Abstract
Sphingosine-1-phosphate (S1P), the bioactive product of sphingosine kinase (SK) activation, is a survival factor for endothelial cells. The mechanism of SK-mediated survival was investigated in endothelial cells with moderately raised intracellular SK activity. Overexpression of SK mediated survival primarily through the activation of the phosphatidyl inositol 3-kinase (PI-3K)/protein kinase B (Akt/PKB) pathway and an associated up-regulation of the antiapoptotic protein B cell lymphoma gene 2 (Bcl-2) and down-regulation of the proapoptotic protein bisindolylmaleimide (Bcl-2 interacting mediator of cell death; Bim). In addition there was an up-regulation and dephosphorylation of the junctional molecule platelet endothelial cell adhesion molecule-1 (PECAM-1), which was obligatory for activation of the PI-3K/Akt pathway, for SK-induced cell survival, and for the changes in the apoptosis-related proteins. Thus, raised intracellular SK activity induced a molecule involved in cell–cell interactions to augment cell survival through a PI-3K/Akt–dependent pathway. This is distinct from the activation of both PI-3K/Akt and mitogen-activated protein kinase (MAPK) pathways seen with exogenously added S1P. Cells overexpressing SK showed enhanced survival under conditions of serum deprivation and absence of attachment to extracellular matrix, suggesting a role for SK in the regulation of vascular phenomena that occur under conditions of stress, such as angiogenesis and survival in unattached states, as would be required for a circulating endothelial cell.
Introduction
Endothelial cell (EC) survival is regulated by cell-matrix and cell–cell interactions and by growth factors. Cell–matrix attachments are mediated by the integrins, and integrin ligation triggers downstream signaling events that inhibit apoptosis or anoikis.1,2 Two cell-cell adhesion molecules are critical in the control of endothelial cell survival. The junctional molecule platelet endothelial cell adhesion molecule-1 (PECAM-1; CD31), a 130-kDa member of the immunoglobulin–immunoreceptor tyrosine-based inhibitory motif (Ig-ITIM) family of inhibitory receptors, possesses a functional cytoplasmic ITIM domain, is constitutively and abundantly expressed on normal endothelium3 regulating leukocyte transmigration4 and acts as a structural protein in cell-cell attachments. PECAM-1 is also able to suppress programmed cell death5-9 by virtue of homophilic interactions. VE-cadherin, an endothelial-specific major structural protein involved in adherens junctions, is also involved in mediating the antiapoptotic effects of vascular endothelial cell growth factor10 (VEGF). VEGF and basic fibroblast growth factor (bFGF) prevent EC apoptosis through activation of the phosphatidyl inositol 3-kinase (PI-3K)/protein kinase B (Akt/PKB) pathway and the Raf/MEK/MAPK (relative activity factor/mitogen-induced extracellular kinase/mitogen-activated protein kinase) pathway, both of which are critical pathways promoting cell survival.11-16 Serum is also a recognized trophic factor for ECs since they undergo apoptosis within 24 hours of serum deprivation.16 The protective factors in serum have been attributed to lysophosphatidic acid, sphingosine-1-phosphate (S1P),17-19 and high-density lipoproteins.20
The lipid mediator S1P is formed by the phosphorylation of membrane-associated sphingosine by sphingosine kinase (SK). S1P is stored in high concentrations in the granules in platelets, released upon activation, and present at high concentrations in the serum.21 It has been argued that S1P may have dual sites of action, one extracellularly as a ligand for the S1P family of G-protein–coupled receptors (GPCRs) and the other as an intracellular second messenger.22-25 S1P is known to regulate diverse biologic processes including cell proliferation, survival, and differentiation (for reviews see Pyne and Pyne26 and Spiegel and Milstien27 ) and acts to antagonize the proapoptotic effects of ceramide, an intermediate in the sphingomyelin pathway.28-30 To this end, the constitutively active endogenous SK, by keeping cellular levels of ceramide in check and promoting the formation of S1P, is an important determinant of the fate of cells. Indeed, overexpression of SK can promote an oncogenic phenotype in cells,31 consistent with its effects on cell survival.
In ECs, S1P promotes migration, proliferation, adherens junction assembly, and capillary tube formation with these effects mediated through endothelial differentiation gene 1 (EDG1) and EDG3 and involving Ras homology (Rho) and Ras-related C3 Botulinum toxin substrate (Rac) signaling pathways.32-38 S1P induces EC morphogenesis in vitro and in vivo,33 and studies in EDG1 knockout animals have shown that this receptor is essential for vascular maturation.39 S1P also results in the stimulation of the inflammatory phenotype in ECs, with the induction of the adhesion molecules vascular cell adhesion molecule (VCAM) and E-selectin. The selective inhibitor of SK, dimethyl sphingosine (DMS), blocks tumor necrosis factor (TNF)–induced adhesion molecule expression.40 S1P also rescues the cells from TNF- and serum deprivation–induced apoptosis41 via EDG1 and G(i) signaling, activation of extracellular signal-related kinase 1/2 (ERK1/2) and PI-3K, and subsequent Akt-induced endothelial nitric oxide synthase (eNOS) production.7,36,42-44 Furthermore, DMS blocks apoptosis in ECs, strengthening the concept that SK is a powerful mediator of EC function, regulating inflammatory responses and cell survival.
To this end, we wished to explore the possible intracellular role of SK in primary ECs. Although we hypothesized that, in ECs, raised intracellular levels of SK should promote cell survival, the question remained as to whether this was mediated through pathways similar to that for EDG-triggered cell survival. We found that, in contrast to exogenous S1P, which activates both the MAPK and PI-3K/Akt pathways, the cell survival pathway induced by intracellular SK activity is attributable to activation of the PI-3K/Akt pathway and not ERK. This selective activation of the PI-3K/Akt pathway is mediated through PECAM-1, which is up-regulated in levels and inhibited in its phosphorylation by intracellular SK. Thus our results have revealed a novel pathway of SK-mediated EC survival and support the hypothesis that regulation of SK levels and activity will be critical for determining endothelial cell fate.
Materials and methods
Endothelial cells
Human umbilical vein endothelial cells (HUVECs) were isolated and cultured as previously described45 with medium supplemented with 50 μg/mL endothelial growth supplement (Collaborative Research, Cambridge, MA) and 50 μg/mL heparin (Sigma, St Louis, MO).
Adenovirus production and generation of HUVECs overexpressing SK1
The AdEasy system (Qbiogene, Carlsbad, CA) was used to produce recombinant adenovirus carrying human SK1 (hSK1; or empty vector; EV) according to the Qbiogene Version 1.4 AdEasy Vector system manual.46 The 293 cells were cultured in 25-cm2 flasks in complete Dulbecco modified Eagle medium (CSL Biosciences, Parkville, Australia) containing 10% fetal calf serum (FCS). Virus was amplified in 293 cells and purified on a cesium chloride gradient with centrifugation. The viral titer was determined using the tissue culture infectious dose50 (TCID50) method according to the manufacturer's protocol. Transfection of HUVECs was achieved by infection with adenoviral preparations of SK (to yield ECsSK) or EV (to yield ECsEV) using equivalent plaque forming units (pfu's)/cell, which yielded a similar level of green fluorescent protein (GFP) expression. Cells were used for functional assays 1 to 3 days after infection. Overexpression of SK was confirmed by Western blot and SK activity assay.
Western blotting
Sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis was performed on cell lysates using 12% acrylamide gels. Immunocomplexes were detected using enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ).
Antibodies
Commercially available antibodies were M2 mouse anti-FLAG antibody (Sigma); rabbit polyclonal anti–phospho-Akt; rabbit polyclonal anti-Akt (Cell Signaling Technology, Beverly, MA); antiphosphotyrosine; anti–β-catenin (Cell Signaling Technology); rabbit polyclonal anti-ERK1/2 and rabbit polyclonal antiactive MAPK (Promega, Madison, WI); mouse monoclonal anti–cyclin D1 or –cyclin E; rabbit polyclonal anti-A1 (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit polyclonal antibodies Bax, Bcl-2, and Bim (BD Pharmingen, San Diego, CA); and mouse monoclonal anti–VEGF-RII (Sigma).
Rabbit anti–PECAM-1 antibody was an affinity-purified antibody as previously described.47 A goat anti-PECAM antibody (Santa Cruz Biotechnology) was used for Western blots as indicated. Two different murine monoclonal antibodies directed against PECAM-1 were used: 51-6F6, which was prepared at the Hanson Institute,45 and IgG2a HEK7 antibody,48 which was kindly supplied by Prof William Muller (Cornell University, NY).
SK activity
SK activity was determined as previously described.40 Briefly, d-erythro-sphingosine and [γ-32P] adenosine triphosphate ([γ-32P]ATP) were used as substrates and were incubated with whole-cell lysates made from ultracentrifugated preparations. The labeled lipids were extracted and resolved by 2 thin-layer chromatography (TLC) separations in the solvents CHCL3/CH3OH/NH4OH (65:35:8) and CHCL3/CH3OH/HAc (9:1:1), respectively. The radioactive spots were quantified by Phosphorimaging Typhoon 9410 (Molecular Dynamics, Buckinghamshire, UK).
Flow cytometry
Flow cytometric analysis of cell surface expression of PECAM-1 and VE-cadherin was performed as previously described45 using 10 μg/mL mouse monoclonal primary antibodies to PECAM-1 (51-6F6) or VE-cadherin (55-7H1) generated in our laboratory. The secondary antibody used was goat anti–mouse IgG R-phycoerythrin conjugate (Southern Biotech, Birmingham, AL). The median fluorescence intensity was determined using a Coulter Epics Profile XL flow cytometer (Fullerton, CA).
Measurement of caspase-3 activity
Cell lysates were prepared and the assay was performed essentially as given in the manufacturer's protocol (Calbiochem-Novabiochem, Darmstadt, Germany). Fluorescence was measured at excitation and emission wave-lengths of 385 nm and 460 nm and normalized for the protein concentration.
Immunofluorescent staining of apoptotic cells
Cells were seeded into fibronectin-coated LabTek slides (Nalge Nunc, Naperville, IL) at 6 × 104 cells per well in medium comprising varying concentrations of FCS and incubated at 37°C for 24 hours. The cells were stained with DAPI (4,6 diamidino-2-phenylindole)–methanol (Roche, Manheim, Germany). The percentage of apoptotic cells in consecutive fields was calculated from counts made on at least 100 cells. Cells were visualized at 20 × magnification. VPlan FL lens on an Olympus BX51 microscope (Olympus, Tokyo, Japan), with an Olympus U-RFL-T fluorescence light source, and with a Photometric Coolsnap fx camera (Roper Scientific, Trenton, NJ). Acquisition software was Precision Digital Imaging System V++ (Auckland, New Zealand), and images were manipulated using Adobe Photoshop 5.0 (Adobe Systems, San Jose, CA).
Cell survival
Endothelial cells were plated into gelatin-coated 96-well microtiter trays at 3 × 103 cells per well in serum-free medium. MTS (3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium; Promega) was used to measure cell viability. Optical density at 490 nm was measured on day 0 and day 2.
Cell suspension
Cells were plated in nontissue culture, nonadhesive 96-well microtiter trays coated with 1% bovine serum albumin at 8 × 103 cells per well in serum-free medium.
Small interfering RNA transfection
HUVECs were plated in tissue culture flasks and grown to 50% confluence. Cells were then changed into EBM II medium (Clonetics, Walkersville, MD) 24 hours after transfection. Small interfering RNA (siRNA) targeted to human SK1 [r(GAGCUGCAAGGCCUUGCCC)d(TT) and r(GGGCAAGGCCUUGCAGCUC)d(tt)] and control nonsilencing siRNA [r(UUCUCCGAACGUGUCACGU)d(TT) and r(ACGUGACACGUUCGGAGAA)d(TT)] were transfected into HUVECs using the Amaxa nucleofector kit according to the manufacturer's protocol (Amaxa Biosystems, Koeln, Germany). After transfection, cells were grow in EBM II medium for another 24 hours before the experiment.
Total RNA isolation and reverse transcriptase–polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from transfected HUVECs using TRIzol (Invitrogen Life Technologies, Carlsbad, CA). First-strand cDNAwas synthesized from 1 μg total RNA using Omniscript reverse transcriptase (QIAGEN, Valencia, CA) and oligo-dT primer (Geneworks, Adelaide, Australia). SK1s were amplified on a PTC-100 Programmable Thermal Controller (MJ Research, Waltham, MA) with an internal glyceraldehyde phosphate dehydrogenase (GAPDH) control. The primers used to amplify were SK1 (sense) 5′-TTG-AAC-CAT-TAT-GCT-GGCTATGA and SK1 (antisense) 5′-GCA-GGT-GTC-TTG-GAA-CCC; GAPDH (sense) 5′-TGG-TAA-AGT-GGA-TAT-TGT-TGCC and GAPDH (antisense) 5′-TGT-TGC-TGT-AGC-CAA-ATTCG.
Statistical analysis
Analyses, stratified over replicate experiments, were performed by analysis of variance (ANOVA) style regression using Statistica Version 6.1 (Statsoft, Tulsa, OK).
Results
Overexpression of Sk-1 enhances cell survival in suspension and resistance to serum deprivation
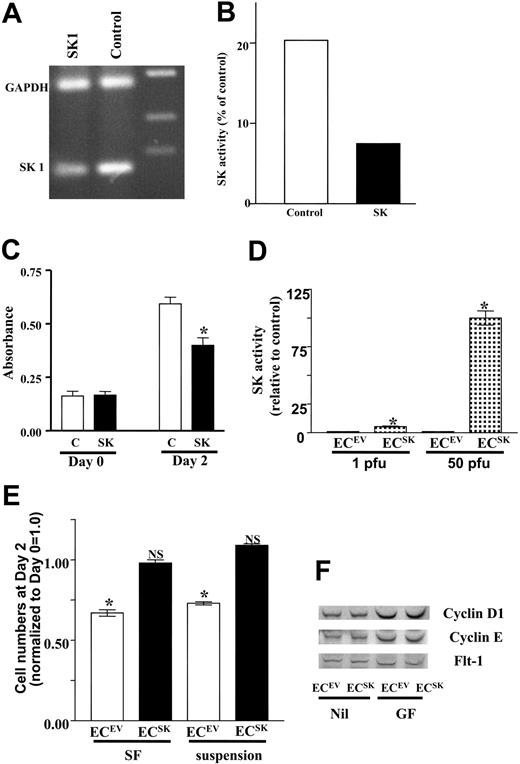
To confirm the importance of SK in EC survival, we sought to determine the effects of regulation of SK levels in these cells. Transfection of siRNA for SK into ECs resulted in a reduction in mRNA levels for SK and SK activity (Figure 1A-B) and a significant decrease in cell survival (Figure 1C), confirming SK as an important regulator of EC survival.
SK promotes endothelial cell survival. (A) Levels of mRNA for SK as measured by RT-PCR. HUVECs were transfected with siRNA for SK (SK1) or control nonsilencing siRNA (C). The results from 1 experiment representative of 3 performed. (B) Levels of SK activity in 1 experiment of 3 performed and (panel C) cell survival after siRNA treatment (as given in panel A) measured by MTS at time of plating and after 2 days. The absorbance at 490 nM reflects the number of viable cells. □ represents control; ▪, SK. The mean ± SEM of quadruplicate determinations from 1 experiment representative of 3 performed. *P less than 0.05 compared with C. (D) SK activity in HUVECs infected with 1 pfu/cell or 50 pfu/cell of adenoviral supernatant carrying SK or control EV are shown as the composite analysis of 2 separate endothelial cell lines assayed in duplicate and normalized to control. *P less than .001 SK compared with EV at equivalent pfu/cell. Bars represent 95% confidence intervals. (E) The survival of ECsSK (▪) or ECsEV (□) as reflected by the optical density at 490 nm in the absence of fetal calf serum (SF) and in the absence of both fetal calf serum and attachment to extracellular matrix (suspension). The SF data represent the pooled data of 43 observations derived from 9 separate experiments, and the suspension data show the pooled data of 10 observations from 2 separate experiments. The cell number at day 2 normalized to day 0 = 1 is given. *P less than .001 compared with ECsEVat day 0. NS indicates no significant difference compared with ECsSK at day 0. Bars represent 95% confidence intervals. (F) Cyclin D1 and E expression by Western blot under unstimulated conditions (Nil) and after stimulation with growth factor (GF) for 24 hours. The membrane was counter-blotted with antibody to fetal liver tyrosine kinase 1 (Flt-1) as a loading control.
SK promotes endothelial cell survival. (A) Levels of mRNA for SK as measured by RT-PCR. HUVECs were transfected with siRNA for SK (SK1) or control nonsilencing siRNA (C). The results from 1 experiment representative of 3 performed. (B) Levels of SK activity in 1 experiment of 3 performed and (panel C) cell survival after siRNA treatment (as given in panel A) measured by MTS at time of plating and after 2 days. The absorbance at 490 nM reflects the number of viable cells. □ represents control; ▪, SK. The mean ± SEM of quadruplicate determinations from 1 experiment representative of 3 performed. *P less than 0.05 compared with C. (D) SK activity in HUVECs infected with 1 pfu/cell or 50 pfu/cell of adenoviral supernatant carrying SK or control EV are shown as the composite analysis of 2 separate endothelial cell lines assayed in duplicate and normalized to control. *P less than .001 SK compared with EV at equivalent pfu/cell. Bars represent 95% confidence intervals. (E) The survival of ECsSK (▪) or ECsEV (□) as reflected by the optical density at 490 nm in the absence of fetal calf serum (SF) and in the absence of both fetal calf serum and attachment to extracellular matrix (suspension). The SF data represent the pooled data of 43 observations derived from 9 separate experiments, and the suspension data show the pooled data of 10 observations from 2 separate experiments. The cell number at day 2 normalized to day 0 = 1 is given. *P less than .001 compared with ECsEVat day 0. NS indicates no significant difference compared with ECsSK at day 0. Bars represent 95% confidence intervals. (F) Cyclin D1 and E expression by Western blot under unstimulated conditions (Nil) and after stimulation with growth factor (GF) for 24 hours. The membrane was counter-blotted with antibody to fetal liver tyrosine kinase 1 (Flt-1) as a loading control.
Overexpression of SK (ECSK), using an adenovirus method for gene delivery, resulted in an approximate 5-fold increase in SK activity above empty vector control (ECsEV) when the cells were infected with 1 pfu/cell and a 100-fold increase in activity with 50 pfu/cell (Figure 1D). Since the 5-fold increase in activity with 1 pfu/cell is a similar level to that induced with known activators of ECs,40 we analyzed the functional effects of this level of overexpression.
ECsSK showed enhanced survival in both serum-free (SF) conditions and when grown in suspension compared with ECsEV (Figure 1E). Cell numbers of ECsEV were maintained for 24 hours but rapidly dropped off thereafter. One day after plating, the cells overexpressing SK had increased in number, and after 2 days in SF or nonadherent conditions more ECsSK survived compared with ECsEV. Measurement of cyclins E and D showed no change in levels between ECsSK compared with ECsEV (Figure 1F), thus suggesting that the alteration in number of ECsSK may be due to an antiapoptotic effect rather than a change in the proliferative potential.
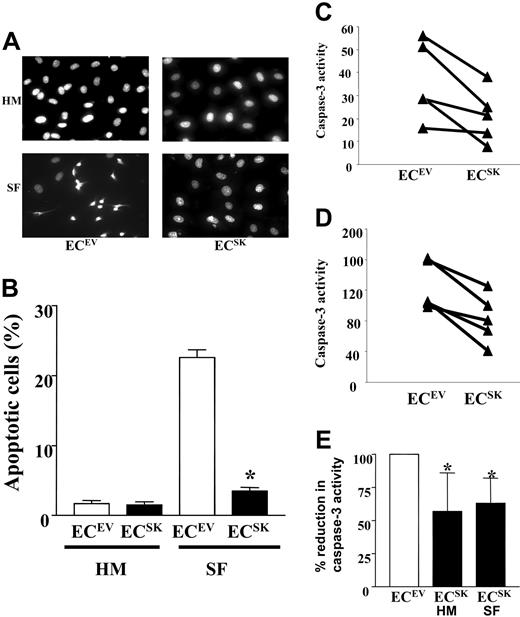
The SK-overexpression–induced resistance to serum deprivation was due to an inhibition of apoptosis. Figure 2A (and quantification in Figure 2B) shows that under basal conditions (HUVE medium; HM) there was no difference in the number of apoptotic cells, as measured by DAPI staining, between ECsSK and ECsEV. However, after 2 days in SF conditions (SF), there was a high proportion of apoptotic cells in ECsEV but not in ECsSK. The results of DAPI staining were confirmed by measurement of caspase-3 activity under basal conditions and in response to 24 hours of serum deprivation. Overexpression of SK was shown to reduce basal caspase-3 activity (Figure 2C) and to confer further resistance to caspase-3 activation induced by serum deprivation (Figure 2D).
SK prevents EC apoptosis. (A) DAPI stain performed on ECsSK and ECsEV in culture medium supplemented with 20% FCS (HUVE medium; HM) or SF medium. (B) Quantification of 5 consecutive microscopic fields to a total cell count of 100 from 2 separate experiments. Bars represent SEM. *P less than .001 compared with ECsEV under SF conditions. (C) Caspase-3 activity in ECsSK and ECsEV in 5 separate endothelial cell lines measured under basal culture conditions (HM) or (D) after 24 hours of serum deprivation. Panel E shows the pooled data from the 5 endothelial cell lines. Bars represent 95% confidence intervals. *P less than .05 for ECsSK under basal or serum-free conditions compared with corresponding ECsEV, normalized to 100.
SK prevents EC apoptosis. (A) DAPI stain performed on ECsSK and ECsEV in culture medium supplemented with 20% FCS (HUVE medium; HM) or SF medium. (B) Quantification of 5 consecutive microscopic fields to a total cell count of 100 from 2 separate experiments. Bars represent SEM. *P less than .001 compared with ECsEV under SF conditions. (C) Caspase-3 activity in ECsSK and ECsEV in 5 separate endothelial cell lines measured under basal culture conditions (HM) or (D) after 24 hours of serum deprivation. Panel E shows the pooled data from the 5 endothelial cell lines. Bars represent 95% confidence intervals. *P less than .05 for ECsSK under basal or serum-free conditions compared with corresponding ECsEV, normalized to 100.
Sphingosine kinase induces PECAM-1 expression and dephosphorylation
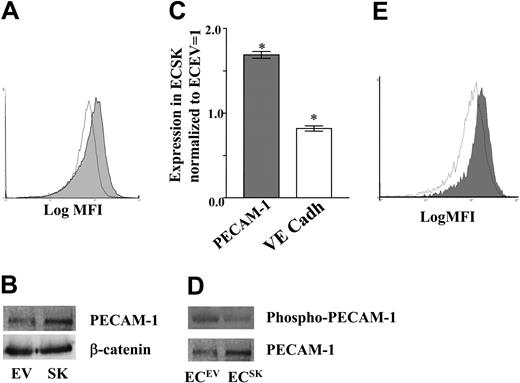
Since adhesion molecules such as PECAM and VE-cadherin regulate cell survival, we sought to determine whether overexpression of SK results in changes in the levels or function of either of these junctional molecules. There was a consistent increase in the cell surface expression of PECAM as measured by flow cytometry in ECsSK (Figure 3A) that was also seen for total protein as measured by Western blots (Figure 3B). In 5 lines analyzed there was a 69% (95% confidence interval [CI] 65%-73%) increase in PECAM (P < .001) with an 18% (95% CI 16%-21%) reduction in VE-cadherin expression (Figure 3C). There was no change in β-catenin (Figure 3B), a signaling molecule associated with both PECAM and VE-cadherin.49 In ECs, PECAM-1 is phosphorylated on tyrosine residues and phosphorylation is associated with a decrease in cell-cell adhesion.49,50 ECsSK showed a reduced phosphorylation of PECAM-1 (Figure 3D). In 3 separate endothelial cell lines, the mean fold percentage reduction in the proportion of PECAM-1, which was phosphorylated for ECsSK compared with ECsEV, was 48% (95% CI 28%-63%) when normalized to actin. Furthermore, when plated under SF conditions for 48 hours, there was still a consistent increase in PECAM-1 expression in ECsSK versus ECsEV (Figure 3E). When PECAM-1 phosphorylation was measured under SF conditions, no significant differences between ECsSK and ECsSK were observed. However, this is most likely due to the fact that incubations of HUVECs in SF conditions quickly disrupt cell-cell interactions, a situation that is also known to induce PECAM phosphorylation.49,50
SK regulates PECAM-1 expression and phosphorylation levels. (A) Flow cytometry profile of the cell surface expression of PECAM-1 in ECsEV (open curve) and ECsSK (shaded curve) analyzed 48 hours after infection. MFI indicates mean fluorescent intensity. (B) Western blot for PECAM-1 and β-catenin expression in these cells. (C) Cell surface expression of PECAM (▦) and VE-cadherin (VE Cadh; □), as indicated by the median fluorescence intensity, in ECsSK and ECsEV. The figure shows the pooled data from 3 separate experiments normalized to EV = 1. *P less than .001 for ECsSK compared with ECsEV. Bars represent 95% confidence intervals. (D) PECAM-1 phosphorylation (top) as detected by an antiphosphotyrosine antibody and total PECAM-1 (bottom) in ECs. (E) Flow cytometry profile of cell surface expression of PECAM-1 on ECsEV (open curve) and ECsSK (shaded curve) analyzed 96 hours after infection including 48 hours of growth in SF conditions.
SK regulates PECAM-1 expression and phosphorylation levels. (A) Flow cytometry profile of the cell surface expression of PECAM-1 in ECsEV (open curve) and ECsSK (shaded curve) analyzed 48 hours after infection. MFI indicates mean fluorescent intensity. (B) Western blot for PECAM-1 and β-catenin expression in these cells. (C) Cell surface expression of PECAM (▦) and VE-cadherin (VE Cadh; □), as indicated by the median fluorescence intensity, in ECsSK and ECsEV. The figure shows the pooled data from 3 separate experiments normalized to EV = 1. *P less than .001 for ECsSK compared with ECsEV. Bars represent 95% confidence intervals. (D) PECAM-1 phosphorylation (top) as detected by an antiphosphotyrosine antibody and total PECAM-1 (bottom) in ECs. (E) Flow cytometry profile of cell surface expression of PECAM-1 on ECsEV (open curve) and ECsSK (shaded curve) analyzed 96 hours after infection including 48 hours of growth in SF conditions.
Analysis of PECAM-1 mRNA levels showed no change between ECsEV and ECsSK, suggesting a posttranscriptional change is responsible for the increase in PECAM-1 expression in the ECsSK. In addition, there was no significant alteration in the ratio of the cellular distribution of PECAM between the cytosolic and membrane fractions in these 2 different cell populations, suggesting that the increase was not a result of a change in PECAM-1 localization (data not shown).
SK-induced survival is mediated by PECAM-1
We next tested, by the use of antibodies, whether the changes in PECAM-1 were responsible for SK-induced EC survival. A well-characterized47 affinity-purified rabbit polyclonal anti–PECAM-1 antibody significantly reduced the survival of ECsSK in both suspension (Figure 4A) and under SF conditions (Figure 4B), whereas normal rabbit serum had no effect. The effect was seen with 2 different preparations of affinity-purified polyclonal antibody that had been raised independently. Two murine monoclonal antibodies directed to PECAM-1 (6F6 and HEK7) were also used. The 6F6 is directed to domain 1 of PECAM and regulates EC–EC interactions,45 whereas HEK7 binds domains 1 and 2 and activates leukocytes and inhibits transmigration.48,51 Both antibodies significantly enhanced the ability of ECsEV to survive in SF conditions to levels similar to those seen in ECsSK but had no further effect on these SK-overexpressing cells (Figure 4A). A murine monoclonal antibody directed to VE-cadherin (7H1) had no effect in altering survival of cells overexpressing SK or EV control. The ability of the polyclonal but not monoclonal antibody to reverse the PECAM-mediated survival by ECsSK suggested that the polyclonal antibody may disrupt cell-cell interactions whereas the monoclonal antibodies may mimic PECAM–PECAM engagement. Consistent with this possibility, the monoclonal antibody 6F6 decreased the phosphorylation of PECAM-1, whereas the polyclonal antibody increased the phosphorylation (Figure 4C) either when analyzed using normal ECs (Figure 4C) or in ECEV and ECSK populations (Figure 4D) cultured for 6 hours in SF conditions.
PECAM-1 signaling is involved in SK-mediated cell survival. The effect of altering PECAM-1 signaling on cell survival as measured by the MTS assay of ECsSK (▪) and ECsEV (□) in (A) suspension and in (B) serum-free conditions. The effect on cell survival of 20 μg/mL rabbit polyclonal anti–PECAM-1 antibody (RP) and 20 μg/mL of 6F6 added at plating. Panel A shows the pooled data of 10 observations from 2 separate experiments normalized to day 0 = 1. Bars represent 95% confidence intervals. *P less than .001 compared with normal rabbit serum (NRS)–treated corresponding ECsEV or ECsSK at day 2. Panel B shows the results of 1 experiment representative of 3 performed. □ indicates no antibody; ▪, polyclonal antibody; and ▦, monoclonal 6F6. Mean ± SEM of quadruplicate determinations is given. *P less than .001 compared with ECsSK at day 2. (C) Western blot showing phosphorylation of PECAM (P-PECAM) in normal ECs after treatment for 10 minutes with control (Nil), the monoclonal antibody (6F6), or polyclonal anti-PECAM antibody (RP). Total PECAM is given as a loading control (bottom). Cells were grown in normal medium. (D) Western blot of the phosphorylation of PECAM-1 (P-PECAM) in ECsEV and ECsSK after growth for 6 hours in SF conditions and then 10 minutes treatment with either control or anti-PECAM antibodies. Actin is given as a loading control. (E) The effect of incubation of antibodies to PECAM on the cell surface expression of PECAM on normal ECs, ECsEV, and ECsSK. Cells were incubated under SF conditions with either no treatment, NIL (□), polyclonal rabbit antibody to PECAM (RP; ▪), or monoclonal antibody to PECAM (6F6; ▨) for 6 hours, harvested, and stained for flow cytometry analysis. The data for the mean ± SEM pooled from 4 experiments. *P less than .001 compared with no antibody control. Inset shows a Western blot for PECAM in ECsSK after antibody treatment and probed with a goat anti-PECAM antibody. Similar effects were seen in ECsEV (not shown).
PECAM-1 signaling is involved in SK-mediated cell survival. The effect of altering PECAM-1 signaling on cell survival as measured by the MTS assay of ECsSK (▪) and ECsEV (□) in (A) suspension and in (B) serum-free conditions. The effect on cell survival of 20 μg/mL rabbit polyclonal anti–PECAM-1 antibody (RP) and 20 μg/mL of 6F6 added at plating. Panel A shows the pooled data of 10 observations from 2 separate experiments normalized to day 0 = 1. Bars represent 95% confidence intervals. *P less than .001 compared with normal rabbit serum (NRS)–treated corresponding ECsEV or ECsSK at day 2. Panel B shows the results of 1 experiment representative of 3 performed. □ indicates no antibody; ▪, polyclonal antibody; and ▦, monoclonal 6F6. Mean ± SEM of quadruplicate determinations is given. *P less than .001 compared with ECsSK at day 2. (C) Western blot showing phosphorylation of PECAM (P-PECAM) in normal ECs after treatment for 10 minutes with control (Nil), the monoclonal antibody (6F6), or polyclonal anti-PECAM antibody (RP). Total PECAM is given as a loading control (bottom). Cells were grown in normal medium. (D) Western blot of the phosphorylation of PECAM-1 (P-PECAM) in ECsEV and ECsSK after growth for 6 hours in SF conditions and then 10 minutes treatment with either control or anti-PECAM antibodies. Actin is given as a loading control. (E) The effect of incubation of antibodies to PECAM on the cell surface expression of PECAM on normal ECs, ECsEV, and ECsSK. Cells were incubated under SF conditions with either no treatment, NIL (□), polyclonal rabbit antibody to PECAM (RP; ▪), or monoclonal antibody to PECAM (6F6; ▨) for 6 hours, harvested, and stained for flow cytometry analysis. The data for the mean ± SEM pooled from 4 experiments. *P less than .001 compared with no antibody control. Inset shows a Western blot for PECAM in ECsSK after antibody treatment and probed with a goat anti-PECAM antibody. Similar effects were seen in ECsEV (not shown).
Some antibodies have the ability to stimulate antigen clearance from the cell surface. To test whether this possibility is operating here, normal ECs cultured in full medium and EV- and SK-transfected cells, subjected to serum deprivation, were cultured in the presence of the antibodies and PECAM cell surface expression was subsequently measured. As shown in Figure 4E, pretreatment of cells with rabbit anti–PECAM-1 but not 6F6 resulted in a significant reduction in the cell surface expression of PECAM-1, which was also seen for total PECAM as detected by Western blots (Figure 4E inset). Clearance of antigen took place within 6 hours and normal levels returned by 12 hours (data not shown). Furthermore, HEK7 and 7H1 also did not alter PECAM-1 cell surface expression or total PECAM nor did a number of other monoclonal antibodies directed to differing domains of PECAM-1 (data not shown). Although we have not definitively proven that antigen clearance is responsible for the differing effects between the polyclonal and monoclonal antibodies, there does exist a correlation between the effects on cell survival and their ability to clear PECAM-1 from the cell surface.
Exogenously added S1P mediates cell survival through S1P receptors. To determine whether overexpression of SK leads to S1P receptor–mediated effects by an exogenous action of S1P, the effect of inhibiting GPCRs with pertussis toxin on cell survival was examined. SK-mediated cell survival was not inhibited in the presence of pertussis toxin (Table 1) consistent with an intracellular site of action of S1P. Also, exogenously added S1P had no effect on the level of PECAM-1 expression (data not shown), further suggesting that S1P receptor activation is not involved in the PECAM-1–mediated changes in cell survival.
Overexpression of SK activates the PI-3K/Akt pathway
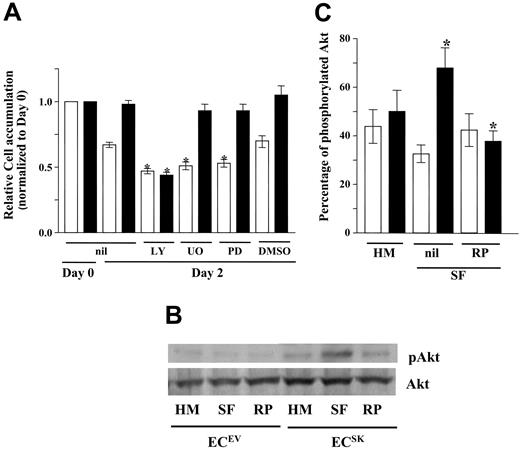
Survival factors such as growth factor and attachment to extracellular matrix influence cell survival through a number of pathways that include the PI-3K/Akt and the rat sarcoma viral oncogene homolog (Ras)–MAPK pathways.52-54 S1P stimulation is known to activate both of these signaling pathways. To determine whether these pathways are involved in the increased survival induced by SK overexpression, phosphorylation of Akt and ERK1/2 was assessed. Under basal conditions there was no significant difference in the percentage of phosphorylated Akt (p-Akt) in ECsSK compared with ECsEV (P = .47), as shown in Figure 5A. A reduction in the phosphorylation of Akt in response to serum deprivation was seen in ECsEV, as would be expected in normal ECs. ECsSK however responded to the stress of serum deprivation by a maintenance in phosphorylation of Akt. Thus, in SF conditions, ECsSK showed significantly greater phosphorylation of Akt than ECsEV, indicating activation of this pathway. This was confirmed and quantified in 5 separate endothelial cell lines (Figure 5B). In contrast to these effects on the PI-3K/Akt pathway by SK, neither ECsSK nor ECsEV were able to activate the MAPK pathway in response to the stress of serum deprivation, although the ability to activate this pathway in response to exogenous stimulation with S1P was preserved (Figure 5C).
SK activates the PI-3K/Akt pathway but not the MAPK pathway. (A) Western blot of the phosphorylation of Akt (p-Akt) and total Akt (Akt) in ECsSK and ECsEV under basal conditions (HM) and in response to 6 hours of serum deprivation (SF). (B) The pooled data from 5 separate endothelial cell lines quantified for p-Akt compared with total Akt. *P less than .05 for ECsSK (▪) compared with ECsEV (□) in SF conditions. Bars represent SEM. (C) Phosphorylation of ERK1/2 (p-ERK1/2) and total ERK1/2 expression in cells taken from basal conditions (HM), after 6 hours of serum deprivation (SF), and in cells subjected to serum deprivation for 6 hours and then stimulated with 5 μM S1P for 10 minutes.
SK activates the PI-3K/Akt pathway but not the MAPK pathway. (A) Western blot of the phosphorylation of Akt (p-Akt) and total Akt (Akt) in ECsSK and ECsEV under basal conditions (HM) and in response to 6 hours of serum deprivation (SF). (B) The pooled data from 5 separate endothelial cell lines quantified for p-Akt compared with total Akt. *P less than .05 for ECsSK (▪) compared with ECsEV (□) in SF conditions. Bars represent SEM. (C) Phosphorylation of ERK1/2 (p-ERK1/2) and total ERK1/2 expression in cells taken from basal conditions (HM), after 6 hours of serum deprivation (SF), and in cells subjected to serum deprivation for 6 hours and then stimulated with 5 μM S1P for 10 minutes.
To determine whether the ability of ECsSK to engage the PI-3K/Akt pathway had functional consequences, the effect of inhibiting this pathway with the inhibitor LY294002 and the MAPK pathway (with inhibitors UO126 and PD98059) on SK-induced cell survival was investigated. While LY294002, UO126, and PD98059 all significantly reduced cell survival of ECsEV, ECSK-induced survival was inhibited by LY294002 but not by UO126 or PD98059 (Figure 6A). This together with the results in Figure 5 suggests that SK-induced cell survival is mediated through the PI-3K pathway and that the MAPK pathway is not implicated.
Inhibition of the PI-3K/Akt pathway blocks SK-induced cell survival. ECsSK (▪) and ECsEV (□) were treated with 10 mM LY294002 (LY) to block the PI-3K/Akt pathway and 20 mM UO126 (UO) or 20 mM PD98059 (PD) to block the MAPK pathway. A vehicle control of equivalent concentration of dimethyl sulfoxide (DMSO) is indicated. The figure shows cell survival by the pooled data of 8 observations from 2 separate experiments, adjusted to day 0 = 1. Bars represent 95% confidence intervals. *P less than .001 compared with corresponding untreated cells overexpressing SK or EV at day 2. (B) Western blot measuring phosphorylated Akt (p-Akt) and total Akt in basal conditions (HM) and after 6 hours of serum deprivation (SF) in ECsSK and ECsEV. The effect of 20 μg/mL of rabbit polyclonal anti–PECAM-1 antibody (RP) added at plating under SF conditions is shown. (C) The pooled data of the quantification of phosphorylated Akt compared with total Akt from 4 separate experiments performed as in panel B for ECsSK (▪) and ECsEV (□). Bars represent SEM. *P less than .05 of untreated ECsSK versus ECsSK treated with RP.
Inhibition of the PI-3K/Akt pathway blocks SK-induced cell survival. ECsSK (▪) and ECsEV (□) were treated with 10 mM LY294002 (LY) to block the PI-3K/Akt pathway and 20 mM UO126 (UO) or 20 mM PD98059 (PD) to block the MAPK pathway. A vehicle control of equivalent concentration of dimethyl sulfoxide (DMSO) is indicated. The figure shows cell survival by the pooled data of 8 observations from 2 separate experiments, adjusted to day 0 = 1. Bars represent 95% confidence intervals. *P less than .001 compared with corresponding untreated cells overexpressing SK or EV at day 2. (B) Western blot measuring phosphorylated Akt (p-Akt) and total Akt in basal conditions (HM) and after 6 hours of serum deprivation (SF) in ECsSK and ECsEV. The effect of 20 μg/mL of rabbit polyclonal anti–PECAM-1 antibody (RP) added at plating under SF conditions is shown. (C) The pooled data of the quantification of phosphorylated Akt compared with total Akt from 4 separate experiments performed as in panel B for ECsSK (▪) and ECsEV (□). Bars represent SEM. *P less than .05 of untreated ECsSK versus ECsSK treated with RP.
Given the changes in PECAM-1, we next investigated whether the engagement of the PI-3K/Akt pathway by SK is dependent upon PECAM-1 signaling. Total Akt and active (phosphorylated) Akt were measured by Western blot under basal conditions and in response to serum deprivation for 6 hours in the presence of rabbit polyclonal anti–PECAM-1 antibody. Results are shown in Figure 6B with quantification shown in Figure 6C. The SK-mediated activation of the Akt pathway in response to serum deprivation is again demonstrated. Rabbit polyclonal anti–PECAM-1 antibody reduced the increase in phosphorylation of Akt for ECsSK similar to the levels seen in control cells but had no effect in ECsEV.
SK signals through PECAM-1 to regulate antiapoptotic proteins
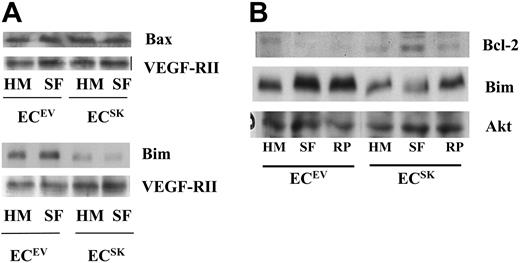
The PI-3K/Akt pathway can mediate some of its survival signals through the Bcl-2 family. Bcl-2, A1, and Bcl-X are members of the antiapoptotic Bcl-2 family which when bound by the pro apoptotic proteins, such as Bax and Bim, are neutralized for their antiapoptotic effects.55,56 We investigated whether raised intracellular SK activity alters the expression of these antiapoptotic and proapoptotic proteins under basal conditions and in response to serum deprivation. In 2 lines tested, neither control cells nor cells overexpressing SK showed a change in Bax expression in response to serum deprivation (Figure 7A). ECsSK showed less Bim expression under basal conditions compared with ECsEV (Figure 7A). In response to serum deprivation, ECsEV showed a further increase in Bim expression, although ECsSK did not.
SK regulates proteins involved in apoptosis. (A) Western blot for the expression of Bax and Bim. (B) Bcl-2 and Bim expression under normal culture conditions (HM) and in response to 6 hours of SF conditions with and without 20 μg/mL of rabbit polyclonal anti–PECAM-1 antibody (RP). The loading control of VEGF-RII expression in panel A and Akt in panel B is shown.
SK regulates proteins involved in apoptosis. (A) Western blot for the expression of Bax and Bim. (B) Bcl-2 and Bim expression under normal culture conditions (HM) and in response to 6 hours of SF conditions with and without 20 μg/mL of rabbit polyclonal anti–PECAM-1 antibody (RP). The loading control of VEGF-RII expression in panel A and Akt in panel B is shown.
A1 expression was not altered in ECsSK under basal conditions or in response to serum deprivation either at the protein level or at the mRNA level as quantified by real-time PCR (data not shown). There was also no regulation of Bcl-X (data not shown). There was a slight increase in Bcl-2 expression in cells overexpressing SK under basal conditions. However, a significant up-regulation in ECsSK was seen under SF conditions (Figure 7B).
To determine whether the regulation of Bim and Bcl-2 is important in the PECAM-1–mediated antiapoptotic effects induced in ECsSK, we tested the effects of the polyclonal anti–PECAM-1 antibody. After such a treatment it was found that the antiapoptotic protein Bcl-2 was down-regulated whereas the proapoptotic protein Bim was up-regulated, consistent with the effects of the antibody on cell survival (Figure 7B).
Discussion
We show here that in primary ECs, SK overexpression results in survival under conditions of stress induced by either growth in SF conditions or growth in suspension. Our studies show 2 important points about the mechanism of this SK-mediated survival pathway in ECs. First, we demonstrate that protection from apoptosis by SK overexpression is mediated through the PI-3K/Akt pathway and not, as is seen for exogenously added S1P, through the PI-3K/Akt and MAPK pathways. Second, we show that the regulation of survival by SK is achieved through a cell junctional protein, PECAM-1. The degree of overexpression of SK to achieve the phenotype was modest (approximately 5-fold) in comparison to what is seen in established cell lines, such as fibroblasts in which 100- to 1000-fold overexpression is not unusual.40,57 In primary ECs, higher levels (over 20-fold) of SK overexpression result in toxicity (J.R.G., unpublished results, 2004). Nevertheless, the phenotype seen in our ECsSK was robust and is likely to correspond to the type of changes seen under physiologic circumstances.40
The enhanced survival of ECsSK was associated with activation of PI-3K/Akt pathway but not of the MAPK pathway (Figure 5). The overexpression of SK resulted in an increase in phosphorylation of Akt in response to SF conditions (Figure 5A-B) without any increase in ERK1/2 activity (Figure 5C). This was in spite of an intact MAPK pathway, as demonstrated by the ability to activate ERK1/2 by the addition of exogenous S1P (Figure 5C). The latter finding is in keeping with the inability of pertussis toxin to inhibit the effects of overexpression of SK (Table 1), suggesting that increased generation of S1P acting through EDG1 or EDG3 (the chief GPCR in ECs) is not the primary mechanism. Intracellular roles of SK/S1P, by an as yet undetermined mechanism, as have been emphasized by Spiegel et al,22-25 are thus likely to be involved, although an S1P-induced decrease in ceramide levels28 also still remains a possibility.
Further evidence that the PI-3K/Akt pathway is specifically linked to SK-induced cell survival is provided by inhibitor studies in which a PI-3K inhibitor, but not the inhibitors of the MAPK pathways, ablated the SK-induced cell survival (Figure 6). The mechanism of enhanced survival mediated by SK/S1P appears cell-type dependent. In human melanocytes, MAPK activation by S1P58 plays a dominant role in protecting from UV irradiation, whereas in osteoblasts activation of PI-3K protects from serum withdrawal.59 The reasons for differential usage of these pathways is not clear, but one possible explanation could be based on the observation that although SK is a constitutively active enzyme, it is able to become further activated by a mechanism that involves phosphorylation on serine 225, altering its membrane localization and increasing its Vmax by 14-fold. The activation of SK is clearly linked to its phosphorylation by ERK1/2 and in turn an increased ERK1/2 phosphorylation in the cytosol.57 Thus the possibility arises that overexpression of unactivated SK may be preferentially linked to Akt activation, with ERK1/2 (as well as Akt) being engaged upon more acute stimulation, such as is provided by TNFα or phorbol myristate acetate (PMA).40 However, the interaction of the PI-3K/Akt pathway with the SK pathway is complex, as Akt may also act upstream of S1P by phosphorylating EDG1 to induce activation of Rac and subsequent stimulation of endothelial cell migration.38
In examining the regulation of cell surface protein by ECsSK we were struck by changes in PECAM-1 expression (Figure 3A-C) and phosphorylation (Figure 3D). By contrast there is no effect on the levels of expression of β-catenin and no major change in VE-cadherin, the other junctional molecule that is specific to endothelial cells and that is also implicated in cell survival. These results raised the possibility that PECAM-1 mediates the increased survival of ECsSK. Antibodies have been very useful in studying PECAM-1 function and are able to inhibit or mimic PECAM-1 interactions.6,7,49 In our system, we used blocking affinity–purified polyclonal rabbit anti–PECAM-1 antibody, which was very effective in reversing the ECSK phenotype of enhanced survival under either SF or suspension conditions (Figure 4) and in mediating the activation of the PI-3K/Akt pathway in response to serum deprivation in ECsSK (Figure 6). In neutrophils, PECAM-1 has been shown to be functionally and physically associated with PI-3K, as PI-3K was coimmunoprecipitated with PECAM-1.60 We have been unable to detect such an interaction in our ECs.
The mechanism of the reversal by the polyclonal antibody is not totally understood and could, at least partially, be through clearance of PECAM-1 from the cell. We demonstrated that even after 1 hour of incubation with the polyclonal antibody there was a significant decrease in expression of PECAM-1. Thus, the loss of expression of PECAM-1 will have major influences on the downstream signaling pathways. The requirement for PECAM signaling was also demonstrated with the monoclonal antibodies, which although they failed to further enhance the ECSK-induced survival, induced an increase in survival in ECsEV, an observation in keeping with previous reports showing that monoclonal antibodies mimic homotypic PECAM interactions, engage PECAM, and prevent apoptosis induced by serum deprivation.6,7
The other striking change in ECsSK was the decreased tyrosine phosphorylation of PECAM-1 (Figure 3D). Resting ECs have low levels of tyrosine phosphorylation47,48 but this can be increased by factors such as VEGF,61 TNF,62 and thrombin,63 which are known permeability-inducing agents and decrease cell-cell interactions. Conversely, phosphorylation is inhibited by the antipermeability agent angiopoietin-164 and in migrating ECs.49 Tyrosine phosphorylation recruits Src homology 2 (SH-2)–containing proteins such as SH-2 domain–containing tyrosine phosphatase (SHP-2) to PECAM-1. However, engagement of PECAM-1 results in the generation of other known signaling events such as intracellular Ca++ and prostacyclin.65 The differential effects of the antibodies on cell survival could therefore also be linked to their differential effects on PECAM-1 phosphorylation and subsequent signaling pathways. Indeed, the monoclonal antibody inhibited and the polyclonal antibody enhanced the PECAM-1 phosphorylation (Figure 4C-D), consistent with a promotion and disruption of cell-cell interactions, respectively.
Our demonstration here of a functional interaction between PECAM-1 and the SK/S1P system is in keeping with the recent finding by Fukuda et al66 showing a physical association, at least in transfected cells, between PECAM-1 and SK1. Moreover, they showed that phosphorylation of PECAM-1 attenuated its association with SK1. Although our initial experiments in HUVECs to demonstrate such an association between PECAM-1 and SK have not been successful, the possibility remains that such an association, which may also include PI-3K/Akt in the complex, may be extremely transient or association takes place in specific subcellular compartments.
The findings documented here are consistent with the known role of PECAM-1 in ECs in suppressing programmed cell death. Homophilic PECAM-1–PECAM-1 interactions, or engagement of PECAM-1 by monoclonal antibodies, have been shown to prevent serum deprivation–induced apoptosis. Endothelial cells from PECAM-1–deficient mice are more susceptible than their PECAM-1–replete counterparts to the apoptotic stimuli of irradiation as well as to the cytotoxic effects of staurosporine.8 Furthermore, in Jurkat T lymphocytes lacking PECAM-1, restoration of PECAM-1 expression was protective against UV-induced apoptosis.8 Thus PECAM-1 is recognized to provide resistance to a broad range of apoptotic stimuli; however, the proteins that support PECAM-1–dependent cell survival are varied. The antiapoptotic proteins A1 and A20 have been implicated in prevention of serum deprivation–induced apoptosis5 whereas they are not involved in cytotoxic stimuli that activate Bax.8 PECAM-1 ligation has also been shown to up-regulate Bcl-2 message levels in adherent endothelial cells.6 In the present study, SK-induced protection from serum deprivation resulted in no change in Bax (Figure 7A), A1, or Bcl-X expression but in a PECAM-1–dependent induction of Bcl-2 and down-regulation in Bim (Figure 7B). Bim functions through binding to antiapoptotic Bcl-2 family members resulting in an inhibition of their antiapoptotic effects.55 Thus it would appear that the SK-induced, PECAM-1–mediated, antiapoptotic effects shown here are generated primarily through a regulation of the proapoptotic protein Bim together with effects on the PI-3K/Akt pathway.
The functional and gene expression heterogeneity of endothelial cells (from various organs or disease states) is just being recognized. The work reported here shows that alteration of the intracellular levels of SK in endothelial cells results in distinct phenotypic changes, with a resetting of signaling pathways and the associated alterations in gene expression. The capacity of ECsSK to survive in suspension suggests that they may have increased capacity to survive in the circulation or in situations such as during angiogenesis where cell-matrix attachments are limited. We demonstrate that intracellular levels of SK regulate endothelial cell fate through targeted alterations in PECAM-1 signaling and activation of the PI-3K/Akt pathway. The dominant role for PECAM-1 in mediating endothelial cell survival suggests a reliance on cell-cell interactions or the activation of intracellular pathways to replicate cell-cell interactions for the survival signals. Our results suggest that therapeutic manipulation not only of intracellular levels of SK but also of its downstream targets in endothelial cells may be one way in which desired phenotypic changes may be created.
Prepublished online as Blood First Edition Paper, January 4, 2005; DOI 10.1182/blood-2004-02-0452.
Supported by a Programme Grant from the National Health and Medical Research Council of Australia and a project grant from the National Heart Foundation. V.L. is a recipient of a National Health and Medical Research Council scholarship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Professor Bill Muller for the generous gift of the HEK7 antibody; Milena Babic, Michelle Parsons, Peter Brautigan, Jenny Drew, and Anna Sapa for excellent technical assistance; and the staff of the Maternity Wards of the Women's and Children's Hospital and Burnside War Memorial Hospital for collection of umbilical cords. We are grateful to Sue Lester (Arthritis Research Laboratory, Hanson Institute) for invaluable help with the statistics.