomer). (F) Quantification of protein expression of PSGL-1 either with or without removal of O- and N-linked glycans. Results are expressed as mean ± SEM. WB indicates Western blot.
Analysis of chemokine receptor expression in the context of altered T-bet levels in primary CD4+ T cells. Real-time PCR analysis (A,C) and flow cytometric (B,D) surface staining of mRNA levels of CXCR3 in T cells activated under different polarizing conditions (mean ± SEM). (A-B) WT and T-bet–/– CD4+ T cells. (C-D) WT and T-bet CD2-Tg CD4+ T cells. (E) Real-time PCR analysis of CXCR3 expression in primary T-bet–/– and T-bet–/– × IFN-γ–/– CD4+ T cells retrovirally transduced with empty retrovirus (RV) or T-bet RV (mean ± SEM). (F) Flow cytometric surface staining of CXCR3 in retrovirally transduced T cells. (G) mRNA levels of CCR5 in WT and T-bet–/– T cells. All real-time PCR results are normalized to β-actin and are expressed as mean ± SEM (*P < .01). Shaded areas represent isotype staining.
Analysis of chemokine receptor expression in the context of altered T-bet levels in primary CD4+ T cells. Real-time PCR analysis (A,C) and flow cytometric (B,D) surface staining of mRNA levels of CXCR3 in T cells activated under different polarizing conditions (mean ± SEM). (A-B) WT and T-bet–/– CD4+ T cells. (C-D) WT and T-bet CD2-Tg CD4+ T cells. (E) Real-time PCR analysis of CXCR3 expression in primary T-bet–/– and T-bet–/– × IFN-γ–/– CD4+ T cells retrovirally transduced with empty retrovirus (RV) or T-bet RV (mean ± SEM). (F) Flow cytometric surface staining of CXCR3 in retrovirally transduced T cells. (G) mRNA levels of CCR5 in WT and T-bet–/– T cells. All real-time PCR results are normalized to β-actin and are expressed as mean ± SEM (*P < .01). Shaded areas represent isotype staining.
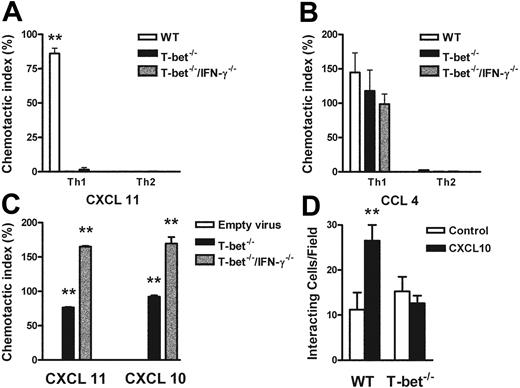
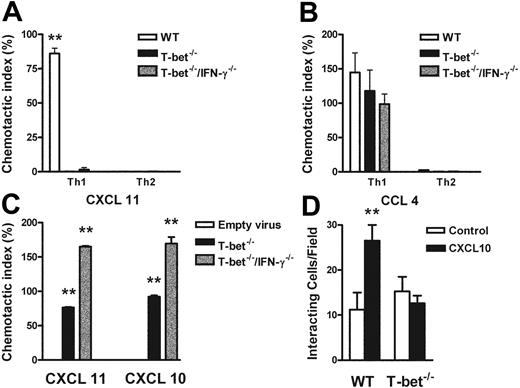
The functional absence of CXCR3 was demonstrated by an impaired response to CXCR3 ligands in transwell chemotaxis assays. T-bet–/– T cells failed to migrate in response to the CXCR3 ligand CXCL11 (I-TAC, Figure 6A) or CXCL-10 (not shown), but migrated comparably to WT CD4+ T cells in response to the CCR5 ligand CCL4 (macrophage inflammatory protein 1β [MIP-1β], Figure 6B). T-bet CD2-Tg T cells migrated comparably to WT T cells to MIP-1β under all polarizing conditions, showing that transgenic overexpression of T-bet did not directly induce expression of functional CCR5 even under Th2-polarizing conditions (Figure S5). Retroviral transduction of T-bet into T-bet–/– and T-bet–/– × IFN-γ–/– CD4+ T cells restored transmigration of these cells to the CXCR3 ligands CXCL11 and CXCL10 when compared with transduction with empty retrovirus (Figure 6C), indicating that T-bet can drive functional expression of CXCR3 by a mechanism independent of its transactivation of IFNG.
Responses of primary CD4+ T cells to chemokine ligands in the context of altered T-bet levels. (A-C) Transmigration of CD4+ T cells in response to recombinant chemokine in the lower chamber of a transwell. (A-B) Chemotaxis of WT, T-bet–/–, and T-bet–/– × IFN-γ–/– T cells to recombinant chemokines. (A) Chemotactic response to CXCL11 (I-TAC, 100 nM). □ indicates WT; ▪, T-bet –/–; ▦, T-bet–/–/IFN-γ–/–.(B) Chemotactic response to CCL4 (MIP-1β, 10 nM). □ indicates WT; ▪, T-bet–/–; ▦, T-bet–/–/IFN-γ–/–. (C) Chemotaxis of retrovirally transduced T-bet (or empty vector control) into T-bet–/– and T-bet–/– × IFN-γ–/– T cells to CXCR3 ligands, CXCL11 (100 nM), and CXCL10 (IP-10, 100 nM). □ indicates empty virus; ▪, T-bet–/–; ▦, T-bet–/–/IFN-γ–/–. (D) Attachment of WT and T-bet–/– T cells to unstimulated endothelial cells under shear flow in the absence or presence of CXCL10 (40 ng/mL) (mean ± SEM). □ indicates control; ▪, CXCL10. **P < .05.
Responses of primary CD4+ T cells to chemokine ligands in the context of altered T-bet levels. (A-C) Transmigration of CD4+ T cells in response to recombinant chemokine in the lower chamber of a transwell. (A-B) Chemotaxis of WT, T-bet–/–, and T-bet–/– × IFN-γ–/– T cells to recombinant chemokines. (A) Chemotactic response to CXCL11 (I-TAC, 100 nM). □ indicates WT; ▪, T-bet –/–; ▦, T-bet–/–/IFN-γ–/–.(B) Chemotactic response to CCL4 (MIP-1β, 10 nM). □ indicates WT; ▪, T-bet–/–; ▦, T-bet–/–/IFN-γ–/–. (C) Chemotaxis of retrovirally transduced T-bet (or empty vector control) into T-bet–/– and T-bet–/– × IFN-γ–/– T cells to CXCR3 ligands, CXCL11 (100 nM), and CXCL10 (IP-10, 100 nM). □ indicates empty virus; ▪, T-bet–/–; ▦, T-bet–/–/IFN-γ–/–. (D) Attachment of WT and T-bet–/– T cells to unstimulated endothelial cells under shear flow in the absence or presence of CXCL10 (40 ng/mL) (mean ± SEM). □ indicates control; ▪, CXCL10. **P < .05.
Chemokines also mediate activation of integrins, resulting in firm adhesion of rolling T cells,25 by inducing high-affinity or high-avidity states via a process of inside-out signaling.39 To demonstrate a requirement for T-bet in this process, we observed the attachment of T cells to unstimulated cardiac ECs that had been treated with PBS or the CXCR3 ligand, CXCL10. In this system, all binding is dependent on β-integrin binding to vascular cell adhesion molecule (VCAM-1).32 In the absence of CXCL10, the binding of WT and T-bet–/– CD4+ Th1 cells was identical. In contrast, after the addition of CXCL10, only WT, and not T-bet–/– Th1 cells, showed increased attachment to ECs under conditions of physiologic shear flow (Figure 6D).
Discussion
Murine and human autoimmune diseases are characterized by infiltration of effector T cells to pathologic sites. T-bet–deficient mice are resistant to a wide range of autoimmune diseases, including type I diabetes, inflammatory colitis and arthritis, lupus nephritis, and experimental autoimmune encephalomyelitis in vivo, but the mechanisms responsible have not been firmly established.5,11,12,30 It is certainly possible that impaired cellular effector function contributes to such resistance. However, the common finding in all of the above models is a reduction of cellular infiltration to inflammatory sites in the absence of T-bet, prompting our interest in the trafficking ability of Th1 cells. If migration of effector cells is defective, then, to a certain extent, the competence of effector function becomes relatively less important. This is illustrated by considering the immunosuppressive capacity of the drug FTY720,40 which prevents lymphocyte egress from lymph nodes by targeting sphingosine-1-phosphate and thus impairs T-cell trafficking. We have shown that in the absence of T-bet, primary CD4+ T cells generated under Th1-polarizing conditions fail to migrate appropriately in vivo due to defects in multiple specific mechanisms in the T-cell trafficking pathway, including sulfation-dependent P-selectin binding and CXCR3-dependent arrest and migration. Th2 cells from T-bet–/– mice also migrated less well to an inflammatory site in vivo, although this was not statistically significant and the relevance of this is unclear. Furthermore, it is entirely possible that further undiscovered mechanisms also contribute to the defective T-cell trafficking that occurs in the absence of T-bet.
Of considerable interest with respect to selectin binding is the specificity of a P-selectin defect with preservation of E-selectin binding. It can therefore be inferred that certain STAT4-dependent processes are functionally preserved. It is also noteworthy that there was minimal, if any, binding of T cells activated under Th2-polarizing conditions either in the absence or presence of T-bet. Hence, some aspects of Th1-cell development are conserved (binding to E-selectin) excluding the notion that these cells are simply default Th2 cells, since Th2 cells do not bind E-selectin. T-bet appears to control the functional expression of TPST-2 and, possibly, TPST-1. Little is known about the control of these 2 critical enzymes, but as the reduction in mRNA was not absolute, we postulate that the mechanism of T-bet control of tyrosine sulfation is unlikely to be at the transcriptional level. However, the expression and cellular location of these enzymes is exquisitely controlled. Active TPSTs in the Golgi do, to a large degree, require tyrosine sulfation in order to function effectively,41 and it remains a possibility that T-bet somehow modulates the posttranslational functioning of either of these enzymes, more likely TPST-2 (see below). Furthermore, the relative contribution of these 2 enzymes to the sulfation of PSGL-1 has not been determined and will require further characterization of mice deficient in one or both of these enzymes. Murine PSGL-1 differs from its human counterpart in certain respects, most pertinently that sulfation of only 1 of 2 tyrosine residues is required in mouse, compared with 2 of 3 tyrosine residues in humans.42-44 Hence, any deficiency in sulfation might be magnified in human T cells. Nevertheless, interruption in the function of either of these 2 enzymes represents an intriguing therapeutic strategy.
Our observations concerning expression and functioning of chemokine receptors reiterate the hypothesis that selective Th1 trafficking responses are impaired in T-bet–/– mice. Previous studies have elucidated a role for overexpressed T-bet in the induction of surface CXCR39,45 on long-term polarized Th2 cells and demonstrated an associated increase in chemotactic function. Furthermore, retroviral transduction of T-bet into polarized Th2 clones induces marked secretion of IFN-γ, the cytokine that has been shown to be key in driving the expression of CXCR3. In light of our data, the fact that IFN-γ drives expression of CXCR3 may be reinterpreted as IFN-γ acting upstream of T-bet to increase its expression; T-bet then induces expression of CXCR3. To our knowledge, this is the first description that deletion of T-bet leads to a reduction in CXCR3 expression with the subsequent abrogation of multiple functions, including lymphocyte arrest on activated endothelium and chemotaxis. These findings are of particular interest since a recent report has suggested that the mechanism by which CD4+CD25+ regulatory T cells act as suppressors is to inhibit IFN-γ and CXCR3 expression in vivo.33 This raises the intriguing possibility that one mechanism of action of CD4+CD25+ T cells may be via suppression of T-bet with subsequent reduction in IFN-γ and CXCR3 expression and consequent impaired T-cell trafficking to inflamed peripheral tissues. The absolute loss in CXCR3 expression would suggest that it is a direct transcriptional target of T-bet. However, we have been unable to detect any enrichment of the CXCR3 promoter by the use of chromatin immunoprecipitation using real-time PCR primers tiled to include 1.5 kb upstream of the transcription start site and primers spanning the intron, although this does not preclude T-bet binding outside of this region (G.M.L. and L.H.G., unpublished observations, January 2005). Conversely, expression and chemotactic function of CCR5, the other archetypal Th1 chemokine, are unaltered. Intriguingly, tyrosine sulfation of this chemokine receptor has also been described for its effective functioning.46 It is possible therefore that unimpaired TPST-1 function can modulate effective sulfation of CCR5 but is not sufficient for PSGL-1. Taken together, these results demonstrate that T-bet exerts a level of control of the trafficking of Th1 lymphocytes that was not previously recognized by existing paradigms.
Specific migration of T cells is a major determinant of the outcome of an appropriate immune response. Our experiments provide evidence that the Th1-specific transcription factor T-bet imprints a migratory program upon developing T cells to ensure appropriate homing to inflammatory sites in vivo via control of P-selectin ligand posttranslational biosynthesis and CXCR3 expression and function, interactions of critical importance in many disease states.47-50 This finding has significant implications for the design of rational treatments for autoimmune, neoplastic, and infectious diseases, and the prevention of rejection of transplanted organs.
Prepublished online as Blood First Edition Paper, July 12, 2005; DOI 10.1182/blood-2005-04-1393.
Supported by National Institutes of Health (NIH) grants AI48126 (L.H.G.), HL36028 (A.H.L.), and HL53993 (F.W.L.); Medical Research Council UK (MRC) grant G108/380 (G.M.L.); and Arthritis Research Council UK (ARC UK) grant R0600 (R.M.R.).
G.M.L. and R.M.R. contributed equally to this work.
An Inside Blood analysis of this article appears at the front of the issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Michael Townsend, Adrian Erlebacher, and Sung-Yun Pai for thoughtful review of the article and Deanna Lamont for technical assistance.

![Figure 4. Posttranslational modification of selectin ligands. (A) Flow cytometric surface staining of CD43a (top), CD43c (middle), and PSGL-1 (bottom) on WT and T-bet–/– T cells activated under Th1- or Th2-polarizing conditions (gated on live CD4+ cells; black = isotype, red = WT, and green = T-bet–/–). (B) Real-time PCR analysis of mRNA levels of FucTVII expressed in CD4+ T cells under Th1- and Th2-polarizing conditions (normalized to β-actin). (C) Real-time PCR analysis of mRNA levels of TPST-1 and TPST-2 expressed in CD4+ T cells under Th1-polarizing conditions (normalized to β-actin). □ indicates WT; ▪, T-bet–/–. (D) Autoradiograph of 35S incorporation into PSGL-1, with or without removal of O- and N-linked glycans. (E) Western blot for PSGL-1 in WT and T-bet–/– (knock out [KO]) CD4+ T cells activated under Th1-polarizing conditions (top panel = PSGL-1 dimer; bottom panel = PSGL-1 monomer). (F) Quantification of protein expression of PSGL-1 either with or without removal of O- and N-linked glycans. Results are expressed as mean ± SEM. WB indicates Western blot.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/10/10.1182_blood-2005-04-1393/6/m_zh80220586590004.jpeg?Expires=1771331301&Signature=rls61Zs2HLZZNv1M1tnTzBNQ2JU3p5VSfFI04mx59-cfy-kz5f2AcAS33aJGqq6V06rBedB5RsQCop7yXDj4zz9sgMbpL7w94sZI5vtLmBp2TrAa~WOzdB4Ek5w4972WC0uLESOe1NNkMwIPJUwG26DGhlcFm6-KoC560qngp7M95nEGHNSBM78IbLmjthOa32xl1uD770SYWkZYJqzw4WouXo2L9fztaY7F28BA~17EPzj36VEjZTjYt0hqraLdCjV5~INmq2TCZxM9FDxgKpdrgWa9Z3YQPwwvEqrBEFJ5j96ZdLfbGLLVfzXJjnFRkLvFwrzS1BtZgQv11KHbxQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. Posttranslational modification of selectin ligands. (A) Flow cytometric surface staining of CD43a (top), CD43c (middle), and PSGL-1 (bottom) on WT and T-bet–/– T cells activated under Th1- or Th2-polarizing conditions (gated on live CD4+ cells; black = isotype, red = WT, and green = T-bet–/–). (B) Real-time PCR analysis of mRNA levels of FucTVII expressed in CD4+ T cells under Th1- and Th2-polarizing conditions (normalized to β-actin). (C) Real-time PCR analysis of mRNA levels of TPST-1 and TPST-2 expressed in CD4+ T cells under Th1-polarizing conditions (normalized to β-actin). □ indicates WT; ▪, T-bet–/–. (D) Autoradiograph of 35S incorporation into PSGL-1, with or without removal of O- and N-linked glycans. (E) Western blot for PSGL-1 in WT and T-bet–/– (knock out [KO]) CD4+ T cells activated under Th1-polarizing conditions (top panel = PSGL-1 dimer; bottom panel = PSGL-1 monomer). (F) Quantification of protein expression of PSGL-1 either with or without removal of O- and N-linked glycans. Results are expressed as mean ± SEM. WB indicates Western blot.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/10/10.1182_blood-2005-04-1393/6/m_zh80220586590004.jpeg?Expires=1771331302&Signature=eVTFqVzCTRKtrdiSXbx9aArwdtxMiTooOTtdnuMRyWg4kugVIpqIpgruEf1qQWw1g98qlG6t9NyRIUVZms2mB094HNGp5qqax5j~cmSjME003TCxKx5j5sCSw8HD1bKaCKIMG6t4GQqVaCVO7diz9PMdws-CA0qC~0szjJAN8ArhqWEBU2MSoEewMgKhBfTnJZWwNuMWmnnp-CLkCaz~fvm97P9eLGUrPH45nbf-ZJg-l2qeW~Qg1o0PzbCZm~IfNeVMSPwKs9M7QRR-wD3tOYexP1piEXqh4Tp7-yJAD1h50GpMTc96nATxNCtvaQtc0O1e-aJTnjUMHlNei8VS2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)