Abstract
Activation-induced cytidine deaminase (AID) is key to initiating somatic hypermutation (SHM) and class switch recombination (CSR), but its mode of action and regulation remains unclear. Since Pax-5 and Id-2 transcription factors play an opposing role in AID regulation, we have studied the expression of Pax-5, Id-2, and prdm-1 genes in 54 chronic lymphocytic leukemia (CLL) B cells. In 21 cases, presence of AID is constantly associated with high expression of the complete form of the Pax-5 gene (Pax-5a) and lower expression of the Id-2 and prdm-1 transcripts. In 33 cases, the absence of AID expression and CSR is associated with a reduction of Pax-5a and the appearance of a spliced form with a deletion in exon 8 (Pax-5/Δ-Ex8). Stimulation with CD40L+interleukin 4 (IL-4) induces CSR, the presence of AID transcripts, up-regulation of Pax-5a and down-regulation of Pax-5/Δ-Ex8, and Id-2 and prdm-1 transcripts. Pax-5a and Pax-5/Δ-Ex8 are translated into 2 isoforms of the B-cell–specific activator protein (BSAP) and both are able to bind the AID-promoter region. Overall, these results suggest that Pax-5/Δ-Ex8 could play an important role in the control of its own transcription and indirectly in AID expression and CSR.
Introduction
B lymphocytes develop from hematopoietic stem cells through an orderly process of differentiation. These events underlie the differentiation from a committed B-cell progenitor to precursor and mature B cells that upon stimulation by antigens undergo terminal differentiation into antibody-secreting plasma and memory B cells. The early phase of B lymphopoiesis is characterized by the sequential rearrangement of immunoglobulin (Ig) heavy chain (H) and light chain (L) genes.1 After rearrangement, Ig variable genes can be diversified by somatic hypermutation (SHM), while the effector functions of the constant domain can be modified by class switch recombination (CSR). The occurrence of these processes depends on activation-induced cytidine deaminase (AID), expressed in B cells from secondary lymphoid organs upon CD40 ligand (CD40L) stimulation.2 Since the absence of AID expression in one form of hyper-IgM syndrome in humans3 and in AID-targeted mice4 abolishes both CSR and SHM, this protein is presumed to play a major role in both processes.5
In addition to AID, a large panel of genes encoding key proteins involved in B-cell development has been characterized in the past years.6 The regulation of these genes depends on a complex interplay involving different transcription factors, some of which are ubiquitous, whereas the others are essentially expressed in B-cell lineages. Despite great progress in this field, the molecular mechanism of CSR and the protein factors involved in the process are still largely unknown. Different transcription factors such as the nuclear factor kappa B (NF-κB)/Rel family,7 E1/2/E47 factors,8 and BSAP/Pax-59 have all been implicated in CSR, but evidence for their direct involvement is still missing or controversial.10,11 The B-cell–specific activator protein (BSAP), encoded by the Pax-5 gene, is a member of a transcription factor multigene family that shares the paired box DNA binding domain12 and a singular mechanism of autoregulation carried out by a C-terminal region composed of activating and inhibitory sequences.13 In mammalians, Pax-5 expression is restricted principally to the B-cell lineage and is it essential for the development of B cells.14 Gene inactivation experiments have demonstrated that Pax-5 is important for maintaining the identity and function of mature B cells in late B lymphopoiesis in addition to its early role in B-lineage commitment.15
Chronic lymphocytic leukemia (CLL) results from the relentless accumulation of small mature, slowly dividing, monoclonal CD5+ B lymphocytes, which can express Ig V genes displaying either a mutated or unmutated profile.16 The mutational status of Ig genes has been shown to be associated with disease prognosis,17,18 suggesting that there are 2 types of CLL. The first arises from relatively less differentiated naive B cells with unmutated VH genes, and has a poor prognosis. The second evolves from more differentiated B lymphocytes with somatically mutated VH genes, and has a better prognosis than the unmutated phenotype. Although CLL B cells have been assumed to be frozen in an immature stage,19 in vitro studies have suggested that these cells are not stationary at this stage of differentiation, since appropriate stimulation can give rise to terminal differentiation20,21 and to CSR.22,23 In a recent work, we have shown that in contrast to normal circulating B cells, AID transcripts are expressed constitutively in some patients with CLL undergoing active CSR, but interestingly, this expression occurs predominately in unmutated CLL B cells.24
The inhibitor of differentiation (Id) protein family plays a regulatory role in the coordination of proliferation and differentiation, through sequestration of helix-loop-helix transcription factors and inhibition of the DNA binding functions of different transcription factors like Pax-5 and Ets proteins.25 In consequence, Id proteins appear to be involved in the CSR process, particularly interacting with the Pax-5 gene.26 Recent work from Gonda et al27 demonstrated that the balance between Pax-5 and Id-2 activities is key to the regulation of AID expression. In addition, it has been reported that expression of Pax-528 and AID29 genes is regulated by the transcriptional repressor Blimp-1 (B-cell lymphocyte–induced maturation protein-1), encoded by the prdm-1 gene.28 To gain insight into the molecular mechanisms underlying AID expression and CSR, we analyzed the expression levels of Pax-5, Id-2, prdm-1, and AID genes in normal human B cells and CLL B cells upon CD40 ligand stimulation, which induces expression of AID.
Our results show that AID expression in CLL B cells is subtly regulated by the differential expression of BSAP isoforms. The presence of AID protein is associated with expression of only the complete form of the Pax-5 gene (Pax-5a), whereas no expression of AID is associated with a reduction of Pax-5a mRNA and the appearance of a second spliced form with a complete deletion of exon 8 (Pax-5/Δ-Ex8). Since the deleted form lacks an important fragment of the autoregulatory activation domain while conserving the inhibitory region, we determined whether the appearance of this isoform was linked to control of Pax-5a and AID expression. In this work, expression of the Pax-5/Δ-Ex8 protein isoform and its capacity to bind to the AID gene promoter was assessed, and expression of Pax-5a, Pax-5/Δ-Ex8, Id-2, and prdm-1 transcripts was analyzed. Our results suggest that a subtle autoregulation of the Pax-5 gene products affects the expression of AID protein and is able to drive, at least in CLL B cells, the process of CSR.
Patients, materials, and methods
Patient samples
After informed consent, peripheral blood was obtained from 54 patients with a typical diagnosis of B-CLL and from 6 healthy control donors. The CLL patient group consisted of 32 males and 22 females, with a median age of 66 years (range 43-90 years). Twenty-eight were in stage A, 15 in stage B, and 11 in stage C. Twenty-seven expressed unmutated VH genes, whereas 27 expressed mutated genes (Table 2). Samples were from Necker Hospital (Paris, France). The diagnosis of B-CLL relied on cytologic features of mature lymphocytes and a characteristic phenotype (CD5+, CD23+, low expression of CD79b and of surface Ig).
Phenotypic and functional studies of B cells
Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation on Ficoll-Hypaque. Phenotypic analysis of CLL B cells was performed with anti-CD19 phycoerythrin (PE) and anti-CD5 fluorescein isothiocyanate (FITC), and the CSR process was evaluated by anti-human μ, δ, γ, and α chains F(ab′)2 conjugated with FITC. All antibodies were from Dako (Paris, France). Data were acquired and analysis performed using an EPICS XL flow cytometer (Beckman Coulter, Roissy, France). For CD40L plus interleukin 4 (IL-4) stimulation, B cells were purified through negative depletion by using a RosetteSep antibody cocktail (CD2, CD3, CD16, CD36, CD56) directly on peripheral blood as described by the supplier (StemCell Technologies, Vancouver, BC, Canada). B-cell purity was shown to be more than 99% by flow cytometry. Stimulation of 1.106/mL cells was carried out by in vitro culture for 5 days with 1 μg/mL recombinant soluble CD40L trimeric fusion protein, kindly provided by Amgen (Thousand Oaks, CA), and 1000 U/mL of IL-4 (Pharmingen, San Diego, CA). Sorting experiments of B-CLL cells to assess the clonal identity of switch transcripts were performed using the MOFLO cell sorter (Cytomation, Fort Collins, CO) using antibodies from Dako described above to isolate the following 3 different populations of B-CLL cells: (1) the IgM+ subset corresponding to cells coexpressing membrane IgM and IgD as identified by anti–human-μ chain antibodies; (2) cells expressing exclusively IgG (IgG+ subset), and (3) the IgM+/IgG+ subset corresponding to cells simultaneously expressing IgM and IgG, identified by anti–human μ and anti–human γ chain antibodies, respectively.
Analysis of RNA transcripts by RT-PCR
RNA was isolated from 5.106 B cells and cDNA synthesis was performed as described.24 cDNA of healthy and CLL B cells was used for amplification of AID, Pax-5, Id-2, prdm-1, and isotype switch transcripts by reverse transcription–polymerase chain reaction (RT-PCR). The RT-PCR protocol and the primers used for AID were performed as previously described.24 Amplification of the Ig family using 5′-VH leader specific primers together with a consensus JH primer in 3′ was carried out as previously described.30 The primers used for amplification of Pax-5 are depicted in Table 1 and Figure 1 and switch transcripts Id-2 and prdm-1 are also shown in Table 1. The PCR protocol for these genes included 30 cycles of amplification (95°C for 30 seconds, 62°C for 30 seconds, 72°C for 1 minute). As an internal control, amplification of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcripts was performed. All amplification fragments (Pax-5, Id-2, prdm-1, AID, isotype transcripts switch μ/δ/γ/α, and VDJ Ig transcripts) were cloned and sequenced.
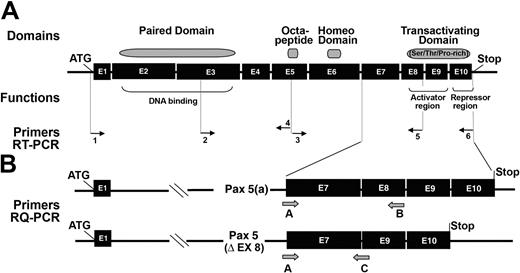
Functional regions and cDNA structures of 2 Pax-5 isoforms. (A) Annealing positions of primers used in RT-PCR, described in Table 1 as 1, 2, etc. (B) Schematic representation of the C-terminal domain of Pax-5a and Pax-5/Δ-Ex8 spliced form displaying positions of primers used in QRT-PCR, described in Table 1 as A, B, etc.
Functional regions and cDNA structures of 2 Pax-5 isoforms. (A) Annealing positions of primers used in RT-PCR, described in Table 1 as 1, 2, etc. (B) Schematic representation of the C-terminal domain of Pax-5a and Pax-5/Δ-Ex8 spliced form displaying positions of primers used in QRT-PCR, described in Table 1 as A, B, etc.
Quantitative real-time PCR
The quantitative real-time PCR (QRT-PCR) assay was performed using the SYBR Green I method on the Light Cycler System (Roche Molecular Biochemical, Mannheim, Germany). PCR was carried out in a 20 μL reaction volume containing 100 ng cDNA, 1X Light Cycler Fast Start Master Mix; 1 mM MgCl2, and specific primers for each amplification of Pax-5a, Pax-5/Δ-Ex8, Id-2, prdm1, AID, and GAPDH (Table 1 and Figure 1). Copy number was calculated with a standard curve generated from serially diluted (10-fold dilutions from 106 to 101 copies) plasmids containing the appropriate confirmed sequence of each gene. After incubation at 95°C for 10 minutes, the cDNA was amplified by 40 cycles of denaturation at 95°C for 15 seconds and a combined annealing/extension at 62°C for 15 seconds for each one. Light Cycler Software (Roche Molecular Biochemical) was used to analyze the fluorescence emission data after PCR. Pax-5a, Pax-5/Δ-Ex8, Id-2, and AID expression levels are expressed in the copy number of the amplified transcript. For prdm1 transcripts, the results are expressed as the ratio equal to mean of gene copy number divided by the mean of GAPDH copy number.
Western blot analysis
Nuclear and cytoplasmic extracts from CLL B cells with or without AID expression were performed by Nuclear Extraction Kit (Chemicon International, Mississauga, ON, Canada). Extracts were separated on 15% and 12% sodium dodecyl sulfate (SDS)–polyacrylamide gel for BSAP and AID protein analysis, respectively. After transfer, membranes were incubated with either anti-AID (C-20) goat polyclonal IgG or anti-Pax-5 (C-20) goat polyclonal IgG, and treated as described.31 All antibodies were from Santa Cruz Biotechnology (Le Perray, France). Finally, a nitrocellulose filter was incubated with SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL) and developed.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts of circulating CLL B cells with or without constitutive AID expression were prepared and protein amounts quantified. Double-stranded oligoprobes were end-labeled using T4 polynucleotide kinase (Biolabs, Beverly, MA), with γ [32P]–adenosine triphosphate (ATP; 260 TBq/mmol; Amersham, Arlington Heights, IL) and then purified over a Sepharose G50 column. The sequences of oligoprobes containing Pa3 promoter region of AID gene described by Gonda et al27 were as follows: 5′-GGGTGATGCTGTCAGGGGAGGAGCCCA AAAGGGCAAGCTC-3′ and 5′GAGCTTGCCCTTTTGGGCTCCTCCCTGACAGCATCACCC-3′. Each sample corresponding to labeled DNA Pa3 oligoprobes was incubated with nuclear extracts and 4 μg poly(dI-dC) nonspecific competitor. Binding reactions were carried out in a total volume of 20 μL, and incubated for 20 minutes at 37°C in buffer containing 25 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 1 mM dithiothreitol (DTT), 10% (vol/vol) glycerol. Loading buffer was added to the reaction mixture and samples were loaded onto a 6% nondenaturating polyacrylamide gel. After electrophoresis, the gel was dried and autoradiographed. Same samples incubated with unlabeled AID-P3 promoter oligoprobes were migrated in an identical position in the other part of the polyacrylamide gel. This half was then transferred to nitrocellulose membrane and incubated with 5 μg goat anti–Pax-5 antibody (C-20) for 3 hours at room temperature. Later, the nitrocellulose was washed with phosphate-buffered saline (PBS) and 0.3% Tween-20, incubated with horseradish peroxidase–labeled anti-goat immunoglobulin (1/10 000), and finally developed according to the enhanced chemiluminescence's method.
Results
Expression profile of AID and switch transcripts in CLL B cells
Table 2 shows that 21 (39%) of 54 CLL cases constitutively express AID; 16 (76%) of 21 AID+ CLL cases were unmutated and 5 (23%) were mutated. In addition, together with the typical AID amplification (transcripts labeled “a” in Figure 4A), 2 additional RNA transcripts (transcripts labeled “b” and “c” in Figure 4A) were amplified in both CLL and normal B cells. The clonal specificity of isotype switch variants was investigated by sequencing the different transcripts following amplification with family-specific primers in 5′ and isotype chain–specific primers in 3′ in the 54 patients with CLL. These experiments substantiated the presence of specific clonal VH switch transcripts in 16 (76%) of 21 patients, which constitutively expressed AID. Clonally unrelated IgG switch transcripts were found in the case of patients 20, 25, 34, and 36 (Table 2).
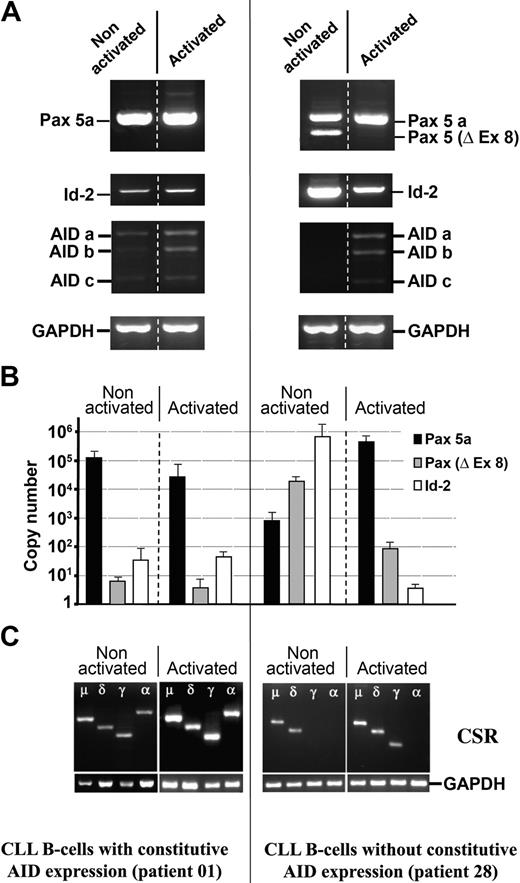
Expression profile of Pax-5a, Pax-5/Δ-Ex8, Id-2, AID, and switch transcripts in CLL B cells before and after stimulation with CD40L+IL-4. (A) Purified B cells from CLL patients 01 and 28 were stimulated in vitro with CD40L+IL-4 molecules, and amplification of Pax-5, Pax-5/Δ-Ex8, Id-2, AID, and GAPDH was analyzed. Splicing variants of the AID gene described in a previous work24 are labeled a, b, and c. (B) Quantification of Pax-5a (▪), Pax-5/Δ-Ex8 (▦), and Id-2 transcripts (□) before and after CD40L+IL-4 stimulation. All amplifications were normalized to GAPDH mRNA and the gene copy number was calculated. (C) CSR analysis by RT-PCR. Amplifications of purified B cells from activated and nonactivated CLL patients (nos. 01 and 28) using Ig isotype–specific primers are depicted.
Expression profile of Pax-5a, Pax-5/Δ-Ex8, Id-2, AID, and switch transcripts in CLL B cells before and after stimulation with CD40L+IL-4. (A) Purified B cells from CLL patients 01 and 28 were stimulated in vitro with CD40L+IL-4 molecules, and amplification of Pax-5, Pax-5/Δ-Ex8, Id-2, AID, and GAPDH was analyzed. Splicing variants of the AID gene described in a previous work24 are labeled a, b, and c. (B) Quantification of Pax-5a (▪), Pax-5/Δ-Ex8 (▦), and Id-2 transcripts (□) before and after CD40L+IL-4 stimulation. All amplifications were normalized to GAPDH mRNA and the gene copy number was calculated. (C) CSR analysis by RT-PCR. Amplifications of purified B cells from activated and nonactivated CLL patients (nos. 01 and 28) using Ig isotype–specific primers are depicted.
Expression profile of Pax-5 and AID transcripts in normal and CLL B cells
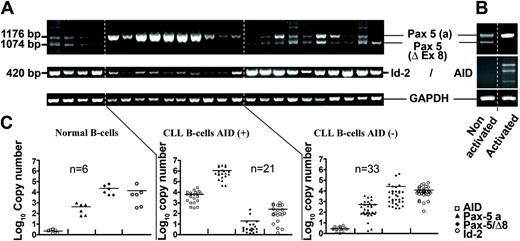
In order to analyze the expression of the Pax-5 gene, we studied B cells from 54 patients with CLL displaying or not displaying constitutive AID expression and B cells from 6 healthy donors to determine the expression profile of Pax-5 transcripts. Figure 2A depicts a representative profile of this expression. Results show that amplification of Pax-5a is predominantly found among CLL B cells with constitutive AID expression (CLL B cell/AID+). Interestingly, normal B cells and CLL B cells without constitutive AID expression (CLL B cell/AID-) display 2 main amplified fragments, one at the expected size of the full-length Pax-5 gene (1177 base pair [bp]) and another at 1074 bp (Figure 2A). Sequencing of these 2 transcripts revealed that the 1177-bp fragment contained the complete previously described form of the gene,14 whereas the 1074-bp amplification corresponded to a spliced form of the Pax-5 gene with a complete deletion of exon 8. Table 2 shows that 19 (90%) of 21 CLL B cell/AID+ patients displayed exclusive expression of Pax-5a (low levels of Pax-5/Δ-Ex8 were found in CLLs 32 and 37). Sixteen (84%) of 19 exhibited an active CSR process. Thirty-one of 33 CLL B cell/AID- cases displayed both Pax-5a and Pax-5/Δ-Ex8 (CLLs 39 and 48 only express Pax-5/Δ-Ex8). Six (100%) of 6 healthy donors and 33 (100%) of 33 CLL B cell/AID- patients expressed the Pax-5 spliced transcript (Pax-5/Δ-Ex8; Figure 2 and Table 2). Additional spliced variants such as Pax-5b, Pax-5d, and Pax-5e have been described in murine B cells.32 However, we cannot amplify Pax-5b transcripts in our experimental conditions, either in normal B cells or in CLL B cells. We have also evaluated by semiquantitative RT-PCR the expression of Pax-5d and Pax-5e but remarkably, no significant difference was found comparing the 2 populations of CLL B cells, whether expressing AID or not (data not shown).
Expression profile and quantification of Pax-5, AID, and Id-2 transcripts in normal and CLL B cells. (A) Semiquantitative gene-specific RT-PCRs of Pax-5a, Pax-5/Δ-Ex8, and Id-2 transcripts in normal B cells and in CLL B cells with or without expression of AID. GAPDH was used as cDNA control (B) mRNA transcript amplifications of the Pax-5 and AID genes before and after CD40+IL-4 stimulation in a representative normal healthy donor. (C) Quantification of AID (□), Pax-5a (▴), Pax-5/Δ-Ex8 ( ), and Id-2 expression transcripts (○) by QRT-PCR normalized to GAPDH mRNA. The gene copy number was calculated with a standard curve generated from serially diluted plasmids containing an amplified fragment of each gene. n = number of study samples.
), and Id-2 expression transcripts (○) by QRT-PCR normalized to GAPDH mRNA. The gene copy number was calculated with a standard curve generated from serially diluted plasmids containing an amplified fragment of each gene. n = number of study samples.
Expression profile and quantification of Pax-5, AID, and Id-2 transcripts in normal and CLL B cells. (A) Semiquantitative gene-specific RT-PCRs of Pax-5a, Pax-5/Δ-Ex8, and Id-2 transcripts in normal B cells and in CLL B cells with or without expression of AID. GAPDH was used as cDNA control (B) mRNA transcript amplifications of the Pax-5 and AID genes before and after CD40+IL-4 stimulation in a representative normal healthy donor. (C) Quantification of AID (□), Pax-5a (▴), Pax-5/Δ-Ex8 ( ), and Id-2 expression transcripts (○) by QRT-PCR normalized to GAPDH mRNA. The gene copy number was calculated with a standard curve generated from serially diluted plasmids containing an amplified fragment of each gene. n = number of study samples.
), and Id-2 expression transcripts (○) by QRT-PCR normalized to GAPDH mRNA. The gene copy number was calculated with a standard curve generated from serially diluted plasmids containing an amplified fragment of each gene. n = number of study samples.
CD40L+IL-4 stimulation represses Pax-5/Δ-Ex8 transcripts and induces the expression of AID transcripts in normal B cells
To gain insight into the putative role of Pax-5/Δ-Ex8 in AID regulation, we have evaluated the respective expression of Pax-5a and Pax-5/Δ-Ex8 in B cells from healthy donors, before and after stimulation by CD40L+IL-4. The results depicted in Figure 2B show a representative expression profile of normal B cells before and after stimulation. Nonactivated normal B cells express both Pax-5a and its spliced form Pax-5/Δ-Ex8 whereas AID transcripts are not detected. In contrast, activated normal B cells exclusively express the complete transcript Pax-5a and become able to express AID transcripts.
Quantitative evaluation of AID, Pax-5a, and Pax-5/Δ-Ex8 transcripts by QRT-PCR
Taking advantage of the deletion of exon 8 in the spliced form, we have designed specific primers in order to quantify the differential expression of Pax-5a and Pax-5/Δ-Ex8 (Figure 1B). As shown in Figure 2C, the average total copy number of Pax transcripts (Pax-5a + Pax-5/Δ-Ex8) was increased 53 times (1.4E+06/2,7E+04) between CLL B cell/AID+ and CLL B cell/AID- patients. In CLL B cell/AID- patients and in healthy controls, the Pax-5/Δ-Ex8 transcript represented 96% and 93% of total Pax-5 transcripts, respectively, whereas in CLL B cell/AID+ patients, the Pax-5/Δ-Ex8 transcript only accounted for 0.001%. The ratio between the percentage of Pax-5/Δ-Ex8 in AID- and AID+ CLL B cells is then increased 1E+05 times (96%; 0.001%). These results corroborate the data of RT-PCR showing that CLL B cell/AID+ displays a greater quantity of Pax-5a transcripts and weak amounts of Pax-5/Δ-Ex8 transcripts (Figure 2C). In contrast, an important augmentation of the Pax-5/Δ-Ex8 spliced form is observed in detriment of Pax-5a transcript in CLL B cell/AID- and in normal B cells, as shown in Figure 2C.
Id-2 transcript is increased in normal and CLL B cells with the presence of Pax-5/Δ-Ex8 and absence of AID expression
We have evaluated the expression of Id-2 transcripts by semiquantitative RT-PCR in both populations of CLL patients and in healthy donors. Initial results were in favor of an increase of Id-2 transcripts either in CLL/AID- or in normal B cells (Figure 2A). For additional confirmation, reverse quantitative (QRT)–PCR was carried out and the results clearly verified an inverted correlation between Pax-5a and Id-2 expression levels. The population of CLL B cells expressing AID and Pax-5a transcripts expresses minimal levels of Id-2 gene transcripts. In contrast, the population of normal and CLL B cells without AID expression, with diminished levels of Pax-5a and with presence of Pax-5/Δ-Ex8 displays an increased Id-2 transcripts expression (Figure 2C).
Analysis of CSR process and translation of AID, Pax-5a, and Pax-5/Δ-Ex8 protein in CLL B cells
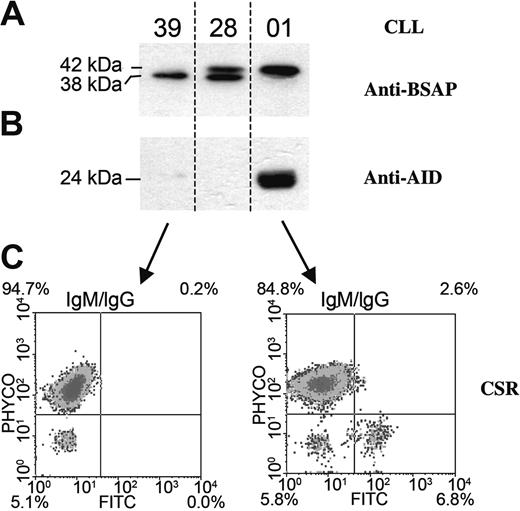
Because a similar profile expression of AID and Pax-5 variants has been observed in normal B cells and in CLL B cell/AID- before and after CD40L+IL-4 stimulation and given the important number of B cells required, the next experiments were exclusively conducted with CLL B cells. With the aim to evaluate the translation of Pax-5/Δ-Ex8 and to determine whether increased Pax-5a expression is linked to AID and CSR, we performed Western blot and flow cytometry analysis. Figure 3 depicts these results. Patient 01 corresponds to a patient with unmutated CLL expressing exclusively Pax-5a and AID transcripts. Western blot results show that anti–BSAP antibody identifies a single band displaying a relative molecular weight of 42 kDa, corresponding to BSAP complete isoform (Figure 3A) and a band corresponding to AID protein with a relative molecular weight of 24 kDa (Figure 3B). In contrast, the 2 other patients with mutated CLL (nos. 28 and 39) do not show expression of AID protein (Figure 3B) and display a different profile with anti-BSAP antibody. CLL patient 28 exhibits 2 bands; the first corresponding to the BSAP complete isoform as suggested by the relative molecular weight of 42 kDa, and the second with a relative molecular weight of 38 kDa. Only expression of this last band was detected for patient 39 (Figure 3A). Specific identification with anti-BSAP antibody of this second band suggest that it probably corresponds to the product of the Pax-5/Δ-Ex8 transcript, which is in agreement with results at the mRNA transcription level. Flow cytometry assays show that CLL B cells from unmutated patient 01, with Pax-5a and AID protein expression, are able to undergo CSR, simultaneously coexpressing clonal VH-μ and VH-γ transcripts (Figure 3C). However, CLL B cells from mutated patient 39 expressing only Pax-5/Δ-Ex8 isoform were found to neither express AID protein nor drive CSR (Figure 3). Absence of CSR process was also found in CLL B cells from mutated patient 28 expressing Pax-5/Δ-Ex8 and decreased Pax-5a (data nor shown).
Evaluation of the CSR process together with BSAP, Pax-5/Δ-Ex8, and AID translation in CLL B cells. Western blot analysis of BSAP, Pax-5/Δ-Ex8 spliced variant (A), and AID protein (B) were detected with specific antibodies from 3 representative patients (nos. 39, 28, and 01). Relative molecular weight is indicated in kilodaltons (kDa). (C) Flow cytometry analysis shows IgM and IgG isotypes in B-CLL lymphocytes from patients 01 and 39, respectively. Histograms show the percentage of cells expressing IgM, IgG, or both IgM/IgG.
Evaluation of the CSR process together with BSAP, Pax-5/Δ-Ex8, and AID translation in CLL B cells. Western blot analysis of BSAP, Pax-5/Δ-Ex8 spliced variant (A), and AID protein (B) were detected with specific antibodies from 3 representative patients (nos. 39, 28, and 01). Relative molecular weight is indicated in kilodaltons (kDa). (C) Flow cytometry analysis shows IgM and IgG isotypes in B-CLL lymphocytes from patients 01 and 39, respectively. Histograms show the percentage of cells expressing IgM, IgG, or both IgM/IgG.
CD40L stimulation enhances the expression of Pax-5a, AID, and CSR, but represses Pax-5/Δ-Ex8 and diminishes the expression of Id-2 transcripts in CLL B cell/AID- patients
We have quantified the expression of Pax-5 and Id-2 genes by QRT-PCR before and after CD40L+IL-4 signaling. This stimulation was carried out in 9 patients with CLL and 3 healthy control donors, shown in Table 3. Figure 4 depicts 2 representative patients with CLL according to either the presence of constitutive AID expression associated with single expression of Pax-5a, lower expression of Id-2 transcripts, and active CSR (unmutated CLL 01); or the absence of constitutive AID expression associated with the expression of Pax-5/Δ-Ex8, lower expression of Pax-5a, higher expression of Id-2 transcripts, and without detectable CSR (mutated CLL 28). The results show that after stimulation of CLL B cell/AID+, patient 01 did not show significant changes in AID, Pax-5a, Id-2, and switch transcripts (Figure 4A-C). Interestingly, after stimulation of CLL B cell/AID- the expression profile of patient 28 changed. Expression of Pax-5a increased, whereas expression of Pax-5/Δ-Ex8 and Id-2 was significantly reduced and AID transcripts were detected (Figure 4A-B). In addition, no switch transcripts were observed in nonstimulated CLL B cell/AID-, but after stimulation this patient was able to undergo CSR (Figure 4C) as it is confirmed by sequence of VDJ-μ, -δ, and -γ fragments (data not shown), and flow cytometry analysis as shown in Figure 3C. The clonal origin of these switch variants was substantiated following cell-sorting experiments for patients 1 and 2 (Table 3). In both cases 98% purified B cells could be obtained and the sequence was preceded in the different lymphocyte subsets expressing IgM+/IgD+ or IgM+/IgG+ or exclusively IgG+. Identical VDJ-Cμ, VDJ-Cδ, and VDJ-Cγ transcripts were found. Taken together, these results suggest that the presence of AID, increase in the expression of Pax-5a, absence of Pax-5/Δ-Ex8, and decrease of Id-2 are the complementary essential events in the process of CSR.
After CD40L+IL-4 activation CLL B cells expressing Pax-5a and AID proteins express lower amounts of prdm1 RNA transcripts
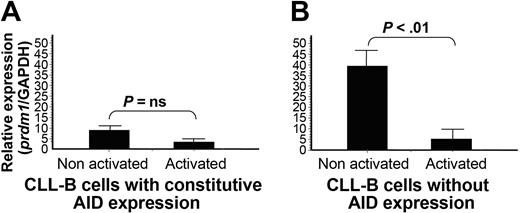
We have analyzed the expression transcripts of the prdm-1 gene by QRT-PCR before and after CD40L+IL-4 stimulation in both populations of CLL B cells. The results show that in CLL B cell/AID+, there are no significant changes in the expression profile of the activated and nonactivated populations (Figure 5A). In contrast, a significant diminution (P < .01) was found after activation of CLL B cell/AID- (Figure 5B), suggesting that expression of the prdm-1 gene is inversely correlated to BSAP and AID protein expression, 2 essential molecules in the CSR process.
Quantitative expression analysis of prdm-1 gene before and after CD40L+IL-4 stimulation of CLL B cells. Peripheral B cells from 7 patients with CLL constitutively expressing AID (A) and 7 patients with CLL without constitutive AID expression (B) were stimulated and cDNA extraction was performed. Transcript expression of prdm-1 mRNA was evaluated by QRT-PCR. Amplifications were normalized to GAPDH mRNA and the gene copy number was calculated by dilution of plasmids calibration curve containing the amplified product of the prdm-1 gene. Results were expressed as the ratio of mean gene copy number divided by the mean GAPDH copy number. The 2-tailed Student t test was performed on the arithmetic mean of each experimental point. A 2-tailed P < .05 was considered significant (ns, not significant). All analyses were done using GraphPad Prism, version 3.0 (GraphPad Software, San Diego, CA).
Quantitative expression analysis of prdm-1 gene before and after CD40L+IL-4 stimulation of CLL B cells. Peripheral B cells from 7 patients with CLL constitutively expressing AID (A) and 7 patients with CLL without constitutive AID expression (B) were stimulated and cDNA extraction was performed. Transcript expression of prdm-1 mRNA was evaluated by QRT-PCR. Amplifications were normalized to GAPDH mRNA and the gene copy number was calculated by dilution of plasmids calibration curve containing the amplified product of the prdm-1 gene. Results were expressed as the ratio of mean gene copy number divided by the mean GAPDH copy number. The 2-tailed Student t test was performed on the arithmetic mean of each experimental point. A 2-tailed P < .05 was considered significant (ns, not significant). All analyses were done using GraphPad Prism, version 3.0 (GraphPad Software, San Diego, CA).
Complete form of Pax-5 and spliced Pax-5/Δ-Ex8 isoforms bind the AID promoter
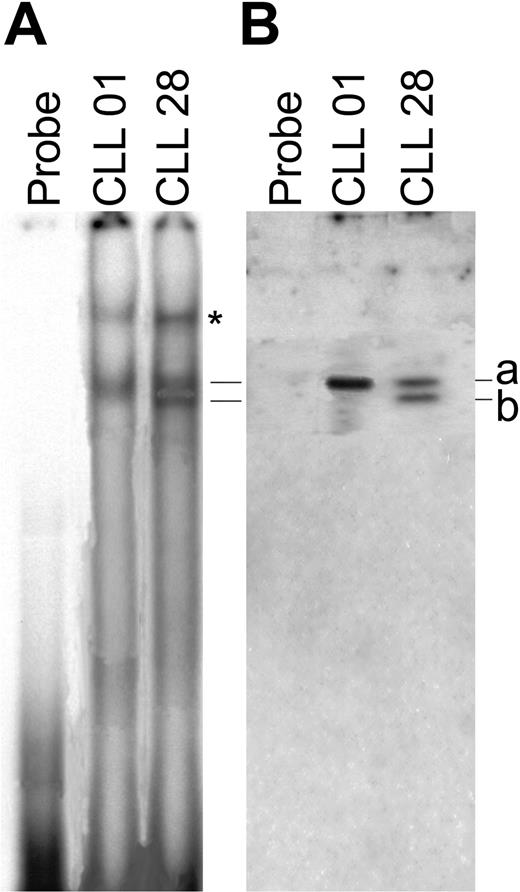
To determine if BSAP in its complete form or in its spliced variant deleted in exon 8 are able to bind the promoter of the AID gene, we have designed oligoprobes bearing the Pa3, and performed EMSA assays. Nuclear extracts prepared from CLL B cell/AID+ with restricted expression of Pax-5a (patient 01) and CLL B cell/AID- with expression of both Pax-5a and Pax-5/Δ-Ex8 (patient 28) were tested for binding to this oligoprobe. After a 3-hour exposure, 2 complexes were detected in CLL B cell/AID+ patient and 3 complexes in CLL B cell/AID- patient (Figure 6A). For control purposes, competitive experiments using different concentrations of the homologous unlabeled fragment (Pa3) were performed. Two complexes (Figure 6, bands a and b) one case from CLL B cell/AID- and one (Figure 6, band a) from CLL B cell/AID+ were inhibited following incubation with 100 ng unlabeled fragment. In addition, 25 ng labeled DNA Pa3 oligoprobes were incubated with increasing amounts of nuclear extracts and a protein-dependent concentration effect was visualized (data not shown). In order to confirm the specificity of these interactions we carried out Western blot analysis of these DNA-protein complexes with C-terminal anti-BSAP antibody. The results show that one of the 2 complexes in a CLL B cell/AID+ patient, no. 01, is specifically recognized by anti-BSAP antibody as shown in Figure 6B, whereas 2 complexes are identified in a CLL B cell/AID- patient, no. 28. These results suggest that spliced form of BSAP (Pax-5/Δ-Ex8) described here is also able to bind the DNA Pa3 region of promoter AID gene.
Binding of Pax-5 and spliced variant Pax-5/Δ-Ex8 to the AID promoter. (A) EMSA of nuclear extracts of CLL B cells from 2 patients was performed. The asterisk indicates a nonspecific band. Oligoprobes containing the P3 promoter region of the AID gene without nuclear extracts was used as control. (B) Western blot analysis with anti–Pax-5 (C-20) goat polyclonal IgG was performed to assess the specificity of the complex detected in EMSA. Solid lines (bands a and b) indicate the Pax-5a– and Pax-5/Δ-Ex8–containing complexes, respectively, in both assays (EMSA and Western blot). One of 3 independent experiments is shown.
Binding of Pax-5 and spliced variant Pax-5/Δ-Ex8 to the AID promoter. (A) EMSA of nuclear extracts of CLL B cells from 2 patients was performed. The asterisk indicates a nonspecific band. Oligoprobes containing the P3 promoter region of the AID gene without nuclear extracts was used as control. (B) Western blot analysis with anti–Pax-5 (C-20) goat polyclonal IgG was performed to assess the specificity of the complex detected in EMSA. Solid lines (bands a and b) indicate the Pax-5a– and Pax-5/Δ-Ex8–containing complexes, respectively, in both assays (EMSA and Western blot). One of 3 independent experiments is shown.
Discussion
Mature B cells, including completed functional V(D)J recombination of both heavy and light chain genes, migrate to the secondary lymphoid organs such as spleen and lymph nodes where they encounter antigens. B lymphocytes activated by antigen stimulation proliferate in lymphoid follicles and form special microenvironments called germinal centers. In this anatomical formation and in close contact with T cells, 2 different types of somatic DNA modification, CSR and SHM, take place. CSR of the Ig heavy chain is the genetic process capable of distributing a particular variable region to different Ig-constant domains, giving to each antibody different effector functions. A number of different processes have been reported to be involved in CSR, including transcription-generated, higher order RNA-DNA structures, specific DNA deamination, some DNA-repair pathways, and a complex interplay of different molecules involving several transcription factors. AID protein has been described as a central molecule in the initiation of CSR and SHM.33 Several reports23,34-36 demonstrated that AID is constitutively expressed in CLL B cells and that this expression predominates among unmutated forms of CLL. In addition, we previously demonstrated that expression of AID transcripts is constantly associated with induction of mutations in the preswitch region and CSR,24 suggesting a functional role for AID protein. These results showing a dissociation between SHM and CSR in CLL are in agreement with the accepted notion that AID would require additional factors to carry out the SHM and CSR process. Since the discovery of AID, the knowledge of CSR has considerably advanced. Presently, it is possible to envisage a mechanistic model of this process linking AID-mediated DNA deamination on the unique primary structure of S regions and the requirements for germ line transcription.37 However, several aspects of CSR both upstream and downstream of AID remain unsolved.
In this work we attempted to gain insight into the multifaceted mechanism of CSR by studying BSAP and Id-2 proteins, since both are key molecules in the regulation of the AID gene.27 Alternative splicing of transcription factors increases the regulatory capacities for gene expression, and this mechanism is commonly used within the several families of transcription factors. The full-length Pax-5a transcript has been identified and characterized in B lymphocytes, and mouse and human cell lines.14,38 In addition to Pax-5a, 3 isoforms of BSAP (Pax-5b, Pax-5d, and Pax-5e) were described in mice by Zwollo et al.32 Pax-5b and Pax-5e have spliced out their second exon, resulting in an incomplete DNA-binding domain, and Pax-5d and Pax-5e have replaced a region containing the transactivating domain with a novel sequence. In this work, we described the expression of the other spliced variant of BSAP with a complete deletion of exon 8, which appears to play an important role in the control of AID expression and CSR process. Although this variant has been previously described in human myeloma plasma B cells by Borson et al,39 data concerning its expression and its putative role are scarce. As concerns differential expression of Pax-5 transcripts, QRT-PCR experiments suggest that a transcriptional control mechanism at splicing level could be implicated in the regulation of this gene. The fact that total Pax-5 transcripts were not significantly changed (53-fold increase) when comparing CLL B cell/AID+ and CLL B cell/AID- patients and that the presence of Pax-5/Δ-Ex8 was augmented 1E+05 times in CLL B cell/AID- patients supports this hypothesis. In addition, the results showing that the presence of Pax-5/Δ-Ex8 is associated with absence of AID expression, diminution of Pax-5a, and increase of Id-2 transcripts, favor the view that this autoregulation mechanism could also play an important role in the expression of AID protein. Western blot and flow cytometry assays carried out in CLL B cells provide additional evidence about the function of the Pax-5/Δ-Ex8 isoform in AID expression and CSR regulation.
It has been demonstrated that CD40L cross-linking induces Pax-5 gene expression40 and that CD40L together with IL-4 is able to induce the expression of AID protein in a synergic collaboration with NFκB and signal transducer and activator of transcription 6 (STAT6).41 In this order, we have evaluated the expression profile of AID, Pax-5, Id-2, and prdm-1 transcripts before and after CD40L+IL-4 stimulation. The results in CLL B cell/AID- patients show that the Pax-5/Δ-Ex8 transcript is drastically repressed upon this stimulation, together with Id-2 and prdm-1, whereas expression of AID and switch transcripts occurs and there is overexpression of Pax-5a. In addition, similar results were observed in the normal human B cells after stimulation, favoring the view that the expression of AID is regulated by a multipartners control mechanism. While CLL B cell/AID- patients were induced to express AID, complete Pax-5a and switch transcripts upon CD40+IL-4 stimulation in CLL B cell/AID+ patients did not result in significant modifications in the expression profile of these genes. It is presently unclear whether this could be related to the fact that CLL B cell/AID+ corresponds to a subpopulation of previously activated B cells. Control experiments before and after stimulation were carried out in unmutated CLL B cell/AID- and mutated CLL B cell/AID+ patients. The results show that the unmutated CLL B cell/AID- patients expressed both isoforms of Pax-5 and increased amounts of Id-2 transcripts as compared with exclusive expression of Pax-5a and low levels of Id-2 transcripts in the mutated CLL B cell/AID+ patients. In the case of unmutated CLL B cell/AID- patients, CD40L stimulation induced AID expression and down-regulation of the Pax-5/Δ-Ex8. In contrast, this stimulation did not result in significant changes in the transcript's expression in the mutated CLL B cell/AID+ patients (data not shown). These stimulation experiments, mimicking B-T cell interactions, resulting in an important decrease of the prdm-1 inhibitory transcript, are in accordance with previous reports suggesting that Blimp-1 negatively regulates the proteins necessary for CSR.42
The Pax family members are transcriptionally regulated by the C-terminal serine/threonine/proline-rich region (Ser/Thr/Pro).13 Dorfler and Busslinger13 demonstrated that this C-terminal region between amino acids (aa) 319 and 358 constitutes the minimal transactivation domain of BSAP. This 40-aa region is placed in the medial region of exon 8 and in the first part of exon 9. Close to this activator region, a small portion of 22 aa, located in exons 9-10, has been identified as the inhibitory domain. The first and last of these exons (8 and 10) code exclusively for transactivating or inhibitory aa residues, respectively, while the second exon (9) contributes to both protein domains13 (Figure 1). In mouse B cells it has been demonstrated that the spliced form of Pax-5d has the ability to act as a transcriptional suppressor and in addition can repress the activity of Pax-5a.43 Pax-5/Δ-Ex8 described in human B cells includes a complete deletion of exon 8 and lacks more than 50% of the sequence described by Dorfler and Busslinger13 as a “minimal transactivation domain” of BSAP. In a recent work27 it has been proposed that Pax-5a up-regulates AID gene expression, through binding to its promoter region. Since the isoform Pax-5/Δ-Ex8 conserves a paired domain for DNA binding function, we performed EMSAs to define the putative function of this isoform in the control of AID expression. Gonda et al27 have described the existence of 4 putative BSAP binding sites (Pa1-Pa4) in the AID gene promoter and unequivocally demonstrated that Pa3 (GGGGAGGAGCCC) is the only sequence indispensable in the transactivation by BSAP. For control purposes, we have sequenced the promoter region of AID in CLL B cells from the 3 patients used in EMSAs, and confirmed that the Pa3 site is identical, as previously reported27 (data not shown). Considering these results, we performed EMSAs using nuclear extracts from these 3 patients with CLL and assessed their capacity to bind the Pa3 oligoprobe. The results of EMSA demonstrated that both the Pax-5a and Pax-5/Δ-Ex8 isoforms are able to bind the AID promoter. The binding specificity was substantiated by Western blot analysis showing that the anti–C-terminal BSAP antibody specifically recognized the DNA-protein complexes constituted by BSAP isoforms and the AID promoter region. This antibody binds the C-terminal region of Pax-5a, Pax-5b, and Pax-5/Δ-Ex8, but not of Pax-5d and Pax-5e, which express a different C-terminal domain. Therefore, since Pax-5b does not have the DNA-binding domain and the Pax-5d and Pax-5e spliced variants are not recognized by this antibody, we can suggest that the double band found in CLL B cell/AID- from patient 28 corresponds to binding of AID promoter region with Pax-5a and Pax-5/Δ-Ex8, respectively. Dorfler and Busslinger13 postulated that the intact C-terminal region of BSAP is required for efficient transcription of the TATA-less promoter. If true, the repressor function of this spliced variant may involve competition with Pax-5a for specific DNA-binding sites of the AID gene promoter, as previously shown for Pax-5d.43 This will mean that once the Pax-5/Δ-Ex8 isoform binds the AID promoter region the expression of this protein is down-regulated, which is consistent with our results in B cells from healthy donors and CLL B cell/AID- cases. However, the potential role of other transcriptional repressors like Blimp-1 and Id-2 should not be excluded. The results showing different expression levels of Id-2 and prdm-1 transcripts suggest that probably a regulation mechanism involving more than one protein would be necessary for the control in the expression of AID protein. In conclusion, our results provide evidence favoring the view that Pax-5/Δ-Ex8, an alternative splicing product of the Pax-5 gene in human B cells, plays an important role in its own transcription and indirectly in AID expression and the CSR process.
Prepublished online as Blood First Edition Paper, November 23, 2004; DOI 10.1182/blood-2004-09-3644.
Supported by grant no. 3261 from Association pour la Recherche sur le Cancer. Pablo Oppezzo is a fellow of the Académie Nationale de Médecine, Paris, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Drs. Alfonso Cayota and Michelle Goodhardt for review and discussion of this manuscript, Muhamed-Kheir Taha for technical assistance in EMSAs, and Reine Bouyssié for helpful secretarial assistance.