Comment on Taylor et al, page 3372
Inhibition of the costimulatory receptor-ligand pair, ICOS: B7RP-1, offers a new powerful tool for tolerance induction in bone marrow transplantation.
Bone marrow transplantation following supralethal radiochemotherapy is associated with dangerous infections due to the slow immune reconstitution during the first year after transplantation.1 Thus, the use of reduced-intensity conditioning, associated with less severe immune ablation, could have remarkable potential in the treatment of a variety of nonmalignant diseases or for the induction of “mixed chimerism” as a prelude for cell therapy in cancer or in organ transplantation.2 However, the marked level of host immune cells surviving mild preparatory regimens represents a difficult barrier for the engraftment of donor cells. Thus, the importance of new approaches that might control the alloreactivity of the graft, without enhancement of graft rejection, cannot be overestimated. For example, the use of purified allogeneic stem cells, which do not pose any risk for graft-versus-host disease (GVHD) and which can continuously present donor type antigens in the host thymus, thereby inducing durable tolerance to donor cells or tissues, represents one of the most desirable goals in transplantation biology. Alternatively, agents that control GVHD might improve the outcome of unseparated (T-cell replete) hematopoietic transplantations.FIG1
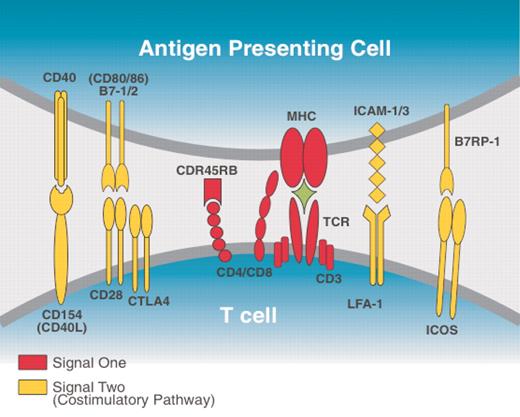
ICOS:B7RP-1 and other major costimulatory receptor–ligand pairs. ICOS is expressed mainly on activated T cells and resting memory cells while its ligand, B7RP-1, is constitutively expressed on APCs and also on fibroblasts and endothelial and epithelial cells. Like the CD28:B7-1/2 pathway, the ICOS-ICOSL pathway can enhance T-cell proliferation, CD154 expression, and cytokine production. Both in vitro and in vivo studies indicate that ICOS-ICOSL costimulation contributes to the production of the effector cytokines interferon γ (IFNγ), tumor necrosis factor α (TNFα), granulocyte-macrophage colony-stimulating factor (GMCSF), interleukin 4 (IL-4), IL-5, IL-13, and IL-10 but little IL-2. It is thought that ICOS-ICOSL costimulation may have a more critical role regulating T-helper 2 (TH2)–cell differentiation than it does regulating T-cell expansion.3-5
ICOS:B7RP-1 and other major costimulatory receptor–ligand pairs. ICOS is expressed mainly on activated T cells and resting memory cells while its ligand, B7RP-1, is constitutively expressed on APCs and also on fibroblasts and endothelial and epithelial cells. Like the CD28:B7-1/2 pathway, the ICOS-ICOSL pathway can enhance T-cell proliferation, CD154 expression, and cytokine production. Both in vitro and in vivo studies indicate that ICOS-ICOSL costimulation contributes to the production of the effector cytokines interferon γ (IFNγ), tumor necrosis factor α (TNFα), granulocyte-macrophage colony-stimulating factor (GMCSF), interleukin 4 (IL-4), IL-5, IL-13, and IL-10 but little IL-2. It is thought that ICOS-ICOSL costimulation may have a more critical role regulating T-helper 2 (TH2)–cell differentiation than it does regulating T-cell expansion.3-5
The so-called costimulatory signal, which is generated by the interaction of a number of nonspecific receptor-ligand pairs between the T cell and its antigen-presenting cell (APC), is crucial for productive T-cell activation. In the absence of costimulatory signals, a T cell encountering an antigen undergoes abortive activation. It does not produce appreciable amounts of cytokines and does not divide but instead becomes anergic or undergoes apoptosis. Several T-cell/APC molecules may serve as receptors/ligands for costimulatory signals, the most characterized of which are the CD28: B7-1/2 and the CD154:CD40 pathways (see the figure).
The new study of Taylor and colleagues demonstrates the efficacy of a new potential intervention at the level of costimulatory receptor–ligand pair, ICOS:B7RP-1. Previous studies with inducible costimulator (ICOS)–deficient mice have shown a critical role for ICOS in T-cell help for immunoglobulin (Ig) class switching, germinal formation, and memory B-cell development.6,7 By using such mice, the authors further show in the present study the important role in alloreactivity of ICOS. Moreover, by using antibodies directed against ICOS they were able to prevent GVHD as well as graft rejection. This type of antibody represents an important addition to the more-studied anti-CD40L, anti-B7, cytotoxic T-lymphocyte antigen 4 (CTLA-4)–Ig, and anti–lymphocyte function-associated antigen 1 (anti-LFA1), all proven to be effective costimulatory blocking agents.
Considering the marked barrier presented by the numerous host-type T cells remaining after reduced-intensity conditioning, or by the level of alloreactive T cells present in bone marrow transplants, it has become apparent that combining different blocking agents might be required for effective tolerance induction in vivo. In particular, considering that thrombotic complications found to be associated in initial clinical trials with anti-CD40L8 have reduced the enthusiasm for this drug, new agents such as anti-ICOS antibody likely enhance the chance for achieving the long-awaited goal of durable allogeneic stem cell engraftment without GVHD under low-toxicity conditioning. ▪