Comment on Hoefnagel et al, page 3671
In this issue, Hoefnagel and colleagues identify anatomic site as a major predictor of the gene expression profile for primary cutaneous large B-cell lymphomas, which segregate into GCB and ABC categories with attendant good and poor prognoses.
Lymphomas derived from large or “transformed” B cells have long been recognized as heterogeneous. However, anatomic site has been underappreciated as a parameter for the classification of diffuse large B-cell lymphoma (DLBCL). Although DLBCLs present in a variety of nodal and extranodal sites, many studies fail to take anatomic site into consideration when evaluating biologic data. The pendulum is now swinging in the other direction. Primary mediastinal large B-cell lymphoma, long recognized as a distinct clinicopathologic entity, was recently shown to have a gene expression profile distinct from other DLBCLs.1,2
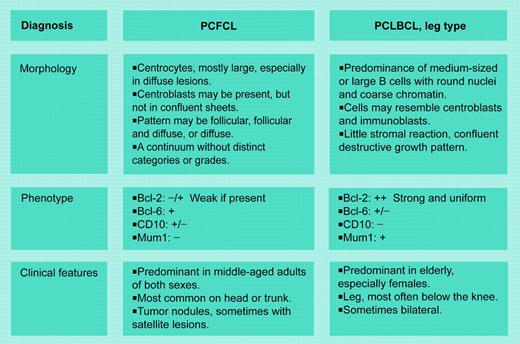
Clinicians have recognized that localized primary cutaneous large B-cell lymphomas (PCLBCLs) can be treated conservatively, with local radiation therapy and excellent outcome in most patients.3 However, when Vermeer et al4 proposed that large B-cell lymphomas of the lower leg were distinct, their observations were greeted with considerable skepticism. Why should lymphomas presenting on the lower leg differ from those on the trunk or scalp? That fundamental question is unanswered; however, the current study lends further support to these distinctions. Using gene expression profiling they show that PCLBCLs of the leg and primary cutaneous follicle center cell lymphomas (PCFCCLs) correspond to activated B-cell (ABC) and germinal center B-cell (GCB) types, respectively. These data correlate with other morphologic and immunophenotypic features. PCLBCLs of the leg are composed of cohesive, round cells with little stromal or cellular reaction; they express Bcl-2 (B-cell lymphoma-2) and multiple myeloma oncogene-1/interferon regulatory factor 4 (Mum1/IRF4) proteins; and have a high proliferative rate. Conversely, PCFCCLs are cytologically heterogeneous, often with numerous admixed T cells, a prominent stromal component, and negative staining for Bcl-2 and Mum1/IRF4.
Should anatomic site take precedence over other features? Grange et al5 found that Bcl-2 expression and round cell morphology were more important than site alone, suggesting that the “leg-type” of DLBCL may occur in other locations and that defining the disease entity is the preeminent predictor. These concepts have been incorporated in the World Health Organization-European Organization of Research and Treatment of Cancer (WHO-EORTC) classification of cutaneous lymphomas, which delineates a PCLBCL, leg type, to encompass this tumor on the lower leg and other sites.6
Interestingly, Hoefnagel and colleagues found discordance between Bcl-2 expression by gene array and protein expression within the tumor cells. While they offer a number of explanations, it may be that the Bcl-2 signal in PCFCCLs is derived from background reactive cells rather than tumor cells. Although they attempted to use a restricted set of 7450 B-cell signature genes to focus on the expression profile of the tumor cells, the importance of the background cellular milieu still fell out as a significant discriminator. Genes associated with the “reactive antitumor immune response” were substantially up-regulated in PCFCCLs. This observation reflects the characteristic stromal response seen in these neoplasms and is further indication of the importance of the cellular environment in tumor biology. Notably, Rosenwald et al7 also identified the lymph node signature, associated with the cellular matrix, macrophages, and natural killer cells, as a positive outcome predictor for mainly nodal DLBCLs. More recently, survival in follicular lymphomas also was shown to correlate with tumor-infiltrating immune cells.8 Future studies must focus not only on the intrinsic biology of the tumor cells but also on the host response and tumor environment to further our understanding of lymphoma pathogenesis and progression. ▪
WHO/EORTC schema for primary cutaneous follicle center lymphoma (PCFCL) and PCLBCL, leg type. Adapted from Willemze et al.6
WHO/EORTC schema for primary cutaneous follicle center lymphoma (PCFCL) and PCLBCL, leg type. Adapted from Willemze et al.6