Comment on Saudemont et al, page 2428
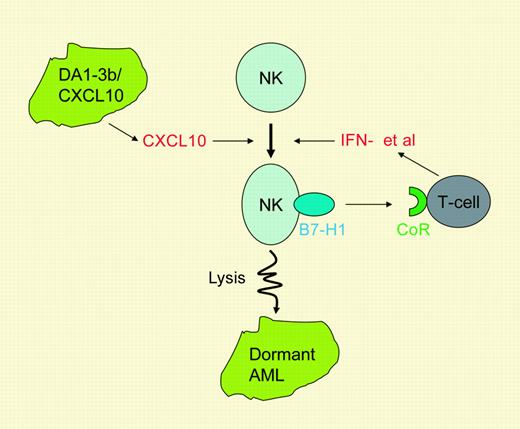
Dormant leukemia cells are selected to express B7-H1 and become resistant to T cells, whereas their susceptibility to NK cells remains intact. In contrast, NK cells expressing B7-H1 stimulate T-cell activation, which in turn keeps NK cells active. As a consequence, NK cells become the major player in eradicating dormant leukemia cells.
B7-H1 is a member of the immunoglobulin superfamily molecules. The expression of B7-H1 protein is limited to the macrophage lineage of cells in normal tissues, although its mRNA transcription is found in a broad range of tissues, indicating tight posttranscription control. Surface expression of B7-H1, however, could be induced by various cytokines, including interferon. Upon binding to its receptors on T cells, B7-H1 regulates activation and differentiation of T cells in both positive and negative fashion.1 Programmed death 1 (PD-1) is a receptor for B7-H1 and is shown to mediate the inhibition of T-cell response in peripheral tissues. The existence of an additional receptor that is involved in T-cell activation and apoptosis has been suggested.2 In contrast to limited cell-surface expression of B7-H1 in normal tissues, B7-H1 protein is abundant in various human cancers. Tumor-associated B7-H1 employs several mechanisms, such as inducing apoptosis of antigen-specific T cells to evade immune responses.3
In this issue of Blood, Saudemont and colleagues have shown that the mouse acute myeloid leukemia line DA1-3b, upon transfection to express CXCL10, induced a protective and therapeutic immunity against challenge of wild-type DA1-3b. More importantly, immunization by CXCL10-transfected leukemia cells as a cancer vaccine prevents the formation of tumor dormancy, a status similar to minimal residual diseases in clinic. The same group of investigators showed previously that dormant leukemia cells became resistant to cytolytic T-cell-mediated destruction in vitro and in vivo.4 This is largely due to selection of B7-H1+ and B7.1+ leukemia cells because blockade of B7-H1 and/or B7.1 significantly abrogates the resistance. In this report, a consequence of immunization by CXCL10-transfected leukemia cells is the recruitment and activation of natural killer (NK) cells in the lesion. Whereas dormant leukemia cells are resistant to T-cell-mediated cytotoxicity, they remain sensitive to NK cell lysis. These experiments support that NK cells are major effector cells against dormant leukemia cells after cancer vaccines.
NK cells are found to express a high level of B7-H1 after the immunization, and it is somewhat surprising that, in contrast to an inhibitory role of leukemia-associated B7-H1 on T cells, NK-associated B7-H1 promotes activation of T cells. Interestingly, activation of T cells by NK-associated B7-H1 appears to mediate through an alternative receptor rather than through PD-1. Because dormant leukemia cells are resistant to lysis of T cells, the main function of T cells in this model appears to be to activate or maintain activity of tumoricidal NK cells by providing interferon-γ and other cytokines (see figure). This hypothesis remains to be tested.
The role of B7-H1 in the connection of NK and T-cell immunity against tumor dormancy. Immunization by DA1-3b/CXCL10 cells activates NK cells and leads to expression of B7-H1. NK-associated B7-H1 activates T cells through an as-yet-unidentified counter-receptor (CoR). Activated T cells secrete cytokines such as interferon-γ to enhance NK activity, leading to destruction of dormant DA1-3b leukemia cells. These findings establish an important B7-H1 connection between innate and adaptive immunity.
The role of B7-H1 in the connection of NK and T-cell immunity against tumor dormancy. Immunization by DA1-3b/CXCL10 cells activates NK cells and leads to expression of B7-H1. NK-associated B7-H1 activates T cells through an as-yet-unidentified counter-receptor (CoR). Activated T cells secrete cytokines such as interferon-γ to enhance NK activity, leading to destruction of dormant DA1-3b leukemia cells. These findings establish an important B7-H1 connection between innate and adaptive immunity.
Clinical implications of the findings presented in this paper are several-fold. Cancer cells in minimal residual diseases may overexpress B7-H1 and/or other cell-surface molecules, which confer resistance of tumor cells to T-cell-mediated immunity. Cancer vaccines or cancer immunotherapy based on tumor antigen-specific T cells might not be sufficient to eradicate these residual cancer cells. A combined therapeutic approach, including selective blockade of tumor-associated B7-H1, should be considered. In addition, although there are various approaches to activate innate immunity components, including NK cells, it is important to select those approaches that not only activate innate immunity but also connect innate immunity to adaptive immunity. As demonstrated in this study, interleukin-12, a potent activator of NK cells, is less potent in the eradication of tumor dormancy and it is likely that this is partially due to the inability of this cytokine to induce B7-H1 on NK cells. ▪