Comment on Schuettrumpf et al, page 2316
Schuettrumpf and colleagues report in this issue of Blood an innovative mouse model of gene therapy for hemophilia B that could substantially improve the efficacy of gene transfer.
Hemophilia B, an X-linked bleeding disorder characterized by a deficiency of coagulation factor IX (F.IX), is a preferred model for gene-based therapy because, in patients lacking this factor, 1% of normal levels could substantially reduce spontaneous bleeding. In past years, several groups1 have demonstrated sustained expression of clotting factors in animal models of hemophilia. The goal of the ongoing clinical studies is to determine whether these results can safely and efficiently be extended to humans. However, achieving and maintaining therapeutic F.IX levels is still a difficult task. In hemophilia B patients, intra-muscular injection of adeno-associated viral (AVV) vectors, encoding F.IX driven by viral- and tissue-specific promoters, has produced only subtherapeutic doses.
Schuettrumpf and colleagues have devised a mouse model of gene therapy for hemophilia B based on transduction by AAV vectors of mutant F.IX molecules characterized by advantageous biologic properties. Basic molecular biology and biochemistry studies conducted in the 1990s have found that lysine 5 and valine 10 are essential for interaction of F.IX with endothelial cells (collagen IV),2 and that the R338A change modulates the catalytic efficiency (3-fold increase in kcat and a 2-fold decrease in Km) of activated F.IX bound to its cofactor, factor VIII.3 This could improve F.IX biodistribution and availability to procoagulant macromolecular complexes by impairing F.IX binding to useless targets in the extracelluar space.
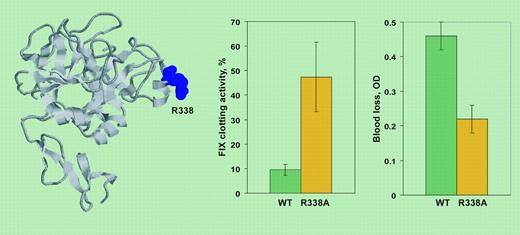
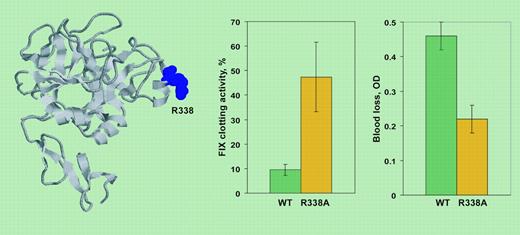
The optimized release of F.IX into the circulation and the gain of biologic activity were tested in mice and the results obtained (see figure) exceeded expectations. The variant with low-affinity to extracellular matrix (L5A/V10K) reached levels 2- to 5-fold higher than wild type, whereas the catalytically improved variant (R388A) showed a 2- to 6-fold-higher specific activity. Gene transfer of these mutant F.IX molecules, particularly with the catalytic variant, provided effective hemostasis in vivo upon a very crude challenge, the tailclipping assay. Moreover, injection of AAV-F.IX variant sin mice tolerant to wild-type F.IX did not cause antibody formation, a major problem in treatment of hemophilia patients with null mutations.
On the left, F.IX structure derived from 1RFN PDB (Protein Data Bank) entry. Residue R338 (spacefilling) was mutated to A for gene transfer. On the right, F.IX clotting activity and blood loss in a tailclipping assay 4 weeks after delivery of AAV-wild type F.IX or AAV-R338A F.IX to hemophilia B mice. Activity is reported as percentage of normal mice. OD indicates absorbance of hemoglobin in the saline solution in which the tail was placed.
On the left, F.IX structure derived from 1RFN PDB (Protein Data Bank) entry. Residue R338 (spacefilling) was mutated to A for gene transfer. On the right, F.IX clotting activity and blood loss in a tailclipping assay 4 weeks after delivery of AAV-wild type F.IX or AAV-R338A F.IX to hemophilia B mice. Activity is reported as percentage of normal mice. OD indicates absorbance of hemoglobin in the saline solution in which the tail was placed.
The potential of this approach has not been fully exploited: the amino acid changes improving biodistribution and catalytic properties, located in different F.IX domains, could be combined with each other in the same F.IX molecule, eventually producing additive effects. More robust AAV vectors,4 able to increase the number of transduced cells in the liver, could further boost the efficiency of gene transfer. It remains to be established whether new F.IX molecules and AAV vectors will be so powerful in humans.
The combination of protein molecular biology and biochemistry with viral vector technology provides a promising strategy to improve the efficacy for a variety of gene-based therapies in hematology. ▪