Abstract
Exposure of endothelial cells to recombinant tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced a modest (2-fold) increase of HL-60 cell adhesion as compared to TNF-α (40-fold) or interleukin 1β (IL-1β; 20-fold). However, pretreatment of endothelial cultures with TRAIL determined a significant reduction of the proadhesive activity induced by both TNF-α and IL-1β. Unexpectedly, the antiadhesive activity of TRAIL was not due to interference with the nuclear factor κB (NF-κB)-mediated up-regulation of surface intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin adhesion molecules in response to inflammatory cytokines. In searching for the molecular mechanism underlying this biologic activity of TRAIL, a cDNA microarray analysis was performed. TRAIL pretreatment variably down-modulated the mRNA steady-state levels of several TNF-α-induced chemokines, and, in particular, it abrogated the TNF-α-mediated up-regulation of CCL8 and CXCL10. Of note, the addition of optimal concentrations of recombinant CCL8 plus CXCL10 to endothelial cultures completely restored the proadhesive activity of TNF-α. Moreover, experiments performed with agonistic anti-TRAIL receptor antibodies demonstrated that both TRAIL-R1 and TRAIL-R2 contributed, although at different levels, to TRAIL-induced chemokine modulation. Taken together, our data suggest that TRAIL might play an important role in modulating leukocyte/endothelial cell adhesion by selectively down-regulating CCL8 and CXCL10 chemokines.
Introduction
Vascular endothelium is a dynamic tissue that possesses important secretory and metabolic functions and has a central role in controlling leukocyte migration into different tissues in adult life.1,2 The migration of leukocytes into extravascular tissues involves a cascade of molecular events, including the elaboration of chemotactic factors and chemokines, the response to these factors, the interaction of leukocytes with endothelial cells, and leukocyte transmigration through the blood vessel wall.2-5 The first step in the canonical pathway of leukocyte migration involves transient selectin-mediated interactions between rolling leukocytes and the endothelium. Next, integrins on leukocytes are activated by chemokines that have been produced locally; they are presented on glycosaminoglycans, resulting in firm adhesion between leukocytes and endothelial cells. Finally, leukocytes extravasate through the vascular wall and into the surrounding tissue.2-5 Together with adhesion molecules, chemokines regulate the appropriate “addressing and delivery” of each leukocyte subtype to healthy or diseased body compartments.5 It is also noteworthy that an abnormal increase of leukocyte adhesion to endothelial cells is considered an early step in endothelial cell dysfunction.6-8
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), also known as Apo-2 ligand (L), is a 40-kDa protein that is structurally related to the TNF family of cytokines.9,10 TRAIL is expressed as a type II transmembrane protein; however, its extracellular domain can be proteolytically cleaved from the cell surface and acts as a soluble cytokine.11 TRAIL interacts with 4 high-affinity transmembrane receptors and one soluble receptor belonging to the TNF receptor (R) family. TRAIL-R1 (DR4) and TRAIL-R2 (DR5) contain cytoplasmic “death domains” and mediate proapoptotic signals by activating the apical caspases 8 and 10 via the adaptor protein Fas-associating protein with death domain (FADD). However, increasing experimental data indicate that TRAIL-R1 and TRAIL-R2 can also mediate cell type-dependent prosurvival and proliferation signals mainly by activating the extracellular-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) and nuclear factor κB (NF-κB) pathways.11 The other 3 receptors appear to act as “decoys,” although their function is much less characterized. TRAIL-R3 (DcR1) and TRAIL-R4 (DcR2) have homology to the extracellular domains of TRAIL-R1 and TRAIL-R2. TRAIL-R4 has a truncated cytoplasmic death domain, whereas TRAIL-R3 lacks a cytosolic region and is anchored to the plasma membrane through a glycophospholipid moiety. Both receptors are therefore incapable of transmitting an apoptosis signal, whereas the potential role of these receptors in transducing other intracellular signals is unclear.11 The soluble “decoy” receptor osteoprotegerin (OPG) was initially discovered to bind the TNF superfamily member receptor activator of NF-κB ligand (RANKL). Later, OPG was found to bind also TRAIL, although, similarly to the transmembrane decoy receptors TRAIL-R3 and TRAIL-R4, its role in modulating the biologic activity of TRAIL is not firmly established.11
Whereas TNF-α, the prototype member of the TNF family of cytokines, strongly induces leukocyte adhesion to endothelial cells,1 the role of TRAIL on leukocyte adhesion has not been characterized so far, despite the fact that endothelial cells express all 4 transmembrane TRAIL receptors.12,13 In this context, we have previously demonstrated that recombinant TRAIL promotes the survival/proliferation of endothelial cells through activation of the Akt and ERK/MAPK pathways13,14 and Li et al15 have provided preliminary evidence that TRAIL might have proadhesive activity in endothelial cells. Therefore, the aim of this study was to address the role of TRAIL on endothelial/leukocyte interactions. For this purpose, a series of in vitro studies were performed to investigate the biologic activity of TRAIL used alone and in combination with canonical proinflammatory cytokines, such as TNF-α and interleukin 1β (IL-1β).
Materials and methods
Materials
Recombinant histidine 6-tagged TRAIL was produced as described.13,14 Recombinant TNF-α and IL-1β were purchased from Sigma (Saint Louis, MO); CCL8, CXCL10, CCL20, and CXCL1, were purchased from R&D Systems (Minneapolis, MN); IL-8, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) were purchased from PeproTech (London, United Kingdom). The optimal concentrations of TRAIL, IL-1β, TNF-α, VEGF, and bFGF (10 ng/mL each) were determined in preliminary dose-response experiments. The selective activation of single TRAIL receptors was performed by using agonistic goat anti-human TRAIL-R1, TRAIL-R2, TRAIL-R3, TRAIL-R4 polyclonal antibodies (Abs), and neutralization of IL-8 was performed by using the Ab (clone 6217) anti-IL-8 (all from R&D Systems).
For Western blot analyses, the following Abs were used: anti-IκBα and IκBβ Abs (both from Santa Cruz Biotechnology, Santa Cruz, CA), antitubulin Ab (Sigma). The enhanced chemiluminescence (ECL) reagent detection system was from DuPont-NEN (Boston, MA).
Flow cytometric analyses were performed by fluorescence-activated cell sorting (FACScan; Becton Dickinson, San Jose, CA), using anti-human TNF receptor 1 (TNF-R1) and anti-human TNF-R2 Abs (both from Alexis Biochemical, Lausen, Switzerland); phycoerythrin (PE)-conjugated anti-mouse secondary Ab (Immunotech; Marseille, France); fluorescein isothiocyanate (FITC)-conjugated anti-E-selectin, anti-intercellular adhesion molecule 1 (ICAM-1; Bender Medical System, Vienna, Austria), and antivascular cell adhesion molecule 1 (VCAM-1; Cymbus Biotechnology; Chandlers Ford, United Kingdom) Abs. Nonspecific fluorescence was assessed using normal mouse IgG followed by second layer or by incubation with irrelevant isotype-matched conjugated Abs.
Measurement of chemokines in endothelial cell culture supernatants was performed with chemokine-specific enzyme-linked immunosorbent assays (ELISAs; Search Light Human Chemokine Arrays; Pierce, Rockford, IL), following the manufacturer's instructions.
Cell cultures
Primary human umbilical vein endothelial cells (HUVECs) were obtained as described.13,14 Approval was obtained from the University of Ferrara institutional review board and informed consent was provided according to the Declaration of Helsinki. Cells were grown on 0.2% gelatin-coated tissue-culture plates in M199 endothelial growth medium (EGM; BioWhittaker; Walkersville, MD) supplemented with 20% fetal bovine serum (FBS), 10 μg/mL heparin, and 50 μg/mL endothelial cell growth factor (ECGF). Human aortic endothelial cells (HAECs) were purchased from BioWhittaker and cultured in EGM basal medium supplemented with 2% FBS, 12 μg/mL bovine brain extract (BBE), 1 μg/mL hydrocortisone, and 10 ng/mL ECGF (all from BioWhittaker). In all experiments cells were used between the third and fifth passage in vitro.
The myeloid HL-60 leukemic cell line (American Type Culture Collection, Rockville, MD) was routinely grown in RPMI supplemented with 10% FBS.
Adhesion assay
Confluent HUVECs or HAECs, seeded in 24-well plates, were treated with TRAIL or inflammatory cytokines or both for 18 hours. In other experiments, HUVECs were pretreated for 1, 2, 3, or 4 days with TRAIL before being exposed to inflammatory cytokines for an additional 18 hours. When indicated, endothelial cells were pretreated with agonist anti-TRAIL receptor Abs for 3 days before stimulation with inflammatory cytokines. After treatments, the culture medium was removed and endothelial cultures were washed twice before adding HL-60 (350 × 103/well) to avoid any cytotoxic effect of recombinant TRAIL on HL-60. In selected experiments, recombinant chemokines were added to the endothelial cultures together with HL-60. After 1 hour of coculture of endothelial cells and HL-60, unbound HL-60 cells were removed by gently washing with medium. Endothelial-leukocyte cocultures were photographed under a Nikon Eclipse TE 200-S inverted light microscope (Nikon Instech, Kawasaki, Japan), 10 ×/0.25, using a CoolSnap video camera (Photometrics, Livingston, United Kingdom). Adhered leukocytes were counted/scored in at least 6 random fields for each treatment.
The viability of both endothelial cells and adherent HL-60 was routinely monitored at light microscopy by trypan blue dye exclusion. In some experiments, parallel sets of endothelial cells (treated and untreated) were assessed using 3-[4,5-dimethylthiazol-2-yl]-2.5-diphenyl tetrazolium bromide (MTT) and neutral red staining, performed as described,16 whereas the viability of unbound HL-60 was analyzed by propidium iodide (PI) staining and flow cytometric analysis of apoptosis, performed as described.13
Western blot and assay for NF-κB DNA binding
For Western blot analysis, HUVECs were grown at subconfluence prior to the addition of TRAIL or TNF-α. Cells were harvested in lysis buffer containing 1% Triton X-100, Pefablock (1 mM), aprotinin (10 μg/mL), pepstatin (1 μg/mL), leupeptin (10 μg/mL), NaF (10 mM), and Na3VO4 (1 mM). Protein determination was performed by Bradford assay (Bio-Rad, Richmond, CA). Equal amounts of protein (50 μg) for each sample were migrated in acrylamide gels and blotted onto nitrocellulose filters. Blotted filters were probed with primary Abs for IκBα and IκBβ and for tubulin to verify loading evenness.
NF-κB induction was measured using the Trans-am NF-κB p65 and p50 kit (Active Motif, Rixensart, Belgium), which measures the level of active form of NF-κB contained in cell extracts, able to specifically bind to an oligonucleotide containing the NF-κB consensus site (5′-GGGACTTTCC-3′), attached to a 96-well plate. Assays were performed in triplicate, according to the manufacturer's instructions. NF-κB DNA-binding activity was determined as absorbance values measured by using a microplate reader (Multiskan Ascent; Dasit, Milan, Italy). Increase in fluorescence was linear over extract concentration.
cDNA microarray analysis
RNA was isolated with a Qiagen RNeasy kit (Hilden, Germany) from HUVECs, either left untreated or stimulated with TNF-α, in the absence or presence of a pretreatment for 3 days with TRAIL. Labeled cDNA was hybridized with a customized cDNA microarray containing an array of 367 inflammation-associated genes together with housekeeping genes at the SuperArray core facility (the list of the genes is available elsewhere).17 Analysis of gene expression was done essentially as described.18 Alterations imposed by the different treatments on the basal gene expression were determined as the ratio of relative gene expression compared to unstimulated cells. The fold change in expression between unstimulated and treated cultures was considered significant when greater than 2. However, only those genes that were differentially expressed more than 3-fold were selected to reliably detect differential expression in the reverse transcription-polymerase chain reaction (RT-PCR) confirmation assays (by SingleGene PCR kit, purchased from SuperArray Bioscience, Frederick, MD).
Statistical analysis
For each set of experiments, values are reported as means ± SD. The results were evaluated by using analysis of variance with subsequent comparisons by Student t test. Statistical significance was defined as P less than .05.
Results
Recombinant TRAIL alone induces a modest HL-60 cell adhesion to endothelial cells as compared to proinflammatory cytokines
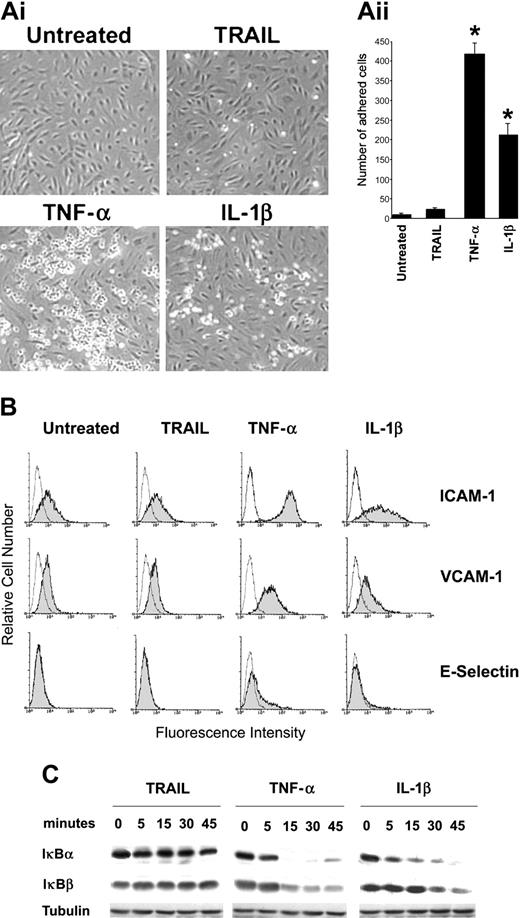
The first group of experiments was designed to investigate whether TRAIL modulates the adhesion properties of endothelial cells. As shown in Figure 1A, in 12 separate experiments, TRAIL (10 ng/mL) induced an approximately 2-fold increase of HL-60 adhesion to HUVECs. However, the TRAIL-induced HL-60 cell adhesion was extremely modest as compared to canonical proinflammatory cytokines, such as TNF-α and IL-1β (both used at 10 ng/mL), which induced approximately 40-fold (P < .01) and 20-fold (P < .01) increase, respectively, over the background levels. Moreover, at variance with TNF-α or IL-1β, TRAIL did not modulate the surface expression of adhesion molecules ICAM-1, VCAM-1, and E-selectin (Figure 1B) and it did not activate the NF-κB pathway (Figure 1C) in endothelial cells.
TRAIL and inflammatory cytokine-mediated leukocyte adhesion. HUVECs were either left untreated or exposed to TRAIL, TNF-α, or IL-1β. (Ai) HL-60 cell adherence on HUVECs treated (for 18 hours) as indicated. Representative fields of the cultures are shown (original magnification × 10). (Aii) Cell adhesion was calculated as described in “Materials and methods” and is expressed as mean ± SD of 12 experiments, each performed in triplicate. *P < .05. (B) HUVECs were exposed to cytokines for 18 hours and expression levels of leukocyte adhesion molecules (ICAM-1, VCAM-1, and E-selectin) were evaluated by flow cytometry. The control (open) histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control Abs. One of 8 experiments with similar results is shown. (C) HUVECs were stimulated with TRAIL, TNF-α, or IL-1β for the indicated time intervals (0-45 minutes), and cell lysates were analyzed for degradation of IκBα and IκBβ by Western blotting. Equal loading of protein in each lane was confirmed by staining with the Ab to tubulin. One of 4 experiments with similar results is shown.
TRAIL and inflammatory cytokine-mediated leukocyte adhesion. HUVECs were either left untreated or exposed to TRAIL, TNF-α, or IL-1β. (Ai) HL-60 cell adherence on HUVECs treated (for 18 hours) as indicated. Representative fields of the cultures are shown (original magnification × 10). (Aii) Cell adhesion was calculated as described in “Materials and methods” and is expressed as mean ± SD of 12 experiments, each performed in triplicate. *P < .05. (B) HUVECs were exposed to cytokines for 18 hours and expression levels of leukocyte adhesion molecules (ICAM-1, VCAM-1, and E-selectin) were evaluated by flow cytometry. The control (open) histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control Abs. One of 8 experiments with similar results is shown. (C) HUVECs were stimulated with TRAIL, TNF-α, or IL-1β for the indicated time intervals (0-45 minutes), and cell lysates were analyzed for degradation of IκBα and IκBβ by Western blotting. Equal loading of protein in each lane was confirmed by staining with the Ab to tubulin. One of 4 experiments with similar results is shown.
TRAIL counteracts the TNF-α- and IL-1β-induced leukocyte adhesion to endothelial cells
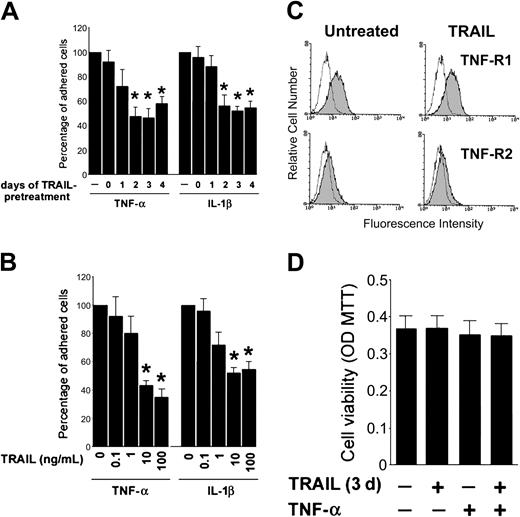
To examine the effect of TRAIL on the biologic response induced by proinflammatory cytokines, HUVECs were cultured with TRAIL, added in combination with TNF-α or IL-1β. When TRAIL was added at the same time with TNF-α or IL-1β, it modestly affected the proadhesive activity of both inflammatory cytokines (Figure 2A). On the other hand, a 2- to 4-day preexposure to TRAIL before addition to inflammatory cytokines induced a clear-cut (P < .05) decrease in the number of adherent HL-60 cells to HUVECs (Figure 2A). This was a dose-dependent effect and showed a plateau at a TRAIL concentration of 10 ng/mL (Figure 2B). Of note, the antiadhesive activity of TRAIL in TNF-α-treated cultures was not due to a down-regulation of surface TNF-R1 or TNF-R2 in HUVECs, as shown by flow cytometric analysis performed 3 days after TRAIL treatment (Figure 2C). The possibility that the observed effects were due to cellular toxicity induced by TRAIL or TRAIL plus TNF-α was ruled out by assessing endothelial cell viability following treatments. As expected on the basis of previous studies of our and other groups of investigators12-14 incubation of endothelial cells for 2 to 4 days with TRAIL, irrespective of TNF-α addition, did not induce any significant cytotoxicity as determined by MTT staining (Figure 2D). Moreover, the decreased adhesiveness of HL-60 cells also to HAECs in response to TNF-α (data not shown) allowed us to exclude the possibility that the anti-inflammatory activity of TRAIL was restricted to HUVECs.
TRAIL pretreatment alters inflammatory cytokine-mediated leukocyte adhesion. HUVECs were either left untreated or preexposed to TRAIL before stimulation with TNF-α or IL-1β for 18 hours. (A) Cells were treated with TRAIL (10 ng/mL) at the time of exposure to inflammatory cytokines (day 0) or 1 to 4 days before. (B) Dose-response effect of 3 days of TRAIL pretreatment (used at the indicated concentrations) was evaluated on HL-60 cell adhesion to HUVECs stimulated by either TNF-α or IL-1β. In panels A-B, cell adhesion in the absence of TRAIL pretreatment was set as 100%. In panels A-B, results are expressed as means ± SD of 3 independent experiments, each performed in triplicate. *P < .05. (C) TRAIL pretreatment does not affect surface expression of TNF receptors (TNF-R1 and TNF-R2). The control (open) histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control Abs. One of 4 experiments with similar results is shown. (D) Viability of endothelial cultures (treated as indicated) was assessed by MTT assay. Data are shown as average optical density (OD) ± SD.
TRAIL pretreatment alters inflammatory cytokine-mediated leukocyte adhesion. HUVECs were either left untreated or preexposed to TRAIL before stimulation with TNF-α or IL-1β for 18 hours. (A) Cells were treated with TRAIL (10 ng/mL) at the time of exposure to inflammatory cytokines (day 0) or 1 to 4 days before. (B) Dose-response effect of 3 days of TRAIL pretreatment (used at the indicated concentrations) was evaluated on HL-60 cell adhesion to HUVECs stimulated by either TNF-α or IL-1β. In panels A-B, cell adhesion in the absence of TRAIL pretreatment was set as 100%. In panels A-B, results are expressed as means ± SD of 3 independent experiments, each performed in triplicate. *P < .05. (C) TRAIL pretreatment does not affect surface expression of TNF receptors (TNF-R1 and TNF-R2). The control (open) histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control Abs. One of 4 experiments with similar results is shown. (D) Viability of endothelial cultures (treated as indicated) was assessed by MTT assay. Data are shown as average optical density (OD) ± SD.
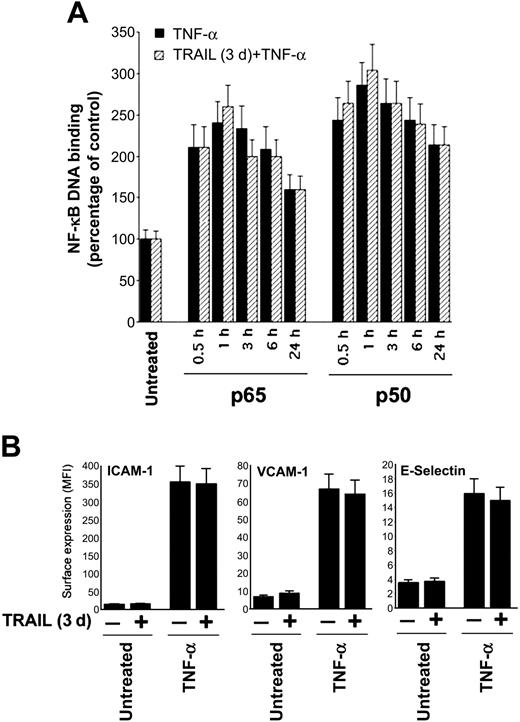
Next, experiments were designed to elucidate whether TRAIL interfered with the ability of TNF-α to activate the NF-κB pathway, which plays a key role in the regulation of ICAM-1, VCAM-1, and E-selectin surface adhesion molecules. As shown in Figure 3A, TRAIL did not modulate the NF-κB pathway and, consistently, did not affect the surface expression of ICAM-1, VCAM-1, and E-selectin induced by TNF-α (Figure 3B).
TRAIL pretreatment does not affect TNF-α-induced NF-κB activation. HUVECs were either left untreated or pretreated with TRAIL for 3 days before stimulation with TNF-α. (A) NF-κB-p65 and NF-κB-p50 DNA-binding activity was determined at the indicated time points as absorbance values and is expressed as percentage of untreated control. ▪ indicates TNF-α; ▨, pretreated with TRAIL. (B) Analysis of surface leukocyte adhesion molecules (VCAM-1, ICAM-1, E-selectin) was performed by flow cytometry and is reported as mean fluorescence intensity (MFI). Results are expressed as means ± SD of 3 (for panel A) or 8 (for panel B) independent experiments.
TRAIL pretreatment does not affect TNF-α-induced NF-κB activation. HUVECs were either left untreated or pretreated with TRAIL for 3 days before stimulation with TNF-α. (A) NF-κB-p65 and NF-κB-p50 DNA-binding activity was determined at the indicated time points as absorbance values and is expressed as percentage of untreated control. ▪ indicates TNF-α; ▨, pretreated with TRAIL. (B) Analysis of surface leukocyte adhesion molecules (VCAM-1, ICAM-1, E-selectin) was performed by flow cytometry and is reported as mean fluorescence intensity (MFI). Results are expressed as means ± SD of 3 (for panel A) or 8 (for panel B) independent experiments.
The anti-inflammatory activity of TRAIL is mediated by a down-regulation of the mRNA steady-state levels of CCL8 and CXCL10 chemokines
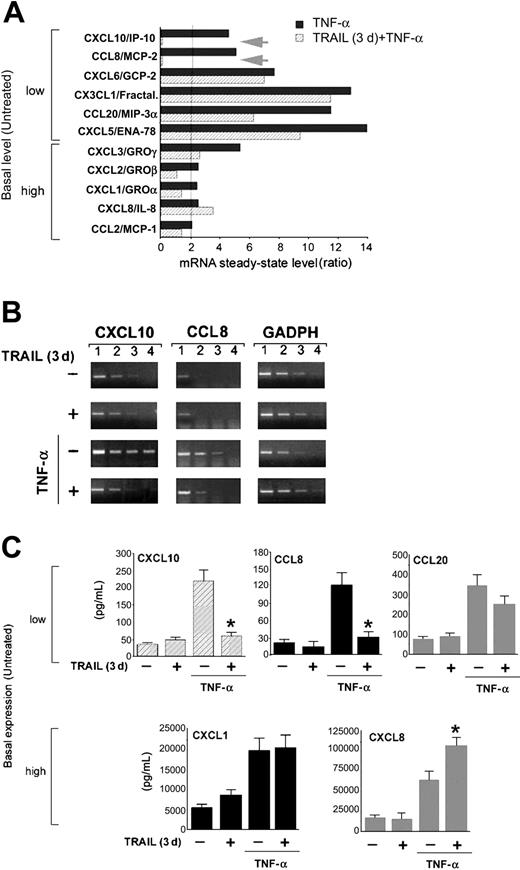
Because chemokines play a major role in promoting the adhesiveness of leukocytes to endothelial cells,1-5,19 the expression profile of a set of chemokines17 was next analyzed by cDNA microarray in unstimulated HUVECs, HUVECs treated with TNF-α for 18 hours, and HUVECs treated with TNF-α for 18 hours after a preexposure to TRAIL for 3 days. As shown in Figure 4A, TNF-α induced an increase (from 2- to 14-fold) of the mRNA steady-state levels of several chemokines with respect to unstimulated cultures. The TNF-α-induced chemokines were divided into 2 groups on the basis of their low or high basal steady-state mRNA levels in unstimulated endothelial cells. Among the low basal steady-state mRNA group of chemokines, TRAIL partially decreased the expression of CCL20/macrophage inflammatory protein 3α (MIP-3α) and CXCL5/epithelial neutrophil-activating protein 78 (ENA-78), whereas it completely abrogated the TNF-α-mediated up-regulation of CXCL10/interferon-inducible protein 10 (IP-10) and CCL8/monocyte chemotactic protein 2 (MPC-2) (Figure 4A). Among the high basal steady-state mRNA group of chemokines, TRAIL modestly affected the expression of CXCL1/GROα, CXCL2/GROβ, CXCL3/GROγ, and CCL2/MPC-1, whereas it increased the expression CXCL8/IL-8 (Figure 4A).
The antiadhesive activity of TRAIL is mediated by down-regulation of chemokines. Differential chemokine gene expression was assessed by cDNA microarray analysis in HUVECs, either left untreated or pretreated with TRAIL for 3 days, before stimulation with TNF-α. (A) Ratios represent TNF-α (▪) or TRAIL (3 days) plus TNF-α (▨) values divided by untreated values. The cut-off of 2-fold of induction is shown as a vertical line. Arrows indicate the genes most significantly down-modulated by the TRAIL pretreatment. (B) Microarray results were validated by semiquantitative RT-PCR (1-4, serial scalar dilutions of the RNA templates; GAPDH, housekeeping gene). (C) Chemokine release by HUVECs either left untreated or pretreated with TRAIL for 3 days, before stimulation with TNF-α, was measured by ELISA. Results are expressed as means ± SD of 3 independent experiments, each performed in triplicate. *P < .05.
The antiadhesive activity of TRAIL is mediated by down-regulation of chemokines. Differential chemokine gene expression was assessed by cDNA microarray analysis in HUVECs, either left untreated or pretreated with TRAIL for 3 days, before stimulation with TNF-α. (A) Ratios represent TNF-α (▪) or TRAIL (3 days) plus TNF-α (▨) values divided by untreated values. The cut-off of 2-fold of induction is shown as a vertical line. Arrows indicate the genes most significantly down-modulated by the TRAIL pretreatment. (B) Microarray results were validated by semiquantitative RT-PCR (1-4, serial scalar dilutions of the RNA templates; GAPDH, housekeeping gene). (C) Chemokine release by HUVECs either left untreated or pretreated with TRAIL for 3 days, before stimulation with TNF-α, was measured by ELISA. Results are expressed as means ± SD of 3 independent experiments, each performed in triplicate. *P < .05.
Validation of the microarray results was performed by semiquantitative RT-PCR and accompanied by ELISA measurement of release in culture media for selected chemokines. The most striking effect associated with TRAIL pretreatment at both the mRNA and protein levels (Figure 4A-C) was the almost complete abrogation of the TNF-α-induced steady-state mRNA levels and protein release of CCL8/MCP-2 and CXCL10/IP-10. On the other hand, the release in culture supernatant of other chemokines, belonging to the low (CCL20/MIP-3α) and high (CXCL1/GROα) basal steadystate mRNA groups and whose mRNA level was variably decreased by TRAIL, was unaffected by TRAIL pretreatment (Figure 4C). In addition, and in agreement with the microarray results (Figure 4A), TRAIL pretreatment (P < .05) further increased the release in culture of CXCL8/IL-8 protein in response to TNF-α (Figure 4C).
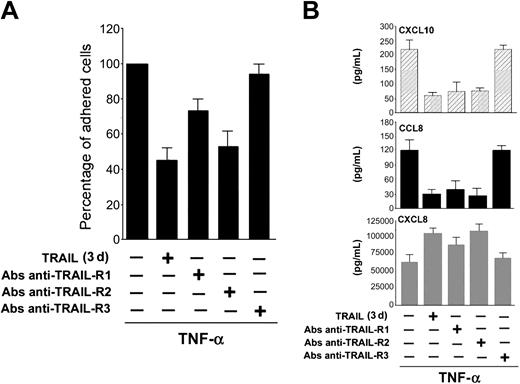
To investigate whether the TRAIL-mediated down-regulation of CCL8/MCP-2 and CXCL10/IP-10 and up-regulation of CXCL8/IL-8 were mediated by a specific TRAIL receptor, HUVECs were challenged with agonistic polyclonal Abs anti-TRAIL receptors, which mimic the interaction between TRAIL and each TRAIL receptor, before exposure to TNF-α. Pretreatment for 3 days with either anti-TRAIL-R1 or anti-TRAIL-R2 Abs significantly (P < .05) decreased, although at different levels, the proadhesive activity of TNF-α, whereas anti-TRAIL-R3 did not show any significant biologic activity (Figure 5A). Moreover, both anti-TRAIL-R1 and anti-TRAIL-R2, but not anti-TRAIL-R3, decreased the release in culture of CCL8/MCP-2 and CXCL10/IP-10 and promoted the release of CXCL8/IL-8 (Figure 5B).
Role of specific TRAIL receptor triggering in TRAIL-mediated antiadhesive activity and modulation of chemokine release. HUVECs were either left untreated or pretreated for 3 days with the indicated agonistic polyclonal Abs anti-TRAIL receptors before addition of TNF-α. (A) TNF-α-induced HL-60 cell adhesion to HUVECs was set as 100%. Results are expressed as means ± SD of 3 independent experiments, each performed in triplicate. (B) Chemokine release was measured by ELISA. Results are expressed as means ± SD of 3 independent experiments, each performed in triplicate.
Role of specific TRAIL receptor triggering in TRAIL-mediated antiadhesive activity and modulation of chemokine release. HUVECs were either left untreated or pretreated for 3 days with the indicated agonistic polyclonal Abs anti-TRAIL receptors before addition of TNF-α. (A) TNF-α-induced HL-60 cell adhesion to HUVECs was set as 100%. Results are expressed as means ± SD of 3 independent experiments, each performed in triplicate. (B) Chemokine release was measured by ELISA. Results are expressed as means ± SD of 3 independent experiments, each performed in triplicate.
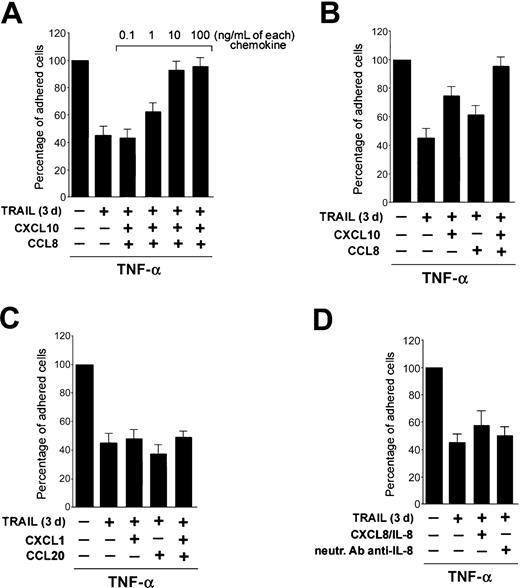
Next, experiments were performed to evaluate the biologic significance of TRAIL interference on TNF-α-induced CCL8/MCP-2, CXCL10/IP-10, and CXCL8/IL-8 steady-state mRNA levels and protein release in mediating the antiadhesive activity of TRAIL. For this purpose, increasing concentrations of recombinant CCL8/MCP-2 plus CXCL10/IP-10 were added to HUVECs preexposed to TRAIL and then treated with TNF-α. As shown in Figure 6A, the addition in culture of CXCL10/IP-10 plus CCL8/MCP-2 dose dependently counteracted the antiadhesive activity of TRAIL in TNF-α-stimulated cultures. Thus, optimal concentrations (10 ng/mL each) of the 2 chemokines added together restored the TNF-α-induced cell adhesion, completely abrogating the antiadhesive effect of TRAIL (Figure 6A-B). In parallel, the addition of CCL8/MCP-2 and CXCL10/IP-10 to untreated or TRAIL-treated endothelial cells showed that these chemokines were unable to increase the adhesive properties of endothelial cells in the absence of the proinflammatory stimulus (data not shown). On the contrary, the addition of other recombinant chemokines (CXCL1/GROα + CCL20/MIP-3α), whose release in culture was not significantly affected by TRAIL pretreatment, did not revert the antiadhesive activity of TRAIL (Figure 6C). Similarly, the addition in culture of recombinant CXCL8/IL-8, which has been shown to promote leukocyte adhesion to endothelial cells20 and is abundantly produced in endothelial cultures treated with TRAIL plus TNF-α, did not revert the TRAIL-mediated inhibition (Figure 6D). Moreover, the possibility that the increased release of CXCL8/IL-8 in response to TRAIL plus TNF-α (Figure 4C) might be paradoxically involved in mediating the antiadhesive activity of TRAIL was excluded (Figure 6D). In fact, the addition in culture of anti-CXCL8/IL-8-neutralizing Abs did not modulate the adhesive activity of TRAIL plus TNF-α-treated cultures (Figure 6D).
The antiadhesive activity of TRAIL is reverted by addition of CXCL10 and CCL8. HUVEC were either left untreated or pretreated for 3 days with TRAIL before addition of TNF-α and adhesion assay. When indicated, recombinant CXCL10 and CCL-8 (A-B), CXCL1 and CCL20 (C), and IL-8 and neutralizing Ab anti-IL-8 (D) were added to the cultures either alone or in combination. TNF-α-induced HL-60 cell adhesion to HUVECs was set as 100%. Results are expressed as means ± SD of 3 to 4 independent experiments, each performed in triplicate.
The antiadhesive activity of TRAIL is reverted by addition of CXCL10 and CCL8. HUVEC were either left untreated or pretreated for 3 days with TRAIL before addition of TNF-α and adhesion assay. When indicated, recombinant CXCL10 and CCL-8 (A-B), CXCL1 and CCL20 (C), and IL-8 and neutralizing Ab anti-IL-8 (D) were added to the cultures either alone or in combination. TNF-α-induced HL-60 cell adhesion to HUVECs was set as 100%. Results are expressed as means ± SD of 3 to 4 independent experiments, each performed in triplicate.
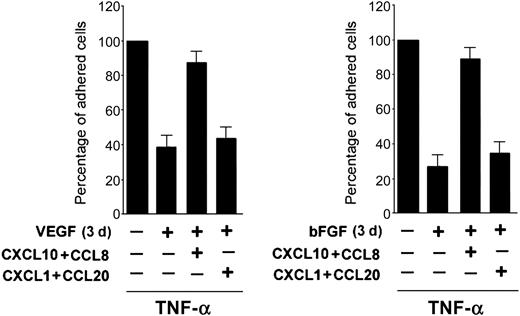
In additional experiments, the critical role of CCL8/MCP-2 and CXCL10/IP-10 in mediating the proadhesive activity of TNF-α was ascertained by analyzing combinations of TNF-α with other antiadhesive cytokines. For this purpose, HUVECs were pretreated for 3 days with the proangiogenic cytokines, bFGF and VEGF, which down-regulate the TNF-α-mediated proadhesive activity.21 As shown in Figure 7, the addition of recombinant CCL8/MCP-2 plus CXCL10/IP-10 reverted the antiadhesive activity of both bFGF and VEGF in TNF-α-treated cultures, therefore suggesting that the control of their release in culture represents a common target for several cytokines able to down-modulate the proadhesive activity of inflammatory cytokines.
CXCL10 plus CCL8 reverts the antiadhesive activity also of VEGF and bFGF. HUVECs were either left untreated or pretreated for 3 days with VEGF or bFGF before addition of TNF-α and adhesion assay. At the time of the HL-60 cell adhesion assay, the indicated recombinant chemokines (10 ng/mL each) were added to the cultures. TNF-α-induced HL-60 cell adhesion to HUVECs was set as 100%. Results are expressed as means ± SD of 4 independent experiments, each performed in triplicate.
CXCL10 plus CCL8 reverts the antiadhesive activity also of VEGF and bFGF. HUVECs were either left untreated or pretreated for 3 days with VEGF or bFGF before addition of TNF-α and adhesion assay. At the time of the HL-60 cell adhesion assay, the indicated recombinant chemokines (10 ng/mL each) were added to the cultures. TNF-α-induced HL-60 cell adhesion to HUVECs was set as 100%. Results are expressed as means ± SD of 4 independent experiments, each performed in triplicate.
Discussion
Our data demonstrated for the first time that TRAIL significantly counteracts the proadhesive activity of canonical inflammatory cytokines, such as TNF-α or IL-1β. However, TRAIL did not significantly modulate the surface level of ICAM-1, VCAM-1, and E-selectin adhesion molecules in HUVECs, either when used alone or in association with TNF-α or IL-1β. Consistently, TRAIL did not affect the ability of TNF-α to potently activate the NF-κB pathway, a prerequisite for the transcriptional up-regulation of ICAM-1, VCAM-1, and E-selectin.22 Nevertheless, TRAIL induced a modest (2-fold) increase of leukocyte adhesion to HUVECs, a finding in agreement with the data of Li et al,15 who described a similar proadhesive activity of TRAIL. However, at variance with Li et al, and consistent with our previous findings,13 we did not observe any TRAIL cytotoxic effect or any TRAIL-induced activation of the NF-κB pathway. Although we do not have a ready explanation for this discrepancy, it might be ascribed to the different time frame examined (5-7 hours of TRAIL treatment in the Li study versus 18-90 hours in our experiments) and endothelial culture conditions. In this respect, it has recently been shown that the susceptibility of endothelial cells to TRAIL cytotoxicity critically depends on the nature of the substrate on which endothelial cells are cultured.23 The fact that TRAIL did not modify the TNF-α-mediated up-regulation of ICAM-1, VCAM-1, and E-selectin likely accounts for the incomplete suppression (approximately 60% inhibition) of the TNF-α-induced proadhesive activity in endothelial cells preexposed to TRAIL.
However, the most striking result of our study was the ability of TRAIL to significantly down-modulate the potent proadhesive activity of inflammatory cytokines through a down-regulation of CCL8/MCP-2 and CXCL10/IP-10 chemokine steady-state mRNA levels and protein release. Chemokines can be divided broadly into 2 categories: (1) inflammatory chemokines, which recruit leukocytes in response to physiologic stress, and (2) homeostatic chemokines, which are responsible for basal leukocyte trafficking and the forming and architecture of secondary lymphoid organs.4 Both CCL8/MCP-2 and CXCL10/IP-10 belong to the group of inflammatory chemokines,24-27 which are expressed in inflamed tissues by resident and infiltrated cells after stimulation by proinflammatory cytokines or during contact with pathogenic agents. This group of chemokines is specialized for the recruitment of effector cells, including monocytes, granulocytes, and effector T cells.4 The ability of TRAIL to down-regulate CCL8/MCP-2 and CXCL10/IP-10 expression and release explains how TRAIL counteracts the proadhesive activity of TNF-α without interfering with the TNF-α-mediated up-regulation of the surface expression of adhesion molecules in endothelial cells. It is noteworthy that although it has been shown that chemokines often show redundant biologic activity,5 the addition in culture of other recombinant chemokines (CXCL1/GROα and CCL20/MIP-3α) was unable to substitute for CCL8/MCP-2 and CXCL10/IP-10. The discrepancy between the levels of endogenous CCL8/MCP-2 and CXCL10/IP-10 detected in culture supernatants of endothelial cells by ELISA and the concentrations of recombinant CCL8/MCP-2 and CXCL10/IP-10 required to fully restore the proadhesive activity of TNF-α can be explained at least in part by the fact that chemokines accumulate mainly on the endothelial cell layer through glycosaminoglycan binding, where they induce conformational changes in the adhesion molecules of endothelial cells, increasing their adhesiveness property.5 The glycosaminoglycan-bound chemokines are not measured by ELISA performed on culture supernatants.
We cannot completely exclude the possibility that the down-regulation of CCL8/MCP-2 and CXCL10/IP-10 might be selectively due to the recruitment of only one of the TRAIL receptors (TRAIL-R1 or TRAIL-R2), able to elicit an intracellular signal transduction pathway. However, this possibility is unlikely because in experiments performed with agonistic anti-TRAIL-R1 and anti-TRAIL-R2 polyclonal Abs, both Abs mimicked the activity of recombinant TRAIL in terms of inhibition of CCL8/MCP-2 and CXCL10/IP-10 and stimulation of CXCL8/IL-8.
Although we have not investigated the molecular mechanisms underlining the TRAIL-mediated down-regulation of CCL8/MCP-2 and CXCL10/IP-10, the involvement of the NF-κB transcription factor family members was excluded. A complex interplay exists between TRAIL and NF-κB in different cell models,28 but we have clearly demonstrated that TRAIL does not induce NF-κB activity in endothelial cells nor does it perturb the TNF-α-mediated induction of NF-κB. To the best of our knowledge, little is known about the effect of TRAIL at the transcription level, but it has been previously shown in a lymphoid model that TRAIL negatively regulates the transcription of an important member of the AP-1 family, c-Fos,29 which might help to explain the observed CCL8/MCP-2 and CXCL10/IP-10 down-regulation.
CCL8/MCP-2 and CXCL10/IP-10 were able to restore the proadhesive activity of TNF-α also in the presence of the proangiogenic cytokines VEGF and bFGF, implying that the modulation of CCL8/MCP-2 and CXCL10/IP-10 represents a critical step in the control of adhesiveness in response to TNF-α. Moreover, these data suggest that modulation of these 2 chemokines represent a general mechanism shared by different cytokines to attenuate the proadhesive activity of TNF-α. Interestingly, also transforming growth factor β1 (TGF-β1) has been previously shown to generally reduce the secretion of several chemokines in TNF-α-activated endothelial cells.24 Thus, in analogy to TGF-β1, which has a role in the later phases of inflammation when repair and tissue regeneration start to occur, also TRAIL may act as a modulator of inflammation. In this respect, of particular interest is our previous demonstration that TRAIL promotes endothelial cell survival and proliferation,13 which are often observed at the end of an inflammatory process in the transition to repair and regeneration.30 In keeping with a potential physiopathologic role of TRAIL in counteracting endothelial cell dysfunction, previous studies have shown that the serum level of OPG, which acts as a soluble decoy receptor for TRAIL, is significantly elevated in patients with chronic inflammation states, such as diabetes mellitus31,32 and coronary artery disease.33
It is noteworthy that the number of chemokines and chemokine receptors is very high and that chemokines have been shown to have redundant biologic functions.1-5 Consistently, it has been demonstrated that mice with functional deletions in a single gene that encode chemokines or their receptors tend to show mild alterations in defensive functions.5 Thus, although further studies are needed to ascertain the potential role of the TRAIL/TRAIL receptors system in modulating the trafficking of leukocytes in vivo, our data demonstrate for the first time that TRAIL has a potent antiadhesive effect on in vitro adhesion promoted by inflammatory cytokines and demonstrate that this biologic activity is mediated by the selective and simultaneous down-regulation of CCL8 and CXCL10.
Prepublished online as Blood First Edition Paper, January 11, 2005; DOI 10.1182/blood-2004-10-4111.
Supported by Italian Fondi per l'Incentivazione della Ricerca di Base (FIRB) grants (P.S. and G.Z.), and by an Associazione Italiana per la Ricerca sul Cancro (AIRC) grant (G.Z.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.