Abstract
Low levels of B-cell-receptor (BCR) expression are the hallmark of tumoral B lymphocytes in B-cell chronic lymphocytic leukemia (B-CLL). These cells also respond inadequately to stimulation through the BCR. This receptor consists of a surface immunoglobulin associated with a CD79a/CD79b heterodimer. We previously showed that the intracellular synthesis of BCR components, from transcription onward, is normal. Here, we investigated the glycosylation status and cellular localization of μ, CD79a, and CD79b chains in 10 CLL patients differing in surface immunoglobulin M (IgM) expression. We reported a severe impairment of the glycosylation and folding of μ and CD79a. These defects were associated with the retention of both chains in the endoplasmic reticulum and lower levels of surface IgM expression. In contrast, no clear impairment of glycosylation and folding was observed for CD79b. No sequence defects were identified for BCR components and for the chaperone proteins involved in BCR folding processes. These data show, for the first time, that lower levels of BCR surface expression observed in CLL are accounted for by an impaired glycosylation and folding of the μ and CD79a chains.
Introduction
The B-cell antigen receptor (BCR) is expressed on the plasma membrane as a disulfide-bonded complex of heavy and light immunoglobulin (Ig) chains associated with the CD79a/CD79b heterodimer. This heterodimer plays a key role in receptor expression and signal transduction, linking the antigen binding Ig chains to intracellular tyrosine kinases of the Src family.1
B-chronic lymphocytic leukemia (B-CLL) is characterized by an accumulation of monoclonal B lymphocytes expressing CD5 and CD23 molecules, but with only small amounts of surface Igs2-4 and CD79b molecules.5 CLL is the only B-cell cancer to display this phenotypic feature that is correlated with defective tyrosine kinase activity, resulting in low levels of tyrosine phosphorylation upon stimulation via the BCR pathway, via an unknown mechanism.6
We previously reported7 that intracellular synthesis of BCR components, including the transcription of the corresponding genes, was normal. We suggested that defective receptor chain assembly might account for the low levels of BCR expression. Proteins comprising several subunits, such as the BCR, require folding and assembly. These processes take place in the endoplasmic reticulum (ER), where the proteins are modified (cleavage of signal peptide, N-glycosylation, formation of disulfide bonds) and folded before they pass into the Golgi apparatus. If folding and maturation are defective, then the quality control (QC) system of the cell makes use of several different mechanisms to prevent the production of non-native proteins. The proteins are retained in the ER and several resident chaperones are responsible for refolding the molecules correctly. In the case of BCR, several chaperones, including calnexin, calreticulin, glucose-regulated protein 78 (GRP78, also known as BiP [Ig heavy chain binding protein]), and GRP94, have been shown to associate with the μ, CD79a, and CD79b chains.8 Improperly folded or assembled proteins may be degraded by ER-associated degradation (ERAD), a multistep process involving selection of the substrates to be degraded, targeting to the translocon and back to the cytosol, for degradation by the ubiquitin proteasome pathway. Some misfolded substrates are not degraded and instead accumulate as aggregates. In some cases, they may venture into the Golgi apparatus, from which they may be retrieved by the ER if successful folding is not achieved.9,10
In this study, we investigated the processing and surface expression of the BCR in the B cells of 10 CLL patients by a combination of flow cytometry, immunofluorescence or electron microscopy, Western blotting, and immunoprecipitation studies.
We observed severe impairment of the glycosylation and folding of μ and CD79a chains, leading to defective assembly and the retention of these chains in the ER compartment, possibly accounting for the low levels of IgM surface expression in malignant B-CLL cells.
Patients, materials, and methods
Patients and cells
Peripheral blood samples were obtained from 10 typical B-CLL patients from Necker Hospital (Paris, France), all of whom gave informed consent for participation in the study. None of these patients had ever received treatment and all were classified as being at Binet stages A, B, or C, before the study, on the basis of established clinical criteria11 (Table 1). There were 7 patients at stage A: 4 had an indolent course with mutated Ig genes (patients 1 through 4), and 3 had an aggressive course (patients 6 through 8) with unmutated (patients 6 and 8) or mutated (patient 7) IgVH genes. There were 2 patients at stage B with aggressive disease and mutated (patient 5) or unmutated (patient 9) IgVH genes. The remaining patient was at stage C and displayed no mutated Ig genes (patient 10). Purified B cells were obtained by negative depletion, using a RosetteSep antibody cocktail (CD2, CD3, CD16, CD36, CD56) directly on peripheral blood, as described by the supplier (StemCell Technologies, Vancouver, BC). Enriched B cells (unro-setted) were isolated by Ficoll density gradient centrifugation and washed twice in phosphate-buffered saline (PBS). B-cell preparations were shown to be more than 95% pure by flow cytometry, using phycoerythrin-conjugated anti-CD19 monoclonal antibodies (mAbs; Beckman/Coulter, Roissy, France). All patients expressed the μ isotype, as shown by flow cytometry (Table 1). VH sequences were established for all patients, as previously reported,12 and are shown in Table 1.
Blood samples were also taken from healthy volunteers included as controls after they gave informed consent. Normal B cells were isolated by the procedure described in the previous paragraph.
Flow cytometry
For flow cytometry, we stained the membranes of 2 to 5 × 106 cells by incubation for 30 minutes on ice with phycoerythrin-labeled rabbit antihuman μ chain antibody from Dako (Glostrup, Denmark) or mouse CD79b (clone CB3.1) antibody from PharMingen (San Diego, CA) in PBS supplemented with 2% fetal calf serum (FCS). Isotype-matched antibodies were used as negative controls. Cell analysis was performed with an Epics XL flow cytometer (Beckman/Coulter). Approximately 5000 to 10 000 events were accumulated to generate a single histogram. The results are presented as the percentage of cells expressing a given marker compared with the isotype staining.
Colocalization studies by immunofluorescence microscopy
Cells were prepared as previously described7 and incubated with primary mouse or rabbit antibodies (Abs) in PBS supplemented with 0.05% saponin and 1 mg/mL bovine serum albumin (BSA) (permeabilization buffer) for 45 minutes at 4°C. The Abs used were from rabbit: anti-human μ chain (Jackson ImmunoResearch Laboratories, West Grove, PA) and anticalnexin (Sigma-Aldrich, St Louis, MO); from mouse: CD79b (clone SN8) (Dako), CD79a, and anticalnexin (PharMingen); or from sheep: anti-trans Golgi network (TGN46) (Serotec, Kidlington, United Kingdom). Cells were then washed in permeabilization buffer and incubated for 1 hour at 4°C with goat anti-mouse IgG1-fluorescein isothiocyanate (FITC; Southern Biotechnology Associates, Birmingham, AL), goat anti-sheep IgG1-FITC (Chemicon International, Temecula, CA), or rabbit antimouse Ab (Jackson ImmunoResearch) in permeabilization buffer. Cells were washed and incubated with goat antirabbit-Alexa 546 and goat antifluorescein-Alexa 488 Abs (Molecular Probes, Eugene, OR) for 1 hour at 4°C. Cells were then mounted in Vectashield (Vector Laboratories, Burlingame, CA), and images were acquired with Zeiss Apotome and Axiovision software (Carl Zeiss, Jena, Germany) using a 63 ×/1.4 objective lens. This system provides an optical slice view reconstructed from fluorescent samples, using a series of “grid projection” acquisitions. Colocalization was analyzed with Imaris 4.05 software (Bitplane AG Scientific Solutions, Zurich, Switzerland).
Cell lysis
Cells (108/mL lysis buffer) were lysed on ice by incubation for 30 minutes with (1) MBS buffer (25 mM morpholinoethanesulfonic acid, 150 mM NaCl [pH 6.5], 0.5% Triton X-100, 1 mM EDTA [ethylenediaminetetraacetic acid], 10 mM NaF, 1 mM sodium orthovanadate, plus protease inhibitors) or (2) digitonin buffer (150 mM NaCl, 1% digitonin from Sigma-Aldrich, 1 mM sodium vanadate, 50 mM NaF, 5 mM EDTA, 25 mM Tris [tris(hydroxymethyl)aminomethane]-HCl [pH 7.5] plus protease inhibitors). Solubilized cells were pelleted by centrifugation at 10 000g for 10 minutes, and supernatants (lysates) were analyzed.
Immunoprecipitation
Supernatants from digitonin lysates were precleared and appropriate mAbs (anti-μ or CD79a) were added. The mixture was shaken for 1 hour at 4°C, 50 μL equilibrated protein G-Sepharose (Pierce, Rockford, IL) was added, and the mixture was incubated for a further hour. Immune complexes were collected in the pellet by a short pulse centrifugation, washed 5 times in lysis buffer, dissociated by boiling the samples with reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and resolved by electrophoresis in a NuPage 10% Bis-Tris Gel (Invitrogen, Groningen, The Netherlands). In some experiments, supernatants from the first centrifugation were recovered and analyzed in reducing conditions.
Western blot analysis
Following electrophoresis, proteins were transferred onto Hybond enhanced chemiluminescence (ECL) membranes (Amersham International, Little Chalfont, United Kingdom). Membranes were blocked by incubation in 3% BSA in PBS and were then incubated overnight with primary antibodies in Tris-buffered saline (25 mM Tris-HCl, 150 mM NaCl, 0.3% Tween 20 [pH 7.5] supplemented with 3% BSA). Membranes were washed 6 times in Tris-buffered saline and incubated with an appropriate horseradish peroxidase-linked secondary antibody (Dako) for 2 hours. Membranes were washed and immunoreactive bands were detected with the ECL system (Pierce). The antibodies used in Western blot experiments were as follows: mouse CD79a and CD79b (PharMingen) and rabbit anti-μ heavy chain (Jackson ImmunoResearch).
Endoglycosidase-H digestion
Proteins were treated with Endoglycosidase-H (Endo-H; Roche Molecular Biochemicals, Mannheim, Germany) in 0.05 M phosphate, 0.02% SDS, 0.1 M 2-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride (PMSF) for 18 hours at 37°C and analyzed by Western blotting, as described in “Western blot analysis.”
Reverse transcriptase (RT)-PCR and sequencing
RNA extraction, cDNA synthesis, and polymerase chain reaction (PCR) were carried out as previously described.7 The cDNAs corresponding to transcripts encoding the constant region of the μ chain, the CD79a molecule, and the chaperones calreticulin, GRP94, and BiP were sequenced with the primers described in Table 2, at an annealing temperature of 62°C. In some cases, several sets of primers were required to obtain the complete sequence.
Immunoelectron microscopy
B-cell samples were fixed in 2% paraformaldehyde (PFA) + 0.1% glutaraldehyde (Sigma-Aldrich) in 0.1 M phosphate buffer (pH 7.4) and embedded in 10% gelatin. The resulting gelatin blocks were infused with 2.3 M sucrose in phosphate buffer for 2 hours at 4°C and snap frozen in liquid nitrogen. Ultrathin cryosections (60 ± 90 nm) were cut and picked up on a mixture of sucrose and methylcellulose. They were labeled with a primary rabbit antibody specific for the human μ chain (Jackson ImmunoResearch) and a gold-conjugated goat IgG antirabbit Ab (6 nm) (British BioCell International, Cardiff, United Kingdom). Sections were examined in a Jeol 1200 EX II electron microscope (Jeol, Tokyo, Japan) using an Eloise MegaView III camera and AnalySIS Pro software version 3.1 (Eloise SARL, Roissy, France). Background labeling with irrelevant antibodies was negligible at an acceleration voltage of 80 kV.
Results
In CLL, levels of surface IgM expression are associated with the glycosylation status of the μ and CD79a chains
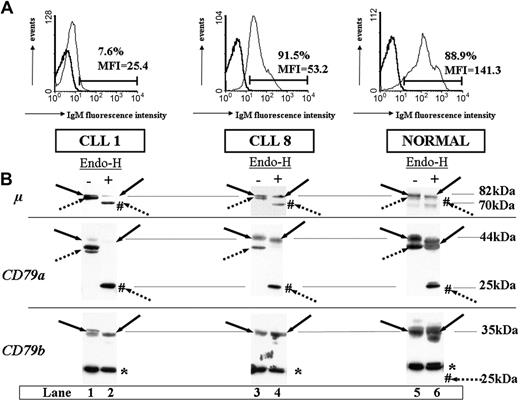
Consistent with previous reports for B-CLL,13,14 all the cases studied expressed lower levels of IgM on their cells than did healthy subjects (Table 1; Figure 1A) and had very low levels of surface CD79b expression (0.3% to 43% in Table 1). However, differences in surface IgM expression were observed among individuals, with very low levels of expression for patients 1 through 6, and higher levels of expression for patients 7 through 10 (the percentage of IgM+ B cells varied from 7.6 to 91.5 and mean fluorescence intensity [MFI] varied from 20.6 to 53.2, Table 1).
Differences in IgM surface expression profiles and BCR component glycosylation status between CLL patients and healthy subjects. (A) Flow cytometry analysis of surface IgM staining. Results from 2 patients, one with weakly expressing IgM (CLL 1 in Table 1) and one expressing IgM more strongly (CLL 8 in Table 1), and from a representative healthy subject are shown. Preparations enriched in B cells were subjected to flow cytometry, comparing isotype control staining (bold line) with test mAb staining (thin line). Fluorescence intensities are shown on a logarithmic scale on the x-axis. We report the percentage of positive cells and mean fluorescence intensity of IgM staining. (B) Glycosylation analysis of BCR components. Cell extracts (20 μg) produced by lysis in MBS were incubated at 37°C in the presence (+) or absence (-) of Endo-H and separated by 10% SDS-PAGE, and the resulting protein bands were transferred to nitrocellulose. Filters were probed with mAbs as follows: rabbit anti-μ heavy chain (μ), mouse anti-CD79a (CD79a), or anti-CD79b (CD79b), and immunoreactive bands were detected with an appropriate horseradish peroxidase-linked secondary antibody. Immature glycosylated (dotted arrows) and mature glycosylated (solid arrows) proteins are indicated for each staining. Forms deglycosylated by Endo-H treatment are indicated by #. Molecular masses are indicated in kilodaltons (kDa). The asterisk indicates a nonspecific band at 30 kDa constantly observed with the anti-CD79b probe from Pharmingen.
Differences in IgM surface expression profiles and BCR component glycosylation status between CLL patients and healthy subjects. (A) Flow cytometry analysis of surface IgM staining. Results from 2 patients, one with weakly expressing IgM (CLL 1 in Table 1) and one expressing IgM more strongly (CLL 8 in Table 1), and from a representative healthy subject are shown. Preparations enriched in B cells were subjected to flow cytometry, comparing isotype control staining (bold line) with test mAb staining (thin line). Fluorescence intensities are shown on a logarithmic scale on the x-axis. We report the percentage of positive cells and mean fluorescence intensity of IgM staining. (B) Glycosylation analysis of BCR components. Cell extracts (20 μg) produced by lysis in MBS were incubated at 37°C in the presence (+) or absence (-) of Endo-H and separated by 10% SDS-PAGE, and the resulting protein bands were transferred to nitrocellulose. Filters were probed with mAbs as follows: rabbit anti-μ heavy chain (μ), mouse anti-CD79a (CD79a), or anti-CD79b (CD79b), and immunoreactive bands were detected with an appropriate horseradish peroxidase-linked secondary antibody. Immature glycosylated (dotted arrows) and mature glycosylated (solid arrows) proteins are indicated for each staining. Forms deglycosylated by Endo-H treatment are indicated by #. Molecular masses are indicated in kilodaltons (kDa). The asterisk indicates a nonspecific band at 30 kDa constantly observed with the anti-CD79b probe from Pharmingen.
We previously showed7 that nascent IgM was not processed to give a mature glycosylated chain in most CLL patients. We investigated the relationship between this defect and surface IgM expression by analyzing the glycosylation status of the various components of the BCR in 10 CLL patients. Figure 1 displays the differences in IgM expression pattern and glycosylation status observed for the BCR components of 2 representative CLL patients (patient 1, with very low levels of surface IgM expression, and patient 8, with higher levels of expression) and a healthy donor. In normal B cells (healthy donor), the 82-kDa form of the μ chain predominated, with the low-molecular-weight form (78 kDa) present in only small amounts (Figure 1B, panel μ, lane 5). The CD79a mAb identified bands at 44 and 40 kDa on immunoblots for normal B cells, and a 35-kDa form predominated with the anti-CD79b probe (Figure 1B, panels CD79a and CD79b, lane 5). In patient 8, we detected bands for the 82-kDa μ chain and the 44-kDa CD79a chain. In contrast, these molecules were found to be weakly expressed in patient 1 (Figure 1B, panels μ and CD79a, lanes 1 and 3). Surprisingly, we found no difference among CLL patients and healthy controls when we probed membranes for CD79b (Figure 1B, panel CD79b, lanes 1, 3, and 5). Of the 10 patients studied, 6 displayed a striking predominance of these low-molecular-weight forms of μ and CD79a, with a pattern similar to that observed for patient 1 in Figure 1 (patients 1 through 6). These patients formed the group 1. Of the 6 patients, 4 had indolent forms of CLL with mutated IgVH genes (patients 1 through 4), 1 had progressive stage B disease with mutated IgVH genes (patient 5), and 1 patient had progressive stage A disease with no mutation (patient 6). The other 4 patients had μ and CD79a chain profiles similar to those of healthy subjects (patients 7 through 10). These patients had progressive forms of the disease and formed the group 2: 3 had no IgVH gene mutations (patients 8 through 10), and the other had a gene mutation (patient 7) and displayed a profile similar to that for patient 8 in Figure 1.
Differences in the electrophoretic mobility of the μ and CD79a chains in reducing conditions (Figure 1B, solid and dotted arrows on lanes 1, 3, and 5) may be accounted for by differences in glycosylation, as previously reported.15 We therefore treated cell extracts with Endo-H to determine whether these differences among CLL patients reflected differences in the glycosylation of the various BCR components. Endo-H cleaves high mannose oligosaccharides associated with glycoproteins present in the ER, and proteins become resistant to digestion after transport to the medial Golgi apparatus. Thus, Endo-H digestion can be used to distinguish ER-resident (immature) from Golgi-processed (mature) glycoforms.16,17 If normal cell extracts were used, only a small proportion of μ and CD79a chains shifted to 70-kDa and 25-kDa bands, respectively, corresponding to immature glycoforms (Figure 1B, panels μ and CD79a, lanes 5 and 6). In contrast, most of the μ and CD79a chains corresponded to immature glycoforms in patients from group 1 (Figure 1B, panels μ and CD79a, lanes 1 and 2, results for patient 1), whereas more mature glycoforms were found for group 2 (Figure 1B, panels μ and CD79a, lanes 3 and 4, results for patient 8). Interestingly, CD79b molecules were completely resistant to Endo-H in healthy subjects and in all CLL cases (Figure 1B, panel CD79b, lanes 1 and 2, 3 and 4, 5 and 6), indicating that these molecules had passed through the Golgi apparatus. These data clearly indicate that the level of IgM expression on the surface of the cells of CLL patients is related to the extent to which the glycosylation of μ and CD79a chains is impaired.
The retention of the μ and CD79a chains in the ER contrasts with the Golgi-processed glycoform patterns of CD79b in CLL patients
The glycosylation defect observed in most of the CLL patients studied may correspond to misfolding of the CD79a and μ chains. Chaperones are present in the ER lumen and are involved in folding, acting as QC sensors in this process. The resident transmembrane ER protein calnexin and its soluble homolog calreticulin act as specific ER chaperone proteins for glycoproteins. They play an important role in the folding of glycoproteins by retaining non-native glycoproteins in the ER until they are correctly folded; in some cases, they target misfolded glycoproteins for degradation.
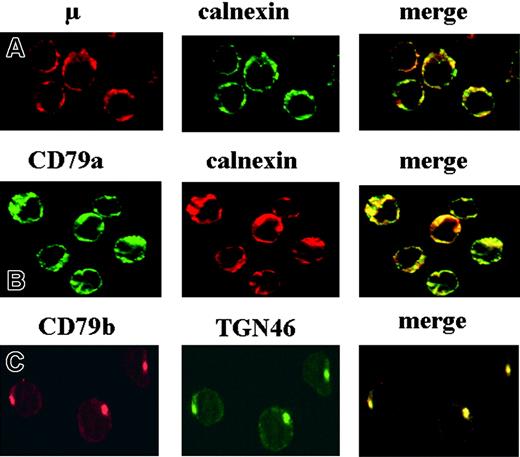
We carried out immunofluorescence microscopy with B cells to determine the cytoplasmic localization of BCR components, taking into account the levels of surface IgM expression of each patient. Independently of the level of surface IgM expression and glycosylation status observed, all patients displayed colocalization of the CD79a or μ chains with the ER-resident protein calnexin, as indicated by the yellow color in merged images (Figure 2A-B). Little if any colocalization was observed with normal B cells (data not shown). In contrast, the CD79b chain was not colocalized with calnexin (data not shown) but was instead detected in the trans-Golgi network, which was detected with the TGN46 marker (Figure 2C).
Analysis of the subcellular localization of the various BCR components by immunofluorescence microscopy. Fixed and permeabilized CLL B cells were incubated with various combinations of mAbs as follows: anti-μ/anticalnexin (A), anti-CD79a/anticalnexin (B), and anti-CD79b/anti-TGN46 (C). Red and green images were collected and merged, with yellow coloration indicating colocalization. These images were obtained with B cells from patient 2 (Table 1).
Analysis of the subcellular localization of the various BCR components by immunofluorescence microscopy. Fixed and permeabilized CLL B cells were incubated with various combinations of mAbs as follows: anti-μ/anticalnexin (A), anti-CD79a/anticalnexin (B), and anti-CD79b/anti-TGN46 (C). Red and green images were collected and merged, with yellow coloration indicating colocalization. These images were obtained with B cells from patient 2 (Table 1).
These data therefore indicate marked retention of the μ and CD79a chains in the ER in all the CLL cases studied.
Only mature glycoforms of BCR components were found to be associated
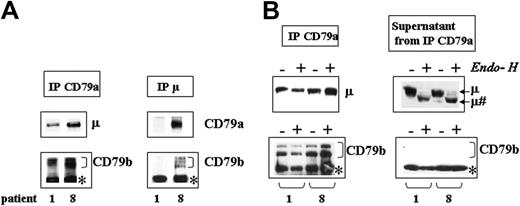
We further explored the defect in the assembly of BCR components by carrying out coimmunoprecipitation studies for 5 patients (patients 1, 2, 3, 8, and 9). Immunoblots of immunoprecipitated CD79a chains showed some association with μ and/or CD79b chains in all patients studied, despite marked differences in the level of IgM export to the cell surface, as shown by much fainter surface staining for IgM obtained for patient 1 (representative of patients 1, 2, and 3) than for patient 8 (representative of patients 8 and 9) (Figure 3A, left panel). In contrast, immunoprecipitation of the μ chains revealed that these chains were associated with CD79a and/or CD79b in patient 8, but not in patient 1 (Figure 3A, right panel).
Analysis, by coimmunoprecipitation assays, of the assembly of BCR components in CLL patients. (A) Analysis of molecules associated with μ or CD79a. B cells from patients 1 and 8 were lysed in digitonin and the resulting cell extract was immunoprecipitated with Abs against CD79a (left panel) or μ (right panel) chains. Immunoprecipitates (IP) were analyzed with anti-μ, anti-CD79a, or anti-CD79b mAbs. (B) Study of the glycosylation status of the molecules associated and unassociated with the CD79a chain. B cells from patients 1 and 8 were lysed in digitonin and the resulting cell extract was immunoprecipitated with anti-CD79a mAb. IP or supernatants from the IP were treated in the presence (+) or the absence (-)of Endo-H and analyzed using anti-μ (top row of blots) or anti-CD79b (bottom row) Abs. μ# indicates the deglycosylated form of μ chains after Endo-H treatment. *Nonspecific band observed with the anti-CD79b probe.
Analysis, by coimmunoprecipitation assays, of the assembly of BCR components in CLL patients. (A) Analysis of molecules associated with μ or CD79a. B cells from patients 1 and 8 were lysed in digitonin and the resulting cell extract was immunoprecipitated with Abs against CD79a (left panel) or μ (right panel) chains. Immunoprecipitates (IP) were analyzed with anti-μ, anti-CD79a, or anti-CD79b mAbs. (B) Study of the glycosylation status of the molecules associated and unassociated with the CD79a chain. B cells from patients 1 and 8 were lysed in digitonin and the resulting cell extract was immunoprecipitated with anti-CD79a mAb. IP or supernatants from the IP were treated in the presence (+) or the absence (-)of Endo-H and analyzed using anti-μ (top row of blots) or anti-CD79b (bottom row) Abs. μ# indicates the deglycosylated form of μ chains after Endo-H treatment. *Nonspecific band observed with the anti-CD79b probe.
We investigated whether the μ and CD79b chains that were able to associate corresponded to mature glycosylated chains, by treating CD79a immunoprecipitates with Endo-H. In both patients studied (1 and 8), the μ and CD79b chains associated with CD79a were in an Endo-H-resistant mature form (Figure 3B, left panel), whereas the μ chains not associated with CD79a and present in the supernatant were Endo-H sensitive (Figure 3B, right panel). In contrast, CD79b chains were not detected in supernatants from CD79a immunoprecipitates for either patient. This suggests that most of the available CD79b was associated with CD79a chains.
Thus, CD79a-CD79b and CD79a-μ associations concerned only mature Golgi-processed glycoforms, and no association was detected between immature Endo-H-sensitive μ and CD79a glycoforms.
A major defect in the glycosylation of CD79a and μ chains may result in the aggregation of μ chains into megavesicles
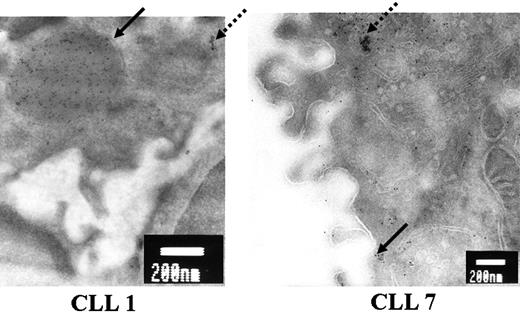
Given the importance of the glycosylation defect associated with marked changes in surface IgM expression, we investigated the possible formation of aggregates of misfolded proteins in patients 1, 2, and 7. Such aggregates were thought likely to occur in patients 1 and 2, and unlikely to occur in patient 7. We used immunogold labeling and electron microscopy to detect aggregates of μ chains. Megavesicles of about 400 nm in diameter (bold arrow), containing large aggregates of μ chains, were detected in B cells from patients 1 and 2 (Figure 4, results for patient 1). In contrast, no vesicles of this size were observed in B cells from patient 7, for whom we observed the export of μ chains to the membrane in small vesicles (bold arrow in Figure 4). We also observed μ chains trapped in the ER in patients 1 and 7 (dotted arrows in Figure 4).
Analysis of μ chain aggregation by electron microscopy. Ultrathin cryosections of B cells from patients 1 and 7 were processed for postembedding immunogold labeling with anti-μ mAb followed by incubation with secondary antibodies conjugated to 6 nm colloidal gold. Bold arrows indicate gold-labeled μ chains in a megavesicle for patient 1 and in a small vesicle moving toward the cell surface for patient 7. Dotted arrows indicate gold-labeled μ chains in the ER in both patients. Bars = 200 nm. Original magnification, × 20 000.
Analysis of μ chain aggregation by electron microscopy. Ultrathin cryosections of B cells from patients 1 and 7 were processed for postembedding immunogold labeling with anti-μ mAb followed by incubation with secondary antibodies conjugated to 6 nm colloidal gold. Bold arrows indicate gold-labeled μ chains in a megavesicle for patient 1 and in a small vesicle moving toward the cell surface for patient 7. Dotted arrows indicate gold-labeled μ chains in the ER in both patients. Bars = 200 nm. Original magnification, × 20 000.
The impaired surface expression of IgM could not be accounted for by structural defects in BCR components and chaperone proteins
We carried out structural studies to check that the glycosylation defects did not result in changes in the structure of the various BCR components or of the major chaperone proteins. In a previous study,18 we sequenced the B29 (CD79b) gene of 20 CLL patients from 10 families and showed the presence of a few silent or replacement mutations, probably corresponding to polymorphisms of the B29 gene, none of which caused structural abnormalities in the CD79b protein. We also detected no mutations in the sequence encoding the transmembrane domain of the μ chain, which is known to be important for the assembly and surface expression of the BCR, or in the calnexin coding sequence.7 However, we checked for the absence of structural abnormalities by sequencing cDNAs corresponding to the transcripts encoding the constant domain of μ, CD79a, and chaperones such as calreticulin, GRP94, and BiP. No such abnormalities were detected for the μ and CD79a chains, thereby excluding the possibility of defects in the N-linked glycosylation sites or, for any of the chaperone proteins tested, in patients 1 and 8 (data not shown).
Discussion
B-CLL cells display a phenotype that is unusual in B-cell cancers, in that the most consistent abnormality observed in this disease is low levels of BCR expression on the cell surface, and low levels of CD79b molecule. However, the mechanism responsible for this phenotype remains unknown.
We show here, for the first time, that the levels of mature glycoforms of the μ and CD79a chains are directly related to levels of surface IgM expression. Unexpectedly, we found no clear evidence of impairment of CD79b glycosylation. We also found that the μ and CD79a chains were retained in the ER compartment.
One possible explanation for such a defect is a mutation affecting a chaperone protein involved in folding, a component of the BCR components, or both. However, we found no evidence of structural defects in the chaperone proteins calnexin,7 calreticulin, BiP, and GRP94 or in the constant domain of μ, CD79a, or CD79b chains.18
We report here an impairment of the glycosylation of μ and CD79a chains, associated with lower levels of expression of IgM at the cell surface. In patients with higher levels of surface IgM expression, we detected no impairment of glycosylation, suggesting that the μ and CD79a chains transited through the medial-Golgi compartment. However, the colocalization of these molecules with calnexin suggests that they may have been misfolded or may have undergone retrograde transport to the ER from the trans-Golgi compartment.19,20 Some colocalization of μ chains with cis-Golgi markers was observed, but only in patients with higher levels of surface IgM expression, consistent with this possibility (data not shown). Nevertheless, the possible involvement of the N-linked glycosylation of both μ and CD79a chains in defective surface expression of IgM in CLL is of particular interest. Indeed, CD79a has been shown to be of key importance in B-cell development,21 and in the stabilization22 and functional properties23 of the BCR. The role played by glycosylation status in the export of receptor proteins with respect to ER QC has also been investigated.24 Our results suggest that abnormal glycosylation profiles are more common in patients with mutations than in those without. This finding requires confirmation by quantitative assays in a larger number of patients. However, a folding defect, as demonstrated by the consistent colocalization of μ and CD79a chains with calnexin, was found in all cases.
Immunoprecipitation studies shed light on the impaired association of BCR components. Indeed, only adequately glycosylated μ and CD79a chains are found to be assembled in CLL patients. We detected only Endo-H-resistant μ and CD79b chains, corresponding to Golgi-processed glycoforms, in CD79a immunoprecipitates regardless of the patient studied and his or her surface IgM expression profile (Figure 3B). Moreover, we were able to recover Endo-H-sensitive CD79a chains from μ-depleted lysates of B-CLL cells (data not shown). The results of our immunoprecipitation studies therefore suggested that there is an ER-resident pool of unassociated immature μ and CD79a chains, possibly corresponding to the calnexin-associated molecules detected by immunofluorescence microscopy in all patients studied (Figure 2A-B). However, CD79b chains were not detected in supernatants from CD79a immunoprecipitates for either patient. This suggests that CD79b chains may therefore limit BCR assembly, at least in patients with CLL. The lack of detection of CD79a and CD79b chains in μ immunoprecipitates, particularly in patients with lower levels of surface IgM expression (Figure 3A, patient 1), and the detection of μ chains in CD79a immunoprecipitates confirm previous observations15 and suggest that μ chains are synthesized in excess over CD79a and CD79b in CLL patients.
It is unclear whether genetic modifications affecting the CD79b molecule occur in CLL. One report described such genetic alterations in the CD79b gene,25 whereas 3 others, including ours,18,26,27 reported no such mutations. Unexpectedly, we detected only Endo-H-resistant Golgi-processed forms of CD79b in all patients (Figure 1B). These data were confirmed by the observed colocalization of these molecules with trans-Golgi markers on immunofluorescence microscopy (Figure 2C). However, the unimpaired folding and structure of CD79b contrast with the low level of surface expression of this molecule in CLL. An increase in the number of short CD79b transcripts lacking the extracellular domain may be responsible for regulating the surface expression of this molecule.28 Overexpression has been reported in CLL patients,7,27,29 but we found no obvious correlation between the amount of mRNA produced and the surface expression of the protein. In Western blots, we detected no significant difference in the amounts of protein produced from short transcripts in CLL and normal B cells (data not shown). Several mechanisms may account for these observations: (1) CD79b chains or CD79a/CD79b complexes may fail to reach the cell surface, being directed instead to the degradation compartment, as reported in studies on human pro-B cells30 or using chimeric proteins31 ; (2) the antigenic epitopes on the CD79b molecule may not be recognized by mAbs, due to changes in the conformation of the BCR complex; or (3) an unknown molecule may interfere with the binding of the antibody. No retention or glycosylation defect was detected for CD79b chains, but the reasons for the very low levels of surface expression of these chains remain unclear and possible involvement in the low level of BCR expression cannot be excluded.
Electron microscopy experiments showed that μ chain aggregates were present in 2 patients with severe defects in IgM export. Such aggregates may form due to the excessive retention of μ chains. This finding is novel and suggests that the processing capacity of the ER has been overwhelmed, resulting in the misfolding and aggregation of nascent proteins. In these extreme conditions, the chaperone system cannot ensure QC in the ER by monitoring and guiding folding, as has been reported in plant and animal cells.32,33 It is unclear whether the megavesicles formed are involved in the removal of aggregates, by degradation34 or transport.35
Many unresolved questions remain concerning the mechanisms underlying these defects in CLL. The patients' B cells express only small numbers of BCR, respond inadequately to BCR ligation, and are frequently committed to the production of natural autoantibodies.36-38 By analogy with a transgenic model,39 in which anergic B cells express only small numbers of BCR and are unresponsive to further stimulation via the BCR pathway, it has been suggested that the B cells of CLL patients are generated by the proliferation of anergic B cells.40 Defective BCR signaling in anergic B cells could then be accounted for by the presence of small numbers of destabilized BCR, with CD79a/CD79b and the μ chain physically dissociated, as reported for lymphoma B cells rendered unresponsive by binding to antigens of moderate to low affinity.22,41 Our detection of mature μ glycoforms in the supernatant of CD79a immunoprecipitates is consistent with these observations.
More than half of all CLL patients harbor somatic mutations of VH genes13,42 that are associated with a good prognosis.43-45 Recent studies analyzing signal competence revealed that patients with CLL who lack such mutations tend to express larger numbers of BCR and to respond more strongly to stimulation than do CLL patients with such mutations. This suggests that the B cells of CLL patients without mutations retain their ability to respond to BCR stimulation, whereas mutated forms result in anergized B cells.46 Our work is consistent with these findings because 5 of the 6 patients with very weak surface IgM expression had mutations, whereas 3 of the 4 with stronger surface IgM expression did not. However, it remains to be determined whether an anergic process can induce the impairments in folding and assembly reported here for CLL B cells.
The interaction of an uncharacterized intracellular protein with a BCR component may also account for the retention of μ and CD79a chains in the ER in CLL, as reported for the K1 protein from human herpes virus 8 (HHV-8) in a B-lymphoma cell line.47 However, in contrast to what we observed here, Lee et al47 found that K1 binding to the μ chain affected transport to the Golgi apparatus rather than BCR assembly in the ER. It is also possible that an unknown protein interacts with the intracellular machinery governing QC mechanisms in the ER compartment. Such a mechanism has been reported for the envelope protein of a neurovirulent virus48 and for the latent membrane protein 2A (LMP2A) of Epstein-Barr virus (EBV). LMP2A has been reported to mimic a constitutive BCR signal, thereby altering BCR expression and inducing the development of the CD5+ lineage in a transgenic mouse model.49-51
Our data provide the first direct evidence of defects in glycosylation and folding in the μ and CD79a chains associated with lower levels of surface IgM expression in CLL B cells. Unexpectedly, we found no similar abnormality for CD79b chains. The export of IgM from the ER to the cell surface therefore seems to depend strongly on glycosylation status and seems to be controlled by a QC mechanism leading to the retention of misfolded proteins. The possible requirement of glucose trimming for the stability of nascent CD79a chains in the ER and for the association of CD79a and CD79b chains, as described for the association of the T-cell-receptor α and β chains,52 requires further investigation.
Prepublished online as Blood First Edition Paper, December 9, 2004; DOI 10.1182/blood-2004-09-3643.
Supported by grant no. 2003005102 from the Fondation de France contre la Leucémie. Pablo Oppezzo is a fellow of the Académie Nationale de Médecine, Paris, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Dr Daniel Scott-Algara for helpful discussion of this manuscript. We particularly thank also Mrs Emmanuelle Perret for help with immunofluorescence microscopy, Mrs Nadège Cayet for technical assistance with electron microscopy, and Mrs Reine Bouyssié for secretarial assistance. We also thank Julie Sappa of Alex Edelman & Associates for correcting the English version of the manuscript.