Abstract
The binding of von Willebrand factor (VWF) to the platelet membrane glycoprotein Ib-IX (GPIb-IX) results in platelet activation. In this study, we sought to clarify previous conflicting reports and to elucidate the mechanism of activation and the precise role of extracellular signal-regulated kinase (Erk) in VWF-induced platelet activation. Erk2 is activated in platelets on stimulation with VWF/ristocetin in a time-dependent manner. VWF-induced Erk2 phosphorylation and thromboxane A2 (TXA2) release were completely blocked by PP2, an Src family kinase inhibitor, suggesting that Erk is downstream of Src family kinases. U73122, a phospholipase C inhibitor, also abolished TXA2 generation and Erk phosphorylation. Although VWF fostered the agglutination of platelets regardless of any additional treatment, the inhibition of mitogen-activated protein kinase kinase (MEK) with U0126 abolished VWF-induced platelet aggregation and thromboxane production in non–aspirin-treated washed platelets. However, in platelets treated with aspirin, VWF failed to cause any aggregation. Thus, we conclude that VWF stimulation of platelets results in phospholipase A2 activation through Erk stimulation and that Src family kinases and phospholipase C play essential roles in this event. We further conclude that VWF-induced platelet aggregation does not directly depend on Erk activation but has an absolute requirement for Src/Erk-mediated TXA2 generation.
Introduction
The platelet activation process is an important component of normal hemostasis.1-5 von Willebrand factor (VWF) is a mandatory component of platelet plug formation at the site of vascular injury and under high shear conditions through its interactions with platelet surface glycoprotein (GP) complex GPIb-V-IX.6 These interactions at the injury site lead to the trapping of platelets to enhance their interaction with collagen and to the further adhesion of platelets. Under high shear conditions of blood flow in the arteries, the adhesion and activation of platelets are dependent on their interactions with VWF. When subendothelium is exposed, the adhesion of platelets is dependent on their interaction with VWF, but it is mainly collagen in the subendothelium that is responsible for the platelet activation process. In vitro, ristocetin or botrocetin can modify the interactions between VWF and GPIb complex to trigger signaling events. Thus, platelets treated with ristocetin or botrocetin in the presence of VWF undergo platelet agglutination followed by platelet activation. Platelet agglutination is a mechanical phenomenon wherein platelets clump together through VWF cross-linking of GPIb molecules on different platelets. However, the subsequent mechanical force on the GPIb complex leads to signaling events that translate into platelet activation.
A number of signaling pathways have been implicated in the stimulation of platelets downstream of GPIb activation by VWF.7 The activation of platelets through the GPIb complex is weak compared with that of platelet agonists such as thrombin, collagen, and adenosine diphosphate (ADP). In a recent study, VWF-GPIb interactions appear first to generate thromboxane A2, leading to ADP secretion and fibrinogen receptor activation.8 VWF-GPIb–mediated platelet activation is delayed and occurs after near completion of agglutination. Despite the years of work conducted in this field, the signaling events downstream of GPIb activation and their contributions to platelet physiology remain controversial.9-14
These variations arose mainly because of the use of different experimental conditions, among them the source of ligands, platelet-rich plasma (PRP) compared with washed platelets, and human platelets compared with mouse platelets. Pathways downstream of GPIb stimulation include activation of the FcRγ chain,14,15 activation of phospholipase C (PLC) and protein kinase C (PKC) molecules,10,11,16 14-3-3ζ, mitogen-activated protein (MAP) kinases, protein kinase G (PKG), Src kinases,17 or phosphoinositide 3 (PI-3) kinase.10,11,16 Recent studies have implicated the MAP kinase extracellular signal-regulated kinase (Erk) in the activation of integrin αIIβ3 by VWF/GPIb stimulation.18 However, subsequent studies by Marshall et al19 disputed the role of Erk or PKG in VWF-mediated fibrinogen receptor activation. Thus, one of the controversies existing to date in the GPIb signaling field is the mechanism of activation of Erk and its role in fibrinogen receptor activation.
In this study, we show that VWF mediates Erk activation that is dependent on Src family kinases. Furthermore, we demonstrate that GPIb-mediated platelet activation leads to thromboxane generation in an Erk-dependent manner. Finally, we show that GPIb-mediated fibrinogen receptor activation depends on generated thromboxane but not directly on Erk activation.
Materials and methods
Materials
Apyrase (type VII), bovine serum albumin (fraction V), GR144053 acetylsalicylic acid, ristocetin monosulfate, and Erk antibodies (both antiphosphospecific and total Erk) were obtained from Sigma (St Louis, MO). PP3, PP2, U0126, and the (PLC) β2 inhibitor U73122 were from Biomol (Plymouth Meeting, PA). Human von Willebrand factor was obtained from Haematologic Technologies (Essex Junction, VT).
Isolation of human platelets
Whole blood was drawn from healthy, consenting human volunteers into tubes containing 0.166 vol ACD (2.5 g sodium citrate, 1.5 g citric acid, and 2 g glucose in 100 mL deionized water). Blood donors were healthy persons recruited from the Temple University staff and student populations. Donors were men and women (approximate ratio, 6:4) of any ethnic background, preferably younger than 40. They were in good health and were not taking any medication, and their blood pressures and hemoglobin levels were within normal limits. Blood was centrifuged (5810R centrifuge; Eppendorf, Hamburg, Germany) at 230g for 20 minutes at room temperature to obtain PRP. PRP was incubated with 1 mM acetylsalicylic acid for 30 minutes at 37°C and was then centrifuged for 10 minutes at 980g at room temperature to pellet the platelets. Platelets were resuspended in Tyrode buffer (138 mM NaCl, 2.7 mM KCl, 1 mM MgCl2,3 mM NaH2PO4, 5 mM glucose, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4, 0.2% bovine serum albumin) containing 0.01 U/mL apyrase. Cells were counted (Z1 Particle Counter; Beckman-Coulter, Miami, FL) and concentration of cells was adjusted to 4 × 108 platelets/mL. All experiments using washed platelets were performed in the absence of extracellular calcium unless otherwise mentioned. In the experiments with thromboxane B2 measurements, the treatment of PRP with acetylsalicylic acid was omitted.
Aggregometry
Aggregation of 0.5 mL washed platelets was analyzed using a P.I.C.A. lumiaggregometer (Chrono-log, Havertown, PA). Aggregation was measured by light transmission under stirring conditions (900 rpm) at 37°C. Agonists were added simultaneously for platelet stimulation; however, platelets were preincubated with each inhibitor (where noted) at 37°C. Each sample was allowed to aggregate for at least 3 minutes. The chart recorder (Kipp and Zonen, Bohemia, NY) was set for 0.2 mm/s. Aggregation tracings are representative of results obtained from 3 separate experiments on 3 different donors.
Measurement of platelet secretion. Platelet secretion was determined by measuring the release of [14C]5-hydroxytryptamine ([14C]5-HT). The activation of labeled [14C]5-HT platelets was performed in a lumiaggregometer (Chrono-Log) at 37°C with stirring and was stopped after 1 minute by the addition of formaldehyde/EDTA (ethylenediaminetetraacetic acid) according to the method of Costa and Murphy.20 Imipramine was added to the HEPES-buffered Tyrode solution at a final concentration of 1 μM to prevent the reuptake of secreted [14C]5-HT. Samples were collected and centrifuged at 10 000g for 1 minute, and the radioactivity of the supernatant was measured using a liquid scintillation counter (LKB; Pharmacia, Uppsala, Sweden). Secretion was normalized and expressed as a percentage to the value in the absence of other reagents (taken as 100%).
Western blot analysis. Platelets were stimulated with agonists in the presence or absence of inhibitors for the appropriate time, and the reaction was stopped by the addition of 3 × sodium dodecyl sulfate (SDS) Laemmli buffer. Platelet samples were boiled for 10 minutes, and proteins were separated by 10% SDS–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride (PVDF) membrane. Nonspecific binding sites were blocked by incubation in Tris-buffered saline-Tween (TBST; 20 mM Tris, 140 mM NaCl, 0.1% [vol/vol] Tween 20) containing 2% (wt/vol) bovine serum albumin (BSA) for 30 minutes at room temperature, and membranes were incubated overnight at 4°C with the primary antibody (1:1000 dilution in TBST with 2% BSA) with gentle agitation. After 3 washes for 5 minutes each with TBST, the membranes were probed with an alkaline phosphatase–labeled secondary antibody (1:5000 dilution in TBST with 2% BSA) for 1 hour at room temperature. After additional washing steps, membranes were incubated with chemiluminescence substrate (CDP-Star; Tropix, Bedford, MA) for 10 minutes at room temperature, and immunoreactivity was detected using a luminescent image analyzer (LAS-1000 CH; Fuji Film, Tokyo, Japan).
Measurement of thromboxane A2 generation. Washed human platelets without aspirin treatment were prepared as noted and brought to a concentration of 4 × 108 platelets/mL. Stimulations were performed in a platelet aggregometer under stirring conditions (900 rpm) at 37°C. The Src family tyrosine kinase inhibitor PP2 and the inactive analog PP3 were added 10 minutes before addition of the agonist. Stimulations were performed for 3.5 minutes, and the reaction was stopped by snap freezing. Samples were stored at –80°C until TXB2 analysis was performed. Levels of TXB2 were determined in duplicate using a Correlate-EIA Thromboxane B2 Enzyme Immunoassay Kit (Assay Designs, Ann Arbor, MI), according to the manufacturer's instructions. The mean ± SE was derived from experiments performed in triplicate using platelets obtained from 3 independent donors.
Results
Effect of Src family tyrosine kinase inhibition on GPIb-mediated phosphorylation of Erk in platelets
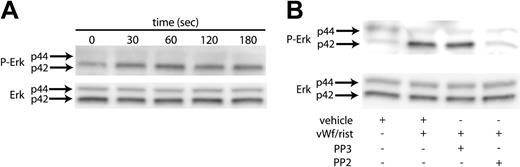
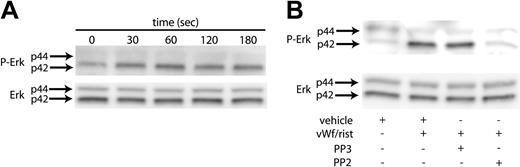
Erk has been reported to be activated in platelets upon GPIb stimulation by VWF.18 We initiated our study to establish conditions to confirm these findings. As shown in Figure 1A, stimulating washed, aspirin-treated platelets with ristocetin/VWF resulted in the phosphorylation of Erk2 in a time-dependent manner. The phosphorylation of Erk started as early as 30 seconds, reached maximal levels at 60 seconds, and was sustained for 3 minutes. Because the Src kinases are the predominant tyrosine kinases found in platelets and have been implicated in VWF-mediated platelet aggregation,19 we investigated whether Src kinases are involved in the phosphorylation of Erk. Platelets have been shown to contain several members of the Src family, which includes Fgr, Fyn, Lck, Lyn, Src, Hck, and Yes. To determine whether Src kinases are involved in the phosphorylation of Erk, we measured Erk phosphorylation in the presence and absence of the Src kinase family inhibitor PP2 (10 μM); the inactive structural analog PP3 (10 μM) was used a negative control. As shown in Figure 1B, the VWF-mediated phosphorylation of Erk was completely abolished by PP2. However, the negative control treatment with PP3 had no effect on the Erk phosphorylation mediated by ristocetin/VWF. Given these results, it is evident that the Src family of tyrosine kinases is activated by GPIb activation and that these kinases are upstream of Erk phosphorylation in platelets.
VWF-mediated Erk phosphorylation in platelets. Platelets were stimulated with VWF/ristocetin (8 and 300 μg/mL, respectively) for various amounts of time (A) or for 1 minute in the absence (vehicle) or presence of the Src kinase inhibitor PP2 or its inactive analog, PP3 (B). Both figures were analyzed to detect either phospho-Erk (top panels) or total Erk (bottom panels), as described in “Materials and methods.” Arrows denote the 2 molecular forms of Erk in platelets.
VWF-mediated Erk phosphorylation in platelets. Platelets were stimulated with VWF/ristocetin (8 and 300 μg/mL, respectively) for various amounts of time (A) or for 1 minute in the absence (vehicle) or presence of the Src kinase inhibitor PP2 or its inactive analog, PP3 (B). Both figures were analyzed to detect either phospho-Erk (top panels) or total Erk (bottom panels), as described in “Materials and methods.” Arrows denote the 2 molecular forms of Erk in platelets.
Role of PLC in the GPIb-mediated phosphorylation of Erk
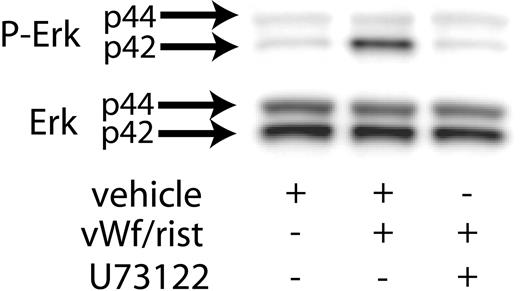
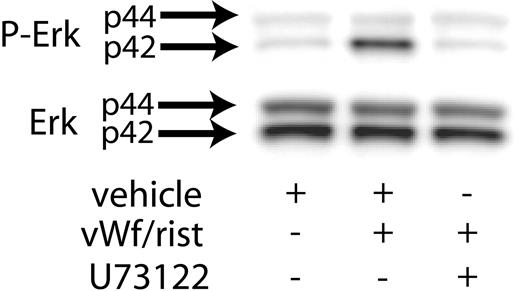
Previous studies have shown that Src kinases activated downstream of GPIb activation lead to the stimulation of PLCγ2.21 Hence, we investigated the role of PLC in VWF-induced Erk phosphorylation. As shown in Figure 2, the presence of the PLC inhibitor U73122 blocked ristocetin/VWF-induced phosphorylation of Erk in human platelets. These results suggest that GPIb-mediated phosphorylation of Erk is dependent on PLCγ2 activation.
Effect of PP2 and U73122 on VWF-induced thromboxane A2 generation in human platelets
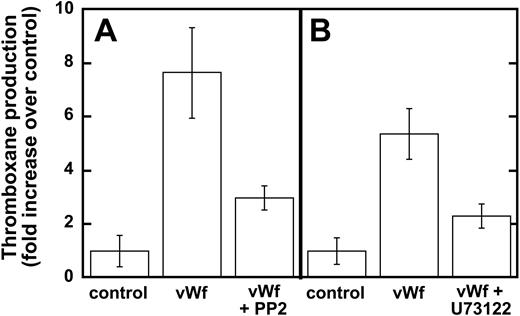
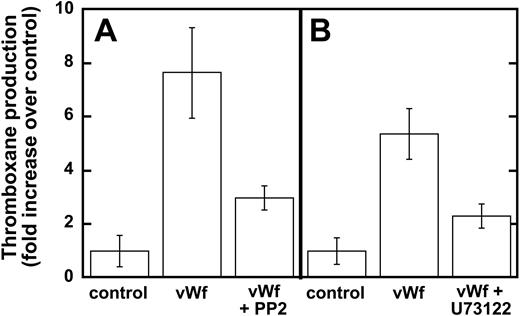
Earlier studies have shown that Src family kinases are important in thromboxane generation downstream of the signaling pathways involving the inhibitory G-protein family (ie Gαi and Gαz)22 and protease-activated receptor stimulation.23,24 Hence, we investigated the role of Src kinases in GPIb-mediated thromboxane A2 generation, and our results show that ristocetin/VWF-mediated thromboxane A2 generation is dramatically inhibited in the presence of PP2 (Figure 3A). Similarly, PLC inhibition leads to the dramatic inhibition of thromboxane generation downstream of GPIb stimulation by VWF (Figure 3B). These data indicate that Src family kinases and PLC play important roles in GPIb-mediated thromboxane A2 generation in human platelets.
Role of Erk in GPIb-mediated thromboxane generation
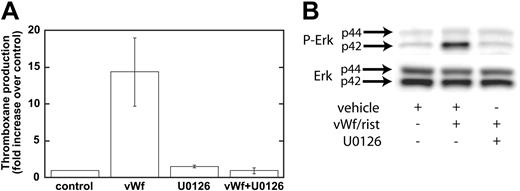
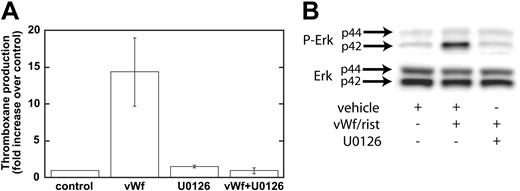
Our data have shown that GPIb-mediated phosphorylation of Erk depends on Src family kinases and on PLC and that the inhibition of Src kinases or PLC leads to the inhibition of thromboxane generation. We next addressed whether Erk is important for thromboxane generation. We used a mitogen-activated protein kinase kinase (MEK) inhibitor, U0126, to block the phosphorylation and activation of Erk downstream of GPIb activation. As shown in Figure 4A, ristocetin/VWF-induced thromboxane generation was completely blocked by U0126 (10 μM). In the control experiments, U0126 blocked the phosphorylation of Erk by risocetin/VWF (Figure 4B). These data indicate that Erk activation downstream of Src and PLC stimulation is essential for GPIb-mediated thromboxane generation. Because recent studies from Liu et al8,25 indicated that VWF/GPIb-mediated dense granule release depends on thromboxane generation, we evaluated the effect of Src family kinase inhibitors and MEK inhibitor on ristocetin/VWF-induced serotonin secretion. In the presence of vehicle or PP3, ristocetin/VWF caused serotonin secretion (11.80% ± 1.03% of maximum), whereas both PP2 and U0126 completely abolished serotonin secretion, confirming the observations of Liu et al.8,25
Inhibition of PLC decreases VWF-mediated Erk phosphorylation. Platelets were stimulated with VWF/ristocetin (8 and 300 μg/mL, respectively) in the absence (vehicle) or presence of the PLC inhibitor U73122. Erk activation was assayed as described in “Materials and methods.”
Inhibition of PLC decreases VWF-mediated Erk phosphorylation. Platelets were stimulated with VWF/ristocetin (8 and 300 μg/mL, respectively) in the absence (vehicle) or presence of the PLC inhibitor U73122. Erk activation was assayed as described in “Materials and methods.”
Inhibition of Src kinases and PLC decreases VWF-mediated thromboxane production. Platelets were unstimulated (control) or stimulated with VWF/ristocetin (8 and 300 μg/mL, respectively) in the absence or presence of either (A) the Src kinase inhibitor PP2 (10 μM) or (B) the PLC inhibitor U73122 (4 μM). Platelets were then processed for measurements of thromboxane production, as described in “Materials and methods.”
Inhibition of Src kinases and PLC decreases VWF-mediated thromboxane production. Platelets were unstimulated (control) or stimulated with VWF/ristocetin (8 and 300 μg/mL, respectively) in the absence or presence of either (A) the Src kinase inhibitor PP2 (10 μM) or (B) the PLC inhibitor U73122 (4 μM). Platelets were then processed for measurements of thromboxane production, as described in “Materials and methods.”
Role of Erk in GPIb-mediated fibrinogen receptor activation
Previous studies have implicated Erk in GPIb-mediated fibrinogen receptor activation.18 However, Marshall et al19 subsequently showed that inhibiting MEK with PD184161 did not inhibit the activation of integrin αIIbβ3. Hence, we investigated whether inhibiting MEK with U0126 has any effect on fibrinogen receptor activation. Given that our earlier data showed that U0126 inhibits GPIb-mediated thromboxane generation, we used untreated and aspirin-treated platelets to further evaluate the role of generated thromboxane on fibrinogen receptor activation. As shown in Figure 5, U0126 abolished the GPIb-mediated aggregation that is normally followed by agglutination in untreated platelets. However, when aspirin-treated platelets were used, 2 important findings were observed. First, ristocetin/VWF caused only primary agglutination in these platelets without causing aggregation. Second, we observed that U0126 did not affect the primary agglutination caused by GPIb stimulation in aspirin-treated platelets. These data indicate that GPIb-mediated thromboxane generation leads to fibrinogen receptor activation and platelet aggregation in untreated platelets and that this inhibition of thromboxane generation by either aspirin or U0126 completely blocks platelet aggregation induced by ristocetin/VWF.
Inhibition of MEK decreases Erk phosphorylation and VWF-mediated thromboxane production. Platelets were treated with the MEK inhibitor U0126 (10 μM) or its vehicle (control) before stimulation with VWF/ristocetin (8 and 300 μg/mL, respectively). Platelets were then processed for measurement of thromboxane production (A) or detection of phospho-Erk (B), as described in “Materials and methods.”
Inhibition of MEK decreases Erk phosphorylation and VWF-mediated thromboxane production. Platelets were treated with the MEK inhibitor U0126 (10 μM) or its vehicle (control) before stimulation with VWF/ristocetin (8 and 300 μg/mL, respectively). Platelets were then processed for measurement of thromboxane production (A) or detection of phospho-Erk (B), as described in “Materials and methods.”
Inhibition of MEK abolishes VWF-mediated platelet aggregation. Platelets were treated with the MEK inhibitor U0126 (10 μM [+] or its vehicle [–] before stimulation with ristocetin (300 μg/mL) and VWF (8 μg/mL) in the absence (–) or presence (+) of previous treatment with 1 mM aspirin. Platelet aggregation was measured as described in “Materials and methods.”
Inhibition of MEK abolishes VWF-mediated platelet aggregation. Platelets were treated with the MEK inhibitor U0126 (10 μM [+] or its vehicle [–] before stimulation with ristocetin (300 μg/mL) and VWF (8 μg/mL) in the absence (–) or presence (+) of previous treatment with 1 mM aspirin. Platelet aggregation was measured as described in “Materials and methods.”
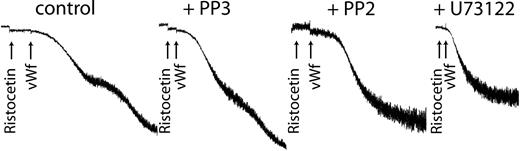
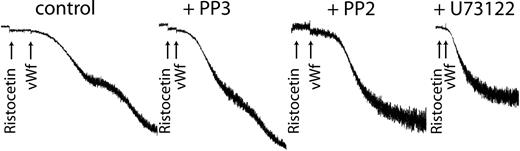
Because we have already shown that inhibiting the activation of Erk or PLC blocked thromboxane generation, we used the respective inhibitors of each to confirm the role of generated thromboxane on GPIb-mediated platelet fibrinogen receptor activation. As shown in Figure 6, both PP2 and U73122 blocked platelet aggregation without affecting the agglutination caused by ristocetin/VWF. These results confirm that generated thromboxane downstream of GPIb activation is essential for the platelet aggregation initiated by ristocetin/VWF.
Discussion
The purpose of this study is to characterize the molecular mechanisms of Erk activation downstream of GPIb stimulation and to evaluate the role of Erk in VWF-induced platelet activation. Many of the signaling events downstream of GPIb are controversial,7 and this study was initiated to clarify one particular controversy that disputed the role of Erk in GPIb-mediated platelet fibrinogen receptor activation.
The idea of whether Erk plays an important role in GPIb-mediated platelet activation has been controversial since studies by Marshall et al26 have shown that GPIb activation by ristocetin/VWF does not lead to Erk activation. Furthermore, they conclude that Erk does not play any important role in GPIb-mediated platelet activation. In contrast, Li et al18 demonstrated that GPIb stimulation leads to Erk activation, which ultimately leads to platelet fibrinogen receptor activation. Our studies support the notion that GPIb stimulation leads to Erk activation, specifically that Src family kinases and PLC are important upstream signaling molecules that regulate the activation of Erk on stimulation by ristocetin/VWF. Marshall et al26 have shown that Src family kinases are important for platelet activation by VWF, but they have not related the role of Src kinases in Erk activation since they fail to detect any Erk activation under their experimental conditions.
The role of PKG is highly disputed by these 2 groups. Evidence from Li et al18 indicates that cyclic guanosine monophosphate (cGMP) is generated downstream of VWF stimulation of GPIb and that leads to activation of PKG. They argue that PKG is the upstream signaling molecule that regulates phosphorylation of Erk as PKG activators cause Erk phosphorylation. However, Marshall et al26 conclude that GPIb activation does not lead to cGMP activation and that the PKG inhibitors used by Li et al might have other targets through which the inhibitors may elicit their effects. In our hands, although PKG inhibitors blocked Erk activation by ristocetin/VWF, PKG activators such as 8-bromo-cGMP failed to cause Erk activation (data not shown).
Inhibition of Src kinases or phospholipase C blocks VWF-mediated platelet aggregation. Platelets were treated with the Src kinase inhibitor PP2 (10 μM), its inactive analog PP3 (10 μM), or the PLC inhibitor U73122 (10 μM) before stimulation with ristocetin (300 μg/mL) and VWF (8 μg/mL). Platelet aggregation was measured as described in “Materials and methods.”
Inhibition of Src kinases or phospholipase C blocks VWF-mediated platelet aggregation. Platelets were treated with the Src kinase inhibitor PP2 (10 μM), its inactive analog PP3 (10 μM), or the PLC inhibitor U73122 (10 μM) before stimulation with ristocetin (300 μg/mL) and VWF (8 μg/mL). Platelet aggregation was measured as described in “Materials and methods.”
Our data consistently demonstrated that inhibiting Erk activation by inhibiting MEK or other upstream signaling events (Src or PLC) led to the blockade of GPIb-mediated thromboxane generation. Unlike the first-generation inhibitors of MEK, such as PD098059, which also blocked the cyclooxygenase pathway, U0126 does not have such effects. Furthermore, inhibiting Erk under similar conditions led to blockade of the thromboxane-mediated second wave of platelet aggregation by ristocetin/VWF. Li et al concluded that Erk does not contribute to thromboxane generation based on the observation that PD98059 or RGDS peptide blocked platelet aggregation to the same extent in aspirin-treated platelets. These results differ from our data but may be attributed to a difference in experimental conditions for aspirin treatment. We consistently used aspirin in PRP and washed platelets prepared from them, and we did not observe thromboxane generation by any agonists (data not shown). However, Li et al used 100 μg/mL aspirin for 5 minutes and had not evaluated whether this treatment was sufficient to block the cyclooxygenase pathway completely. Marshall et al did not observe the effect of MEK inhibition on the GPIb-mediated, thromboxane-dependent second wave of platelet aggregation, most likely because of their use of heparinized blood that contained 2 mM physiologic calcium. It is known that extracellular calcium greatly diminishes thromboxane generation by all agonists except collagen.
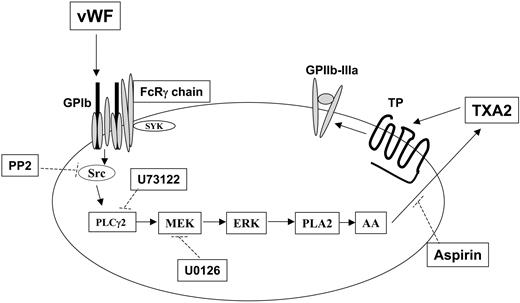
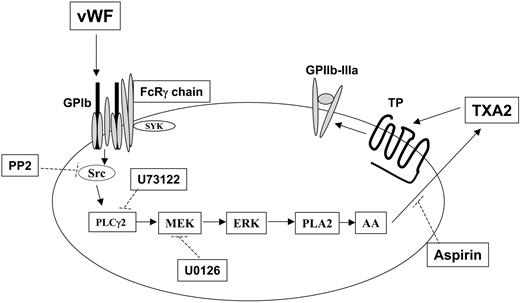
Mechanism of thromboxane production and integrin αIIbβ3 activation after GPIb stimulation in human platelets. Stimulation of GPIb with VWF activates Src, which in turn causes successive activation of phospholipase Cγ2 (PLC2), MEK, and Erk. Erk activation by MEK-mediated phosphorylation activates phospholipase A2 (PLA2), leading to the liberation of arachidonic acid (AA) and its conversion to thromboxane A2 (TXA2) by cyclooxygenase (which is inhibited by aspirin). The released TXA2 then interacts with the G-protein–coupled TP receptor, whose stimulation ultimately contributes to GPIIb–IIIa activation and platelet aggregation. Dotted lines indicate the inhibitory action of various compounds against their respective targets. Note that Src-dependent PLCγ2 activation in response to VWF stimulation in platelets has been previously reported.21,26
Mechanism of thromboxane production and integrin αIIbβ3 activation after GPIb stimulation in human platelets. Stimulation of GPIb with VWF activates Src, which in turn causes successive activation of phospholipase Cγ2 (PLC2), MEK, and Erk. Erk activation by MEK-mediated phosphorylation activates phospholipase A2 (PLA2), leading to the liberation of arachidonic acid (AA) and its conversion to thromboxane A2 (TXA2) by cyclooxygenase (which is inhibited by aspirin). The released TXA2 then interacts with the G-protein–coupled TP receptor, whose stimulation ultimately contributes to GPIIb–IIIa activation and platelet aggregation. Dotted lines indicate the inhibitory action of various compounds against their respective targets. Note that Src-dependent PLCγ2 activation in response to VWF stimulation in platelets has been previously reported.21,26
Thus, given that Erk is important for GPIb-mediated thromboxane generation, we now can evaluate the role of Erk in integrin αIIbβ3 activation. In our study, the use of the fibrinogen receptor antagonist GR144053 and the other inhibitors resulted in similar findings with regard to the stimulation of GPIb with ristocetin/VWF in aspirin-treated platelets. These results indicate that the aggregometry tracings are representative of platelet agglutination rather than platelet aggregation. If the observed phenomena were indeed indicative of fibrinogen receptor activation, the inclusion of GR144053 should have elicited an inhibitory effect on these tracings. On the contrary, the inhibition of Src kinases, PLC, or MEK all blocked aggregation induced by ristocetin/VWF in non–aspirin-treated platelets. Because these molecules are also involved in GPIb-mediated thromboxane production, these data indicate that generated thromboxane is essential for platelet aggregation on GPIb stimulation. Thus, we conclude that Erk does not have a direct role in the GPIb-mediated αIIbβ3 activation in platelets but that it plays an important indirect role through the generation of thromboxane A2 (Figure 7).
In conclusion, ristocetin/VWF-induced activation of GPIb leads to the phosphorylation of Erk through Src family kinases and PLC. This activation of Erk is important for thromboxane generation downstream of GPIb stimulation. Although Erk does not play a direct role in the GPIb-mediated activation of integrin αIIbβ3, it plays an important role indirectly through the generation of thromboxane A2, a more potent platelet agonist.
Prepublished online as Blood First Edition Paper, July 14, 2005; DOI 10.1182/blood-2005-05-1933.
Supported by National Institutes of Health (NIH) research grants HL60683 and HL80444 (S.P.K.) and by NIH training grant HL00777 in thrombosis (A.G., R.T.D.).
A.G. wrote the paper, designed the experiments, performed the research, and analyzed the data. T.M.Q. organized the paper and analyzed the data. R.T.D. designed the experiments and analyzed the data. S.P.K. designed the experiments, analyzed the data, and provided overall direction.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 5. Inhibition of MEK abolishes VWF-mediated platelet aggregation. Platelets were treated with the MEK inhibitor U0126 (10 μM [+] or its vehicle [–] before stimulation with ristocetin (300 μg/mL) and VWF (8 μg/mL) in the absence (–) or presence (+) of previous treatment with 1 mM aspirin. Platelet aggregation was measured as described in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/10/10.1182_blood-2005-05-1933/6/m_zh80220586900005.jpeg?Expires=1769093439&Signature=DcYcDhuoAg2nxxJp3ZUzrImt1hMmNRn1-keX-CEU~CYLOOboXfL1HaCJsB~TU2VSv3WG-itp-knApi1YiANapkujogqxfHfU-8VCsbP3CwjNLFIQfBQvPdv99YU~qKfp9zXt1FelK9NR-LgCi4Rjq365CwNZV3DCIJy2dPDtVlTCudHTO57g9lMfJJw0mFGHs5EyM3Ir-agW7ZG~0wBlx03imijxJtWNW9fhHRYhH8TdFK9GKHzn18kUDDAHtKw-cGMjjWYbtBM9tsT2BEGHsVaDIymYyl35mcyeyPRKCXBwOY~Q3nc6XRYgjLNowYhnprAKpIGSkBgtgY8bH~0HCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Inhibition of MEK abolishes VWF-mediated platelet aggregation. Platelets were treated with the MEK inhibitor U0126 (10 μM [+] or its vehicle [–] before stimulation with ristocetin (300 μg/mL) and VWF (8 μg/mL) in the absence (–) or presence (+) of previous treatment with 1 mM aspirin. Platelet aggregation was measured as described in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/10/10.1182_blood-2005-05-1933/6/m_zh80220586900005.jpeg?Expires=1769093440&Signature=sNyTfZL0g7LHP1ujgqvkkIwihhlo8A77Q51V3WOBBeth5s2a608DiLDxMhwZEeodpT2ie22hqSt6IhO0Rxyh6ghrwmpXUfQ5yW7a-AZsrS0SG2nDZ6PMzgmTtbmEWgwDNkf34v8DYtByrHbsh3tn4ltD7E6tw59qklAEsnMYHw7PqQjaMeFC80ZCU3-qpI90HiTLzrio6H~MKa0NWcLPPYiRovQztSWgn8NpFXXR2SQpD-WY2-cTdohy0-RSuPvUL~7~i~ua0joIUKJhV95pa2OB3AS6s1AGhQ7hnvI1Ik9c9LwGj-EgVHTBcxhxAq3dODyWrESnNQaE0RyEmuiOgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)