Abstract
The mammalian target of rapamycin (mTOR) is a key regulator of growth and survival in many cell types. Its constitutive activation has been involved in the pathogenesis of various cancers. In this study, we show that mTOR inhibition by rapamycin strongly inhibits the growth of the most immature acute myeloid leukemia (AML) cell lines through blockade in G0/G1 phase of the cell cycle. Accordingly, 2 downstream effectors of mTOR, 4E-BP1 and p70S6K, are phosphorylated in a rapamycin-sensitive manner in a series of 23 AML cases. Interestingly, the mTOR inhibitor markedly impairs the clonogenic properties of fresh AML cells while sparing normal hematopoietic progenitors. Moreover, rapamycin induces significant clinical responses in 4 of 9 patients with either refractory/relapsed de novo AML or secondary AML. Overall, our data strongly suggest that mTOR is aberrantly regulated in most AML cells and that rapamycin and analogs, by targeting the clonogenic compartment of the leukemic clone, may be used as new compounds in AML therapy.
Introduction
Acute myeloid leukemia (AML) is a clonal disorder characterized by accumulation of malignant hematopoietic progenitor cells (HPCs) with impaired differentiation program. Despite important progress in the therapy of AML and high rates of complete remission after induction chemotherapy, most patients will relapse and die from the disease. Prevention of relapse is based on intensified programs, including high-dose chemotherapy and autologous or allogenic transplants that can benefit young patients. Thus, outcome of patients older than 60 years has not been improved for decades, underlying the need for potent and less toxic drugs for the treatment of this disease.1
Recent studies have demonstrated that AML cells are characterized by recurrent mutations of genes involved in cell differentiation, survival, and proliferation. A pathogenesis model for AML suggests that mutations of both tyrosine kinase receptors and transcription factors, by conferring survival and/or proliferative advantage (class I mutation) and by impairing cell differentiation (class II mutation), are needed to cause leukemia.2 Fms-like tyrosine kinase 3 (FLT3), c-KIT, and RAS mutations occur in 50% to 60% of AML cases,3-7 leading to aberrant activation of major cell survival or proliferation pathways such as mitogen-activated protein kinase (MAPK), phosphatidylinositol 3–kinase (PI3K)/Akt, signal transducer and activator of transcription (STAT), or nuclear factor κB (NF-kB).8-10 These antiapoptotic signaling pathways also contribute to AML resistance to the cytotoxic agents currently used in this disease.11,12 Thus, therapeutic interference with these pathways represents an attractive strategy in AML therapy. In this context, current clinical trials are evaluating new compounds directly targeting RAS or FLT3 (eg, farnesyl transferase inhibitors; CEP-701 and PKC 412).13,14
Molecules integrating multiple signal transduction pathways may represent relevant therapeutic targets in AML. Mammalian target of rapamycin (mTOR) is a serine/threonine kinase involved in the regulation of cell growth and proliferation by translational control of key proteins such as the cyclin-dependent kinase (CDK) inhibitor p27kip1, retinoblastoma protein, cyclin D1, c-myc, or STAT 3. mTOR is activated by different stimuli including nutrients or growth factors.15,16 Once activated, mTOR can phosphorylate its downstream targets, the ribosomal p70S6 kinase (p70S6K) and the 4E-binding protein 1 (4E-BP1). There are 2 known isoforms of S6K, p70 and p85, generated from differential splicing from a common gene. The p85S6K isoform is identical to p70S6K, except for a 23–amino acid extension at the amino-terminus that specifically targets it to the nucleus.17,18 Although both isoforms are implicated in cell growth regulation, most of the studies have focused on the p70S6K isoform, and the function of the p85S6K is still poorly characterized, particularly in hematopoietic cells. Activated p70S6K phosphorylates the 40S ribosomal protein S6 to initiate the translation of 5′ terminal oligopyrimidine tract-containing mRNAs encoding components of the protein synthesis machinery. 4E-BP1 is phosphorylated and inactivated by mTOR in response to a growth signal. Phospho–4E-BP1 dissociates from the eukaryotic initiation factor 4E (eIF-4E), a translation initiation factor that subsequently binds the cap structure of 5′ mRNAs and initiates the translation of transcripts encoding genes involved in cell cycle control.
mTOR activity is potently inhibited by rapamycin (RAPA), an immunosuppressant and antiproliferative agent, clinically used in the setting of solid-organ and hematopoietic transplants.19-21 RAPA binds to the immunophillin FK506 binding protein 12 (FKBP12). This complex directly inhibits mTOR and, in turn, the phosphorylation of p70S6K and 4E-BP1 leading to cell cycle arrest or apoptosis. Antitumoral activity of RAPA and its analogs (CCI-779, RAD001) has been studied in various preclinical solid tumor models, and appears very efficient on tumors displaying activation of the PI3K/AKT pathway.22-24 CCI-779 and RAD001 are under phase 1/2 clinical trials in cancers such as breast or renal carcinoma.25,26 However, the potential antitumoral effect of RAPA in AML has received little attention. At a first glance, preliminary results were not in favor of a therapeutic interest of this compound in AML. Indeed, RAPA was reported to weakly inhibit the growth of myeloid leukemic cell lines such as HL60, U937, and K562,27-29 and a recent study indicates only a modest effect of RAPA alone on survival of fresh AML cells when cultured in liquid medium.30 However, since this compound has been reported to have mainly cytostatic properties, we decided to investigate its potential effect on the growth of leukemic progenitor cells using a clonogenic assay.31 In agreement with the constitutive RAPA-sensitive phosphorylation of p70S6K and 4E-BP1 observed in most AML cells, we found a potent effect of this compound on the clonogenic compartment of leukemic cells. Based on these preclinical rationales, we conducted a pilot clinical study on a small series of 9 patients with either refractory/relapsed de novo AML or secondary AML. Our results support the use of RAPA or RAPA analogs in AML therapy.
Materials and methods
Cells
Fresh AML samples were obtained from patients diagnosed at the Hematology Department of Toulouse University Medical Center (France), after informed consent, and the study was approved by the institutional review board of the Hôpital Purpan, Toulouse, France. AML cells were isolated from bone marrow by Ficoll-Hypaque density-gradient centrifugation and were cryopreserved in Iscoves modified Dulbecco medium (IMDM) with dimethyl sulfoxide (DMSO, 10% final concentration) and fetal calf serum (FCS, 50% final concentration) or immediately processed for clonogenic assays (see “Clonogenic assays”). Leukemias were characterized by morphology (French-American-British classification), karyotype, immunophenotyping, and FLT3 gene mutations of the internal tandem duplication (FLT3-ITD) and activation loop (D835Mt).32,33 Normal bone marrow CD34+ hematopoietic progenitor cells (HPCs) were obtained after informed consent from discarded fragments from hematologically healthy patients who underwent hip surgery. Mononuclear cells from bone marrow were obtained by Ficoll-Hypaque density-gradient centrifugation, after which isolation of HPCs was performed by positive selection of CD34-expressing cells. Briefly, CD34+ HPCs were magnetically labeled using magnetic-activated cell sorter (MACS) CD34 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and then isolated by positive selection through magnetic separation (MS) separation column (Miltenyi Biotec). The purity of the CD34+ cells was evaluated by flow cytometry using CD45 and CD34 monoclonal antibodies and reached 85% to 98%. To assess the stimulation of the mTOR pathway, 1 × 106 CD34+ HPCs were incubated in IMDM 10% BIT (bovine serum albumin, insulin, transferrin) with or without granulocyte-macrophage colony-stimulating factor (GM-CSF, 10 ng/mL), stem cell factor (SCF, 100 ng/mL), and interleukin-3 (IL3, 1 IU/mL) for 24 hours, and were then processed for Western blot analysis (see “Western blotting”). The human leukemic cell lines KG1a, KG1, U937, K562, HL60, and HEL were purchased from American Type Culture Collection (ATCC; Rockville, MD); UT-7GM and UT-7EPO leukemic cell lines were kindly provided by D. Bouscary and D. Duménil (Cochin Hospital, Paris, France). All AML cell lines were incubated in a humidified CO2 incubator (5% CO2, 37°C) and cultured in IMDM containing 20% FCS (KG1a and KG1); RPMI 10% FCS (U937, K562, HL60, HEL); minimum essential medium (MEM) 10% FCS and 1 UI/L erythropoietin (UT-7EPO); or 5 ng/mL GM-CSF (UT-7GM). Rapamycin was purchased from Sigma (St Louis, MO), dissolved in DMSO, and stored at -20°C.
Western blotting
For Western blot, 1 × 106 cells resuspended and washed 2 times in cell culture medium without serum was denatured in Laemmli sample buffer for 5 minutes at 100°C. Proteins were resolved by polyacrylamide sodium dodecyl sulfate gel (SDS-PAGE) and then transferred onto nitrocellulose (membrane Hybond-C super; Amersham Pharmacia Biotech, Piscataway, NJ) using a liquid transfer apparatus (Amersham Pharmacia Biotech). The membrane was blocked overnight at 4°C in Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) containing 1% fat-free milk and 1% bovine serum albumin (BSA). The proteins were detected by blotting with the appropriate monoclonal or polyclonal antibodies in TBS, 0.1% Tween, 1% fat-free milk, and 1% BSA, followed by incubation with either anti–mouse or anti–rabbit immunoglobulin G (IgG) antibody coupled to horseradish peroxidase. Detection was achieved using a chemiluminescent probe (ECL; Amersham Pharmacia Biotech). Antibodies used were as follows: anti–phospho-p70S6K (Thr389), anti–phospho–4E-BP1 (Thr70), anti-p70S6K, and anti–4E-BP1 from Cell Signaling (Beverly, MA); anti-p27 from Santa Cruz Biotechnology (Santa Cruz, CA); and anti-Cdk2 and anti–cyclin E from Upstate Biotechnology (Charlottesville, VA). Horseradish peroxidase–conjugated secondary antibodies against mouse and rabbit immunoglobulins were from Cell Signaling.
Cell cycle analysis and cell viability
Fresh AML cells (2 × 105) were incubated in 96-well flat bottomed plates in IMDM 10% FCS with or without 10% 5637 cell line–conditioned medium (5637-CM; “Clonogenic assay”) with increasing concentrations of RAPA (0, 1, 10, and 100 nM). KG1a cells (5 × 105) were cultured in IMDM 20% FCS with or without 10 nM RAPA. For cell cycle analysis, fresh AML cells and KG1a were washed in phosphate-buffered saline (PBS; NaCl 7.6 g/L; Na2HPO4 0.7 g/L; KH2PO4 0.2 g/L) with 5.5 mM glucose at 4°C and fixed in 70% ethanol overnight at 4°C. Cells were then resuspended in 1 mL PBS containing 50 μg/mL propidium iodide and 100 U/mL RNAse A and incubated for 30 minutes at room temperature. The DNA content was monitored by flow cytometry (EPICS XL-MCL; Beckman Coulter, Villepinte, France). Cell viability was quantified by methyl thiazolyl tetrazolium (MTT) assay (Sigma). Experiments were performed in triplicate.
Clonogenic assays
Sensitivity of leukemic progenitors to rapamycin. After isolation from bone marrow by Ficoll-Hypaque density-gradient centrifugation, AML cells without previous freezing were adjusted to 1 × 105 cells/mL final concentration and grown in H4230 Stem Cell Technologies methyl cellulose medium (Stem Cell Technologies, Vancouver, BC) supplemented with 10% 5637-CM as a stimulant (5637 is a bladder carcinoma cell line secreting numerous cytokines acting on myeloid progenitors including GM-CSF, granulocyte colony-stimulating factor, IL1beta, macrophage colony-stimulating factor, and SCF)34 and increasing concentrations of RAPA (0, 0.1, 1, 10, and 100 nM). In the present study, from leukemic marrow samples, the percentages of blasts after Ficoll separation were between 62% and 100% (median, 92%). The cells were then plated in 35-mm Petri dishes in duplicate and allowed to incubate for 7 days in a humidified CO2 incubator (5% CO2, 37°C). At day 7, the leukemic colonies (more than 20 cells) and clusters (more than 5 cells) were scored under an inverted microscope. However, since in semisolid medium the growth of clusters or colonies appears to be an intrinsic characteristic of each blast cell population, clusters/colonies were referred to as colony-forming unit–leukemia (CFU-L). In each case, the leukemic nature of CFU-L was confirmed by morphologic analysis after Giemsa staining. Moreover, we also performed fluorescence in situ hybridization (FISH) analysis in 4 informative AML samples (nos. 1, 10, 12, and 39) using locus-specific identifier early growth response-1 (LSI EGR1) (5q31)/D5S721, D5S23, LSI core-binding factor β (CBFB) dual color probe (Vysis, Downers Grove; IL) and chromosome 8 Alpha Satellite (D8Z2) probe (Appligene Oncor, Gaithersburg, MD) to detect del 5 (no. 1), inv 16 (no. 12), and trisomy 8 (nos. 10, 39), respectively. As expected, in each case, colony-forming cells displayed identical cytogenetic abnormalities to the primary leukemic cells.
Colony-forming assays of human bone marrow CD34+cells. Fresh CD34+ human bone marrow cells were washed twice in IMDM medium with 10% FCS and resuspended at a concentration of 7 × 103/mL in Stem Cell Technologies' methyl cellulose complete media: H4230 supplemented with 10% 5637-CM (colony-forming unit–granulocyte macrophage [CFU-GM] growth); H4435 (colony-forming unit–monocyte [CFU-M] growth), and H4535 (burst-forming unit–erythroid [BFU-E] growth). RAPA was added to the cells at concentrations of 0, 1, 10, and 100 nM. The cells were then plated in 35-mm Petri dishes and incubated in a humidified CO2 incubator (5% CO2, 37°C) for 14 days. Colonies (more than 50 cells) were then scored under an inverted microscope. When possible (CFU-M), the compound concentrations required to induce 50% colony formation inhibition (IC50) were calculated from a plot of the number of colonies formed versus compound concentration, using G-pad software. Statistical analysis of changes in colony formation was conducted using Fisher and Yates tables. A probability value of P < .05 was taken to represent statistical significance.
Expression of the results. Results are expressed as percent of the control without drug. RAPA IC50s were calculated after linearization of the semilogarithmic dose-effect curve (linear RAPA concentration and logarithmic colony number) using G-pad software.
PI3K products analysis
AML cells, peripheral blood leucocytes from 2 healthy donors, and freshly isolated CD34+ HPCs were incubated in phosphate-free medium containing (32P) orthophosphate (200 μCi [7.4 MBq]/mL) for 8 hours, and lipids were extracted as previously described35 and resolved by thin-layer chromatography. The spots corresponding to phosphatidylinositol-bisphosphate and phosphatidylinositol-trisphosphate were scraped off, deacylated, and quantified by high-performance liquid chromatography (HPLC).36 The elution profile of the phosphoinositides was determined using appropriate standards.
Pilot clinical study
Requirements for patients' enrollment. Inclusion criteria were (i) refractory, relapsed, or poor-risk AML; (ii) age older than 18 years; (iii) Eastern Cooperative Oncology Group (ECOG) performance status less than 4; (iv) no renal or hepatic function impairment (defined by serum creatinine < 150 μM; serum bilirubin < 35 μM; and alanine aminotransferase and aspartate aminotransferease < 4 times normal); and (v) no active infection.
Rapamycin treatment. Patients were treated with sirolimus (Rapamune; Wyeth, Pearl River, NY) (orally) 6 mg as loading dose at day 1 then 2 mg per day for a planned time of 28 days, after informed consent. Treatment was continued in patients with evidence of hematologic response, until progression or toxicity. Patients were treated as outpatients with weekly clinical examinations and biologic monitoring (hematologic and biochemical tests) at least twice a week for the first 28 days of treatment.
Response criteria. The primary study objective was to determine the response rate. Complete response (CR) was defined by an absolute neutrophil count of more than 1.5 × 109/L, a platelet count of 100 × 109/L or more, and less than 5% of blast cells in the marrow. Partial response (PR) was defined as a more than 50% reduction in the absolute number of blood blasts or at least a 50% reduction in the percentage of marrow blasts, determined by cellular morphology using May-Grunwald-Giemsa staining.14
Results
Effect of rapamycin on AML cell lines
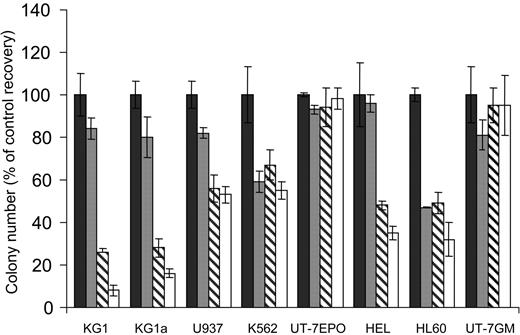
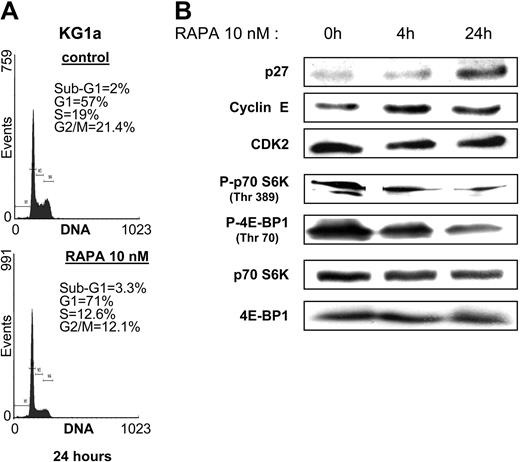
We first analyzed the sensitivity of the monocytic U937, the granulocytic HL60, the megakaryoblastic granulomonocytic differentiated UT-7GM, the erythroid UT-7EPO, the erythro-megakaryocytic HEL, K562, and the more immature myeloblastic CD34+CD38low KG1 and KG1a AML cell lines to increasing concentrations of the mTOR inhibitor RAPA in clonogenic assays. UT-7EPO and UT-7GM were insensitive to relevant concentrations of RAPA. K562, U937, HEL, and HL60 were moderately sensitive, whereas the proliferation of KG1 and KG1a was strongly inhibited by low concentrations of the compound (Figure 1). To investigate the effect of RAPA in KG1a cells in more detail, we performed cell cycle analysis and observed that inhibition of cell growth was due to arrest in G1 phase (Figure 2A). No features of apoptosis could be detected by morphologic (not shown) or cell cycle analysis. Inhibition of cell growth correlated with early up-regulation of the CDK inhibitor p27kip1, whereas levels of cyclin E and CDK2 were not modified (Figure 2B). There were 2 substrates of mTOR, p70S6K and 4E-BP1, phosphorylated in KG1a and RAPA (10 nM) that inhibited their phosphorylation without affecting the expression level of these proteins. These results indicate that RAPA has a strong cytostatic effect on KG1a, the most immature AML cell line tested.
Activity of RAPA on various AML cell lines. KG1, KG1a, U937, K562, UT-7EPO, HEL, HL60, and UT-7GM AML cell lines were grown in clonogenic assays in the presence of increasing doses of RAPA (▪, 0 nM; ▦, 1 nM; ▧, 10 nM; □, 100 nM). Results are presented as percentage of CFU-L for each RAPA-treated AML cell line relative to untreated cells and are mean ± SEM of 3 independent experiments performed in duplicate.
Activity of RAPA on various AML cell lines. KG1, KG1a, U937, K562, UT-7EPO, HEL, HL60, and UT-7GM AML cell lines were grown in clonogenic assays in the presence of increasing doses of RAPA (▪, 0 nM; ▦, 1 nM; ▧, 10 nM; □, 100 nM). Results are presented as percentage of CFU-L for each RAPA-treated AML cell line relative to untreated cells and are mean ± SEM of 3 independent experiments performed in duplicate.
RAPA blocks cell cycle in G1 phase and induces p27kip1 in KG1a cells. (A) KG1a cells were incubated with or without RAPA (10 nM) for 24 hours and then processed for cell cycle analysis. (B) KG1a cells (106 cells) were incubated with or without RAPA (10 nM) for 4 and 24 hours, lysed, and analyzed by Western blotting with the indicated antibodies. Results shown are representative of 3 independent experiments.
RAPA blocks cell cycle in G1 phase and induces p27kip1 in KG1a cells. (A) KG1a cells were incubated with or without RAPA (10 nM) for 24 hours and then processed for cell cycle analysis. (B) KG1a cells (106 cells) were incubated with or without RAPA (10 nM) for 4 and 24 hours, lysed, and analyzed by Western blotting with the indicated antibodies. Results shown are representative of 3 independent experiments.
mTOR targets are constitutively phosphorylated in most AML samples but not in normal HPCs
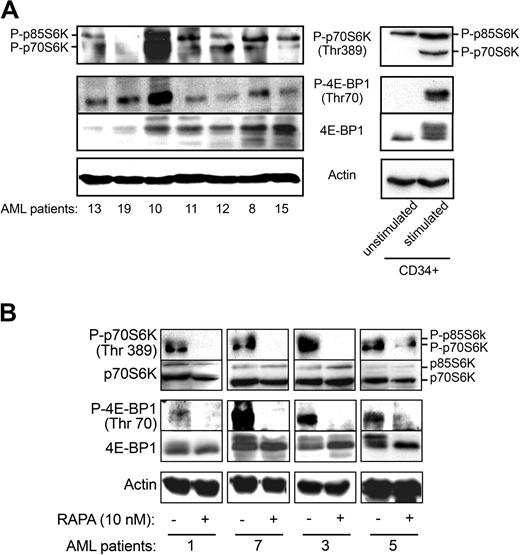
To evaluate the level of activation of mTOR in freshly isolated AML samples, we investigated the phosphorylation status of its targets (Figure 3A). As shown in Figure 3A, both isoforms of S6K (p85 and p70) were phosphorylated on Thr389 in the majority of the cases analyzed (17/22). 4E-BP1 was also constitutively phosphorylated in all cases tested (n = 23), although at variable levels. Conversely, p70S6K and 4E-BP1 were not phosphorylated in normal unstimulated freshly isolated CD34+ HPCs (Figure 3A, right panels), whereas the p85S6K isoform was phosphorylated to some extent. After 24-hour incubation in the presence of cytokines (GM-CSF, SCF, IL3), mTOR targets were clearly phosphorylated, suggesting that this pathway is functional in CD34+ HPCs. Moreover, the levels of PtdIns(3,4,5)P3, a PI3K product required for activation of Akt, an upstream regulator of mTOR, were significantly elevated in 8 AML samples compared with normal peripheral blood leukocytes (6.3 ± 2.6-fold increase in 32PtdIns(3,4,5)P3) or to normal unstimulated CD34+ HPCs where the level of 32PtdIns(3,4,5)P3 was below detection. These results indicate that PI3K is indeed up-regulated in AML cells. Figure 3B shows that treatment of AML samples by RAPA (10 nM) inhibited the phosphorylation of p70S6K on Thr389 and 4E-BP1 on Thr70 without significantly affecting the expression level of these proteins.
4E-BP1 and p70S6 kinase are phosphorylated in primary AML cells. (A) Cell lysates (106 cells) from 7 AML patients and normal unstimulated and stimulated (GM-CSF, SCF, and IL-3 for 24 hours) CD34+ HPCs were analyzed by Western blotting with specific antibodies to evaluate the phosphorylation status of 4E-BP1 and p70S6K. The phosphorylation of 4E-BP1 can also be visualized by a shift in the apparent molecular weight of the protein. Results shown are representative of 23 fresh AML samples tested. (B) Fresh AML cells from 4 patients were incubated with RAPA (10 nM) for 24 hours and analyzed by Western blotting with the indicated antibodies.
4E-BP1 and p70S6 kinase are phosphorylated in primary AML cells. (A) Cell lysates (106 cells) from 7 AML patients and normal unstimulated and stimulated (GM-CSF, SCF, and IL-3 for 24 hours) CD34+ HPCs were analyzed by Western blotting with specific antibodies to evaluate the phosphorylation status of 4E-BP1 and p70S6K. The phosphorylation of 4E-BP1 can also be visualized by a shift in the apparent molecular weight of the protein. Results shown are representative of 23 fresh AML samples tested. (B) Fresh AML cells from 4 patients were incubated with RAPA (10 nM) for 24 hours and analyzed by Western blotting with the indicated antibodies.
Rapamycin inhibits growth of CFU-Ls and CFU-Ms but not CFU-GMs and BFU-Es
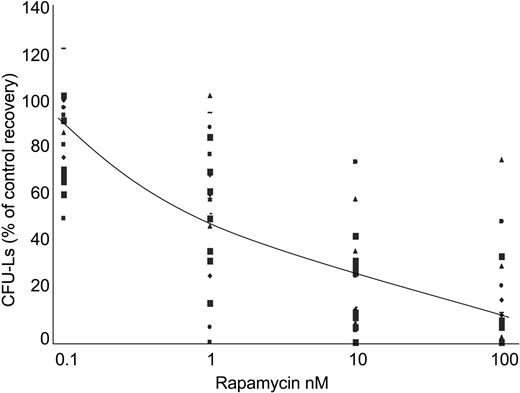
These results prompted us to analyze the effect of RAPA treatment on the viability of AML cells. As previously described with RAD001,30 RAPA induced only a discreet effect on cell survival of terminally differentiated AML cells in liquid culture, probably due to the fact that these cells were mainly in G0/G1 phase of the cell cycle (data not shown). Therefore, to investigate a potential growth-inhibitory effect of RAPA on fresh AML samples, we used a clonogenic assay. AML samples (n = 23) isolated from bone marrow were grown in methylcellulose supplemented with 5637-CM, and incubated with increasing concentrations of RAPA. Clinical and biologic characteristics of patients are described in Table 1. There were 3 patterns of sensitivity to RAPA observed. Of AML samples, 65% (15/23) were extremely sensitive to RAPA (IC50, < 15 nM), whereas 22% (5/23) were moderately sensitive (15 nM < IC50 < 50 nM) and 13% (3/23) were resistant (IC50, > 50 nM) (Table 1; Figure 4). No significant correlation between clinical data, cytogenetic data, and sensitivity to RAPA could be observed. However, most AML samples with mutation of the tyrosine kinase receptor FLT3 (FLT3-ITD) (n = 5/6) were found highly sensitive to RAPA (median IC50, 2.7 nM).
Low doses of RAPA inhibit the growth of AML progenitors. Freshly isolated AML cells from 23 patients were adjusted to 1 × 105 cells/mL final concentration, and grown in methylcellulose medium supplemented with 10% 5637-CM and increasing concentrations (ie, 0, 0.1, 1, 10, and 100 nM) of RAPA. CFU-Ls were scored at day 7. Results are presented as percentage of CFU-L for each RAPA-treated AML samples relative to untreated cells. The average curve is shown. Each symbol represents CFU-L's growth of AML samples at the corresponding concentration.
Low doses of RAPA inhibit the growth of AML progenitors. Freshly isolated AML cells from 23 patients were adjusted to 1 × 105 cells/mL final concentration, and grown in methylcellulose medium supplemented with 10% 5637-CM and increasing concentrations (ie, 0, 0.1, 1, 10, and 100 nM) of RAPA. CFU-Ls were scored at day 7. Results are presented as percentage of CFU-L for each RAPA-treated AML samples relative to untreated cells. The average curve is shown. Each symbol represents CFU-L's growth of AML samples at the corresponding concentration.
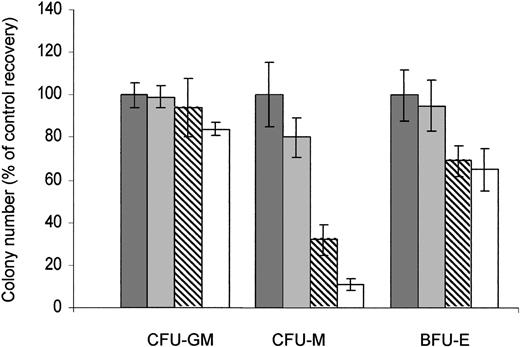
The activity of RAPA on the properties of normal CD34+ HPCs to generate granulo-monocytic (CFU-GM), erythroid (BFU-E), and monocytic (CFU-M) colonies was then investigated. Contrasting with CFU-Ls, no significant change in the number of the colony formation of CFU-GMs (n = 3) was observed upon RAPA exposure, even at the highest concentration. The number of BFU-Es (n = 3) displays only a little decrease, even after 14 days of continuous exposure at 100 nM (the mean percentage of BFU-E colony number formed upon exposure to 1, 10, and 100 nM RAPA relative to untreated cells was 95 ± 7%, 69 ± 10%, and 65 ± 14%, respectively, n = 3). However, RAPA significantly inhibited the colony formation of CFU-M progenitors (n = 6) in a dose-dependent manner (mean IC50, 17.9 ± 13.8 nM) (Figure 5). Altogether, our results demonstrate that in most cases, RAPA inhibits the growth of leukemic progenitors at clinically achievable concentrations, while sparing CFU-GMs and BFU-Es.
RAPA has no effect on CFU-GMs. Normal bone marrow CD34+ HPCs were incubated with appropriate media: H4230 supplemented with 10% 5637-CM to obtain CFU-GMs (n = 3); H4435 to obtain CFU-Ms (n = 6); and H4535 to obtain BFU-Es (n = 3), and in the presence of increasing concentrations of RAPA (▪, 0 nM; ▦, 1 nM; ▧, 10 nM; □, 100 nM). The colonies (more than 50 cells) were scored at day 14. Results are expressed as percentage of control and are mean ± SEM of duplicates.
RAPA has no effect on CFU-GMs. Normal bone marrow CD34+ HPCs were incubated with appropriate media: H4230 supplemented with 10% 5637-CM to obtain CFU-GMs (n = 3); H4435 to obtain CFU-Ms (n = 6); and H4535 to obtain BFU-Es (n = 3), and in the presence of increasing concentrations of RAPA (▪, 0 nM; ▦, 1 nM; ▧, 10 nM; □, 100 nM). The colonies (more than 50 cells) were scored at day 14. Results are expressed as percentage of control and are mean ± SEM of duplicates.
RAPA induces responses in refractory AML patients
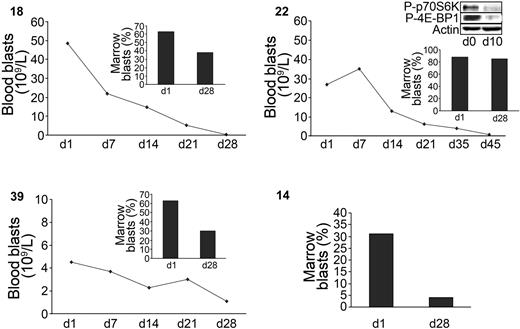
The results of the preclinical study led us to investigate the activity of RAPA in relapsed, refractory, or poor-risk AML patients. At doses used for renal transplant recipients, 9 patients were treated with RAPA for 28 days. Patients' characteristics are described in Table 2. The median age was 72 years (range, 55-77 years). Of the patients, 4 had refractory AML, 4 had relapsed disease (3 after autologous stem cell transplantation), and 1 received no prior therapy (AML secondary to refractory anemia with excess blasts). Whole blood sirolimus concentrations were measured at day 7 and day 21. The range of sirolimus concentrations was highly variable according to patients (trace amounts to 31.4 μg/L). At day 28, partial response, defined as a more than 50% reduction in the absolute number of blood blasts or at least a 50% reduction in the percentage of marrow blasts, occurred in 4 patients, while 4 patients progressed and 1 had stable disease. The median duration of response was 38 days (range, 35-120 days). In 2 responders (39 and 14), the antileukemic activity of RAPA was accompanied by a restoration of normal neutrophil counts and a loss of transfusion requirement. These 2 patients are alive at 8 and 10 months. Moreover, in the responding patient 22, the phosphorylation of p70S6K and 4E-BP1 was inhibited upon RAPA treatment (Figure 6, insert). Altogether, these data demonstrate that RAPA used as a single agent targets mTOR in vivo and induces significant antileukemic responses in poor-risk AML patients.
Antileukemic activity of RAPA in AML patients. Blood and marrow blast responses of 4 sensitive patients under RAPA treatment (numbers in upper left of each panel). Marrow response was evaluated at day 28 as described in “Materials and methods.” A Western blot showing the p70S6-kinase (Thr389) and 4E-BP1 (Thr70) phosphorylation status in patient 22 after 10 days of treatment is included. The percentage of blood blasts of this patient at this date was 90% of WBC.
Antileukemic activity of RAPA in AML patients. Blood and marrow blast responses of 4 sensitive patients under RAPA treatment (numbers in upper left of each panel). Marrow response was evaluated at day 28 as described in “Materials and methods.” A Western blot showing the p70S6-kinase (Thr389) and 4E-BP1 (Thr70) phosphorylation status in patient 22 after 10 days of treatment is included. The percentage of blood blasts of this patient at this date was 90% of WBC.
Discussion
Based on the fact that the mTOR-mediated signaling pathway downstream of PI3K/Akt is often up-regulated in cancer cells,37 the antitumoral effects of RAPA derivatives (eg, CCI-779 or RAD001) are presently evaluated in phase 1/2 clinical trials in various solid tumors. However, the use of mTOR inhibitors in hematologic malignancies is poorly documented.38 In this study, we show that PI3K/mTOR pathway is up-regulated in most AML cases and that RAPA inhibits CFU-Ls at clinically achievable concentrations. RAPA appeared to modulate selectively the proliferative properties of clonogenic leukemic cells as suggested by inhibition of CFU-L formation, while terminally differentiated blasts (AML bulk), which are mainly in G0/G1 phase, were moderately sensitive. In agreement, RAPA up-regulated the cyclin-dependent kinase inhibitor p27 and blocked cell cycle progression in G1 phase in KG1a, a cell line representative of early leukemic hematopoiesis.39 Interestingly, the growth of CFU-GMs was not inhibited by RAPA even at the highest concentrations, contrasting with the high sensitivity of CFU-Ls. We also observed that the mTOR pathway is activated by cytokines in CD34+ cells, but this activation is not essential for their proliferation. Further studies would be necessary to analyze the function of mTOR in normal hematopoiesis. Altogether, our results suggest that the leukemic progenitors are more sensitive to RAPA than their normal counterpart.
This raises the question of how mTOR can be constitutively activated in AML cells. To the best of our knowledge, activating mutations or overexpression of mTOR has not been demonstrated in neoplasia, and the mTOR gene mapping to chromosome 1p36.2 is not targeted by recurrent translocations in AML. Two recent studies have shown that the PI3K/Akt survival pathway is constitutively activated in most AML cells.30,40 In agreement, we found high levels of PI3K products and constitutive phosphorylation of mTOR downstream targets, p70S6K and 4E-BP1, in a series of fresh AML samples. Although the isoform of PI3K involved and its mechanisms of activation are unknown, the production of D3-phosphoinositides is thought to activate Akt and in turn increase mTOR activity. In agreement, PI3K inhibitors block the phosphorylation of p70S6K and 4E-BP1.30 mTOR is known to integrate multiple upstream signals including growth factors, oncogenes, tyrosine kinases receptors, or angiogenic factors. In the setting of AML biology, the activation of this particular pathway may involve several deregulated signaling pathways including aberrant tyrosine kinase receptor signaling pathways such as FLT3,41,42 expression of oncogenic products such as RAS,43 or autocrine production of hematopoietic growth factors such as vascular endothelial growth factor44,45 or SCF.46,47 Induction of mTOR/p70S6K signaling by a tyrosine kinase fusion protein FOP-FGFR1 has been reported in myeloproliferative disorder associated with the t(6;8) chromosomal translocation.48 Most of these aberrant cellular activation mechanisms lead to increased PI3K activity and in turn production of D3-phosphoinositides, an effect potentially reinforced by down-regulation of the phosphatidyl inositol phosphate (PIP3-3) phosphatase and tensin homolog deleted on chromosome 10 (PTEN) expression.30 Thus, mTOR is likely activated in AML through an up-regulated PI3K/Akt pathway; however, other pathways such as the MAPK module, which is constitutively activated in most AML cases,49 may also contribute to its stimulation.
In solid tumors, the relationship between PI3K/Akt activation and sensitivity to RAPA is now well established.22-24 Our study and other recent reports suggest a similar phenomenon in hematopoietic malignancies including multiple myeloma, lymphoma, and leukemia.30,50-52 It is tempting to speculate that the aberrant activation of the PI3K/Akt/mTOR cascade by the oncogenes involved in leukemogenesis sensitizes these cells to drugs targeting this pathway. Although the number of AML samples with mutations of the tyrosine kinase FLT3 is small, our data would suggest that this receptor, known to activate the PI3K/Akt and the MAPK pathways, could sensitize AML cells to mTOR inhibition. Further studies will be necessary to assess this potential role of FLT3, as it may have important clinical implications.14,42,53
Our in vitro studies in established cell lines or in primary cells strongly suggest that proliferation of AML cells could be blocked by inhibition of mTOR at clinically achievable concentrations of RAPA. Given these promising preclinical data, relapsed/refractory or poor-risk AML patients were treated with RAPA. We report here for the first time a clinical effect of a compound targeting mTOR in AML as a single agent. The significant responses obtained within 10 days in 4 of 9 patients suggested that inhibition of the mTOR pathway could be of clinical interest in AML. The antileukemic activity of RAPA was accompanied by a restoration of normal neutrophil counts and a loss of transfusion requirement in 2 responders, in agreement with the selective effect observed in clonogenic assays. RAPA was well tolerated and most patients were treated as outpatients, which is of interest especially for elderly patients. However, whole blood concentrations of RAPA showed a highly variable inter- and intrapatient biodisponibility,54 suggesting that analogs of RAPA, with better pharmacologic properties, could be more efficient.
However, our studies on cell lines and fresh AML cells, as well as the clinical trial, showed primary AML resistance to RAPA. Due to the low number of patients, we did not find any correlation between clinical resistance and bioclinical data or levels of phosphorylation of mTOR targets. Various mechanisms of resistance to RAPA have been described, including FKBP-12 mutations, high eIF-4/4E-BP1 ratio, defective regulation of p27kip1, or c-myc amplification.55,56 Whether these mechanisms are found in AML remains to be determined. Moreover, some AML cells may also activate other signaling pathways able to overpass mTOR inhibition. Interestingly, the most resistant cell line was UT7, either cultured with erythropoietin or GM-CSF, suggesting that RAPA does not interfere with erythropoietin (Epo)57 or GM-CSF signaling in leukemic myeloid cells. From these observations, one can speculate that the inhibitory effect of RAPA on CFU-Ls was not due to GM-CSF signaling interruption. Clearly, further studies will be necessary to assess the mechanisms of resistance to RAPA in AML.
Altogether, our data demonstrate that AML cells are very sensitive to mTOR inhibition both in vitro and in vivo, thus defining the basis for a new promising therapeutic strategy for AML patients using mTOR inhibitors alone or in combination with other pharmacologic inhibitors or cytotoxic drugs.
Prepublished online as Blood First Edition Paper, November 18, 2004; DOI 10.1182/blood-2004-06-2494.
Supported by grants from the Fondation de France (no. 2000002870), the Association de Recherche contre le Cancer (ARECA-Toulouse and contract no. 4794), and the Fédération Nationale des Centres de Lutte Contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Thanks are due to Monique Laroche, Nicole Lhernie, Cécile Viala, and Naïs Houdellier, and Drs Dastugue and Tronchère for excellent technical assistance and helpful discussions.