Abstract
Infusion of either embryonic or mesenchymal stem cells prolongs the survival of organ transplants derived from stem cell donors and prevents graft-versus-host-disease (GVHD). An in-depth mechanistic understanding of this tolerization phenomenon could lead to novel cell-based therapies for transplantation. Here we demonstrate that while human mesenchymal stem cells (hMSCs) can promote superantigen-induced activation of purified T cells, addition of antigen-presenting cells (APCs; either monocytes or dendritic cells) to the cultures inhibits the T-cell responses. This contact- and dose-dependent inhibition is accompanied by secretion of large quantities of interleukin (IL)–10 and aberrant APC maturation, which can be partially overridden by the addition of factors that promote APC maturation (ie, lipopolysaccharide [LPS] or anti-CD40 monoclonal antibody [mAb]). Thus, our data support an immunoregulatory mechanism wherein hMSCs inhibit T cells indirectly by contact-dependent induction of regulatory APCs with T-cell–suppressive properties. Our data may reveal a physiologic phenomenon whereby the development of a distinct APC population is regulated by the tissue's cellular microenvironment.
Introduction
Mesenchymal stem cells (MSCs) are multipotential nonhematopoietic progenitor cells of the adult marrow capable of differentiating into various lineages of the mesenchyme.1 Although present at a low frequency in adult bone marrow, MSCs can be isolated and these cells can further replicate as undifferentiated cells in vitro. These cells are characterized by the absence of hematopoietic markers (such as CD45- and CD34-) and the expression of a specific pattern of adhesion molecules (such as CD106+ and CD105+). Under appropriate conditions, MSCs have the potential to differentiate to chondrocytes, tenocytes, skeletal myocytes, neurons, and cells of visceral mesoderm.2
There are several provocative links between stem cells and the induction of specific immune tolerance. More than one stem cell type has been associated with immune tolerance induction, including embryonic, hematopoietic, and mesenchymal stem cells.3-7 These stem cells have been successfully employed for tolerance induction in a variety of rodent and large-animal studies. A previous report8 suggests that MSCs are not only able to evade the immune system, but they can also suppress immune responses directed against third-party cells, even inducing tolerance toward other tissues of the same origin when transplanted following intravenous infusion of MSCs. This and other studies have further demonstrated that MSCs inhibit T-cell activation ex vivo.8-11 A recent case report has suggested that systemic infusion of haploidentical mesenchymal stem cells suppressed a grade IV graft-versus-host disease (GVHD) in a 9-year-old child who had received a bone marrow transplant.12 Nevertheless, the underlying mechanism for this tolerizing phenomenon, including the involved target cells, is not yet known.
Tolerance induction in the periphery is believed to be critical for the prevention of autoimmunity and maintenance of immune homeostasis. Central tolerance has been classically ascribed to clonal deletion of self-reactive T cells in the thymus upon interaction with self-antigens. However, central tolerance is incomplete because not all self-antigens gain access to the thymus, and several self-reactive lymphocytes escape central deletion. In the past several years there has been growing evidence supporting this notion, revealing subpopulations of cells representing different arms of the immune system, as potential regulators of the immune system. These specific groups include T-cell subtypes (such as CD4+CD25+ T cells), as well as a unique fraction of dendritic cells (DCs) described as semimature DCs, all of whom were shown to possess immune-modulating properties. Therefore, “sentinels” in the periphery of the body are essential to maintain tolerance as well as immunity. These tolerogenic effectors, while constitutively active in autoimmunity prevention, may play a pivotal role in maternal-fetal nonrejection, as well as in immune evasion of tumors and metastases.
Taken together, these studies on MSCs with their potential immunoregulatory activities create a compelling case for further pursuing stem cells as cellular tolerogens in the periphery. Given the uniqueness and potential importance of this observation, in this study we explore the mechanism underlying the immunoregulatory properties of human MSCs (hMSCs) and their ability to inhibit T-cell activation. Our data have uncovered a unique immunoregulatory mechanism wherein hMSCs induce regulatory antigen-presenting cells (APCs) that in turn inhibit T-cell activation.
Methods and materials
Cells
Cells were purified from the venous blood of healthy donors. Either CD4+ or CD8+ T cells or CD14+ cells were isolated by negative selection using the RosetteSep enrichment cocktail (StemCell Technologies, Vancouver, BC, Canada). hMSCs were obtained from discarded bone tissues from patients undergoing total hip replacement surgeries, under approval of the Hadassah Medical Center Helsinki Ethics Committee following informed consent. The hMSCs were separated from other bone-marrow–residing cells by plastic adherence, and were then grown under tissue-culture conditions as previously described.10,11 The cells were maintained in a low-glucose Dulbecco-modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 2 mM glutamine, and penicillin/streptomycin (Biological Industries, Beit-Haemek, Israel). For DC generation, CD14+ cells were plated in RPMI 1640 (Biological Industries) containing 1% autologous plasma, 0.1 μg/mL interleukin (IL)–4 and 0.1 μg/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; PeproTech, Rocky Hill, NJ). Every 2 days 0.3 mL was removed and 0.5 mL media containing plasma and cytokines were added. By day 7, more than 90% of the cells were CD14- and CD11c+. In order to activate DCs, lipopolysaccharides (LPS, 1 μg/mL; Sigma, St Louis, MO) were added to the cells for an additional 24 hours.
Cytokine production
Cultures containing 5 × 104 CD4+ or CD8+ T cells, and the indicated numbers of monocytes or DCs in the absence or presence of the superantigen staphylococcal enterotoxin B (SEB; Sigma), and in the absence or presence of hMSCs, were plated in individual wells of flat-bottom 96-well plates (Corning, Corning, NY). Cells were stimulated for 72 hours and conditioned media were collected. In some experiments either anti-CD40 (BD PharMingen, San Jose, CA), anti–transforming growth factor-β (TGF-β; R&D Systems, Minneapolis, MN) or anti–IL-10 receptor (R&D Systems) antibodies were added. Interferon-γ (IFN-γ), IL-5, tumor necrosis factor-α (TNF-α), TGF-β (R&D Systems), IL-1β, IL-12, and IL-10 (Diaclone, Besancon, France) levels in the conditioned media were assayed by enzyme-linked immunosorbent assay (ELISA).
Flow cytometry of cell-surface markers
CD4+ T cells were cultured with SEB-loaded monocytes for 72 hours in the absence or presence of hMSCs. CD86 (PharMingen), human leukocyte antigen-DR (HLA-DR), CD14 (IQ Products, Groningen, The Netherlands) and CD11c (Dako, Glostrup, Denmark) expression were measured by direct immunofluorescence using conjugated antibodies (R&D Systems), and the immunostained cells (1 × 104 cells/sample) were analyzed on a FACS Caliber flow cytometer (Becton Dickinson, San Jose, CA) using CELLQuest software (Becton Dickinson). The data were calculated as the mean fluorescence intensity.
Proliferation assay
CD4+ T cells and either monocytes, DCs, or whole PBMCs were cultured in flat-bottom 96-well plates (Corning) at 1 × 105 cells in 0.2 mL volume per well in triplicate. Cultures were pulsed with [3H]-methylthymidine (Amersham-Pharmacia Biotech, Buckinghamshire, England) for the last 18 hours. Cells were harvested onto glass-fiber filter paper (Schleicher & Schull, Dassel, Germany), dried, and the incorporated 3H analyzed with a liquid scintillation counter (Wallac, Gaithersburg, MD). Data points are expressed as mean counts per minute (cpm) of triplicate samples.
Results
Several previous studies8-11 have demonstrated that MSCs have immunoregulatory effects on T cells. These studies create a compelling case for further pursuing stem cells as cellular tolerogens. Given the uniqueness and potential importance of this observation, in this study we explore the mechanism underlying the immunoregulatory properties of human MSCs (hMSCs) and their ability to inhibit T-cell activation. To this end, hMSCs were derived from discarded bone tissues obtained from patients undergoing total hip replacement. The hMSCs were separated from other bone-marrow–residing cells by plastic adherence, and then were grown under tissue-culture conditions as previously described,1,13 and tested for their ability to inhibit T-cell activation, as measured by cytokine secretion and T-cell proliferation.
As described previously,10 hMSCs failed to elicit proliferation or secretion of IFN-γ when cocultured with unmatched peripheral blood mononuclear cells (PBMCs; data not shown). Moreover, while significant levels of IFN-γ were detected in the conditioned medium of PBMCs stimulated with either phytohemagglutinin (PHA) or the superantigen SEB, the addition of hMSCs significantly decreased the level of IFN-γ secretion (data not shown). This inhibitory activity of hMSCs (as determined by the inhibition of IFN-γ or proliferation) directly correlated with the number of stem cells in the culture, reaching up to 95% inhibition of the response (Figure 1A) and was independent of the concentration of stimulus (PHA; data not shown) or SEB (Figure 1B). hMSC inhibitory activity was retained following γ irradiation, but not after paraformaldehyde fixation (data not shown) as previously observed.8 As was previously reported,10 there is cell-to-cell contact dependence of hMSC-mediated inhibition based on the absence of inhibition when hMSCs were replaced with their conditioned media (data not shown), or when PBMCs and hMSCs were on opposite sides of a transwell membrane (Figure 1C). Interestingly, IFN-γ secretion as well as proliferation could be partially restored in hMSC-containing cultures by adding either LPS or anti-CD40 monoclonal antibodies (mAbs; Figure 1D). Given that triggering APCs such as monocytes with either LPS or anti-CD40 mAbs are known to promote APC maturation, this finding suggests that hMSCs may somehow interfere with normal APC maturation, thereby indirectly attenuating T-cell activation.
Characterization of hMSC-induced inhibition of IFN-γ secretion. (A) hMSCs inhibit the response of SEB-stimulated T cells in a dose-dependent fashion. PBMCs were stimulated with SEB (1 ng/mL) in the absence or presence of increasing numbers of hMSCs. The levels of IFN-γ in the culture media were determined by ELISA (left panel). Proliferation was determined by [3H]thymidine incorporation (right panel). The data represent the mean values of triplicate samples and standard deviations. (B) The inhibitory activity of hMSCs do not correlate with SEB concentration. PBMCs were stimulated with various concentrations of SEB in the absence or presence of hMSCs (5 × 104 cells). IFN-γ secretion was determined in the conditioned medium. (C) Inhibition of IFN-γ secretion by hMSCs requires cell contact. hMSCs and PBMCs were cocultured together or on opposite sides of a transwell in the presence of SEB (1 ng/mL) for 72 hours. IFN-γ secretion was measured in the conditioned media. (D) Proliferation and IFN-γ inhibition by hMSCs are partially restored by adding LPS or anti-CD40 monoclonal antibodies. PBMCs were cocultured with hMSCs (5 × 104 cells) and SEB (1 ng/mL). Either LPS (1μg/mL; left and right panels) or anti-CD40 mAb (1 μg/mL; middle panel) were added and either IFN-γ secretion or incorporation of [3H]thymidine was determined after 72 hours. The data in panels B-D were calculated as the percent inhibition of IFN-γ secretion in the presence of hMSCs compared with PBMCs stimulated in the absence of hMSCs. The results shown are representative of 3 separate experiments.
Characterization of hMSC-induced inhibition of IFN-γ secretion. (A) hMSCs inhibit the response of SEB-stimulated T cells in a dose-dependent fashion. PBMCs were stimulated with SEB (1 ng/mL) in the absence or presence of increasing numbers of hMSCs. The levels of IFN-γ in the culture media were determined by ELISA (left panel). Proliferation was determined by [3H]thymidine incorporation (right panel). The data represent the mean values of triplicate samples and standard deviations. (B) The inhibitory activity of hMSCs do not correlate with SEB concentration. PBMCs were stimulated with various concentrations of SEB in the absence or presence of hMSCs (5 × 104 cells). IFN-γ secretion was determined in the conditioned medium. (C) Inhibition of IFN-γ secretion by hMSCs requires cell contact. hMSCs and PBMCs were cocultured together or on opposite sides of a transwell in the presence of SEB (1 ng/mL) for 72 hours. IFN-γ secretion was measured in the conditioned media. (D) Proliferation and IFN-γ inhibition by hMSCs are partially restored by adding LPS or anti-CD40 monoclonal antibodies. PBMCs were cocultured with hMSCs (5 × 104 cells) and SEB (1 ng/mL). Either LPS (1μg/mL; left and right panels) or anti-CD40 mAb (1 μg/mL; middle panel) were added and either IFN-γ secretion or incorporation of [3H]thymidine was determined after 72 hours. The data in panels B-D were calculated as the percent inhibition of IFN-γ secretion in the presence of hMSCs compared with PBMCs stimulated in the absence of hMSCs. The results shown are representative of 3 separate experiments.
Monocyte involvement in hMSC-mediated tolerance induction was tested by titrating increasing numbers of monocytes into cocultures of hMSCs and CD4+ T cells in the presence of SEB. In control cocultures, CD4+ T cells were not activated in the absence of monocytes, verifying the purity of the CD4+ T-cell preparations, and addition of purified CD14+ cells (monocytes) increased IFN-γ secretion in a dose-dependent manner (Figure 2A, left panel). While considerable levels of IFN-γ secretion were detected when CD4+ T cells were cocultured with hMSCs in the absence of monocytes, the addition of monocytes to these hMSC-containing cocultures significantly inhibited IFN-γ secretion in a dose-dependent manner (Figure 2A, left panel). Both T-cell proliferation (Figure 2A, middle panel) and IL-5 secretion (Figure 2A, right panel) exhibited similar monocyte dose-dependent inhibition, as seen earlier with IFN-γ. Similar results were also obtained when CD8+ T cells were used instead of CD4+ T cells (Figure 2B). Notably, this suppressive effect was observed in both autologous or allogeneic monocytes (data not shown). Taken together, these data suggest that monocytes play a pivotal role in mediating hMSC inhibitory activity, and that the inhibitory signal from the hMSCs directed toward the APCs is contact dependent (as exemplified in repeated transwell experiments where PBMCs were replaced by purified CD4+ T cells and monocytes; data not shown).
hMSCs convert activating APCs into T-cell inhibitors. Either CD4+ T cells (A,C) or CD8+ T cells (B) were stimulated with SEB (1 ng/mL) in the absence (□) or presence (▪) of hMSCs (5 × 104 cells) and increasing numbers of either monocytes (A,B) or DCs (C). T-cell activation was followed by either IFN-γ secretion, incorporation of [3H]thymidine, or IL-5 secretion to the media. Each panel shows 1 representative experiment of 3.
hMSCs convert activating APCs into T-cell inhibitors. Either CD4+ T cells (A,C) or CD8+ T cells (B) were stimulated with SEB (1 ng/mL) in the absence (□) or presence (▪) of hMSCs (5 × 104 cells) and increasing numbers of either monocytes (A,B) or DCs (C). T-cell activation was followed by either IFN-γ secretion, incorporation of [3H]thymidine, or IL-5 secretion to the media. Each panel shows 1 representative experiment of 3.
These experiments employed monocytes as APCs, showing that they can be conditioned by hMSCs to inhibit T cells. Given that DCs are prototypical professional APCs, we questioned whether DCs can be similarly conditioned by hMSCs. To this end, we repeated the experiments described in Figure 2A, using monocyte-derived DCs in place of monocytes. Peripheral monocytes were cultured with GM-CSF and IL-4 for 7 days according to a standard protocol, and their subsequent ability to inhibit T-cell activation in the presence of hMSCs was tested. Titrating the number of DCs added to the hMSC cocultures inhibited IFN-γ secretion and T-cell proliferation in a dose-dependent manner. Thus, DCs parallel monocytes in their effects on T-cell activation in this hMSC-induced immunoregulatory system. Furthermore, we tested the possibility that this hMSC-elicited inhibitory property of DCs was somehow dependent upon a DC maturation state at the time of hMSC contact. DCs that had been grown with GM-CSF and IL-4 for 7 days were then exposed to LPS for 1 more day, prior to washing and adding them to hMSC cocultures. Inhibition of T-cell activation in these cocultures was significantly reduced compared with DCs that had not been treated with LPS (data not shown). This LPS priming reversed the inhibitory phenotype, presumably by overriding an hMSC-mediated block of DC maturation.
We next tested the hypothesis that hMSCs influence APC maturation, somehow locking them into an immature or semimature phenotype that correlates with T-cell inhibition.14,15 Therefore, we molecularly profiled hMSC-conditioned APCs, comparing them to nonconditioned APCs as controls, looking at both secreted cytokines for which APCs are the main producers using ELISA, and surface molecules by immunofluorescence and flow cytometry.
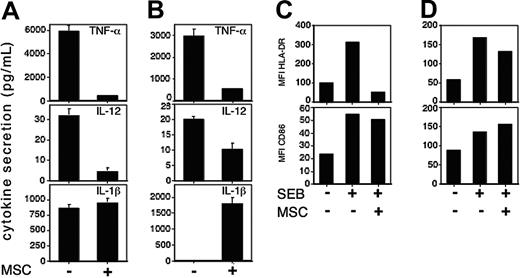
To this end, monocytes were cocultured with CD4+ T cells in the presence or absence of hMSCs, and cytokine secretion was determined in the conditioned media. While unactivated monocytes secreted low levels of IL-12, TNF-α, and IL-1β secretion, these levels were substantially increased upon SEB stimulation. In contrast, stimulation in the presence of hMSCs resulted in significantly lower levels of SEB-induced IL-12 and TNF-α; however, there was no effect on IL-1β secretion (Figure 3A). Similar results were obtained when DCs were used instead of monocytes (Figure 3B), except that low levels of IL-1β were detected exclusively when hMSCs were present (Figure 3B, lower panel).
hMSC-conditioned APCs exhibit a particular pattern of activation markers. Purified CD4+ T cells were stimulated with SEB (1 ng/mL) in the presence of either monocytes (A,C) or DCs (B,D) in the absence (-) or presence (+) of hMSCs (5 × 104 cells of each cell type were plated). After 72 hours, conditioned media were collected and the levels of the indicated cytokines in the media were determined using the appropriate ELISA kit (A,B). The data represent the mean values of triplicate samples and standard deviations. A representative of 4 separate experiments is shown. The cells were collected and the levels of CD86 and HLA-DR surface expression either on CD14+ cells (C) or CD11c+ cells (D) were determined by immunofluorescence and flow cytometry. Data are presented as mean fluorescent intensity of CD86 and HLA-DR staining. One of 3 experiments is shown. *P < .001, based on t test.
hMSC-conditioned APCs exhibit a particular pattern of activation markers. Purified CD4+ T cells were stimulated with SEB (1 ng/mL) in the presence of either monocytes (A,C) or DCs (B,D) in the absence (-) or presence (+) of hMSCs (5 × 104 cells of each cell type were plated). After 72 hours, conditioned media were collected and the levels of the indicated cytokines in the media were determined using the appropriate ELISA kit (A,B). The data represent the mean values of triplicate samples and standard deviations. A representative of 4 separate experiments is shown. The cells were collected and the levels of CD86 and HLA-DR surface expression either on CD14+ cells (C) or CD11c+ cells (D) were determined by immunofluorescence and flow cytometry. Data are presented as mean fluorescent intensity of CD86 and HLA-DR staining. One of 3 experiments is shown. *P < .001, based on t test.
We further looked for the expression of the surface molecule on CD14+ (monocytes; Figure 3C) and CD11c+ (DCs; Figure 3D) cells following coculture with hMSCs. Both monocytes and DCs expressed relatively low levels of CD86 and HLA-DR, which were increased upon SEB stimulation. HLA-DR up-regulation in monocytes was significantly reduced by hMSCs (Figure 3C, upper panel), and to a lesser degree in DCs (Figure 3D, upper panel). On the other hand, the level of CD86 expression on monocytes and DCs was not significantly affected by the presence of hMSCs (Figure 3C,D; lower panels). These findings seem to represent a unique developmental stage for APCs appearing to be similar to that of immature APCs, yet differing from immature APCs in their IL-1β and CD86 expression. Thus, these data suggest that hMSCs partially block APC maturation, leading to an aberrant APC phenotype with dissociation of otherwise clustered molecular markers. These APCs lack T-cell–activating capacity and possibly produce some inhibitory molecule(s) that can trigger T-cell–inhibitory events.
It is possible that these semimature, suppressive APCs act indirectly through the induction or activation of regulatory T cells. Of the several populations of T cells exhibiting suppressive mechanisms, CD4+CD25+ regulatory T cells are of special interest in our system since they arise spontaneously, and are present in the periphery. To determine whether CD4+CD25+ regulatory T cells play a role in hMSC-mediated inhibition, we depleted CD25+ cells from the purified CD4+ T cells before adding them to cocultures with hMSCs along with graded numbers of monocytes. In accordance with a previous report,10 removing the regulatory CD4+CD25+ T cells did not prevent the hMSC-mediated inhibition (data not shown).
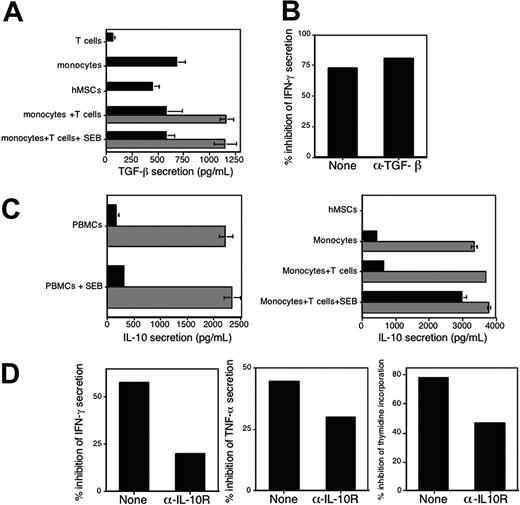
IL-10 and TGF-β are 2 anti-inflammatory cytokine candidates that may play a role in this system. First, IL-10 and TGF-β are potent suppressants of proinflammatory cytokine secretion by APCs (such as IL-12 and TNF-α). Second, IL-10 has been shown to play a role in elicitation of the regulatory DC phenotype and both IL-10 and TGF-β mediate the activity of T regulatory 1 cells that are generated via the regulatory DCs.16 We therefore tested whether these cytokines play a role in hMSC-mediated inhibition. First, the levels of TGF-β were determined in the conditioned media of either PBMCs or monocytes that were cocultured with CD4+ T cells, both in the presence or absence of hMSCs or hMSCs cultured alone (Figure 4A). TGF-β was detected in conditioned media of both hMSCs and immune cells. The level of TGF-β in the cocultures was the expected additive amount of the 2 cell types, indicating that the interaction of immune cells with hMSCs does not result in increased TGF-β secretion (Figure 4A). Furthermore, the addition of neutralizing Abs (specific for human TGF-β1-2) had no effect on the inhibitory activity of hMSCs as determined by IFN-γ (Figure 4B). These data support the notion that TGF-β does not play a major role in hMSC-mediated inhibitory activity. Next, the possible role of IL-10 in mediating hMSC-induced immunomodulation was examined. Interestingly, while PBMCs, monocytes, or both CD4+ T cells and monocytes (but not DCs; data not shown) secreted relatively low levels of IL-10, we detected up to a 10-fold increase in the concentrations of IL-10 in conditioned media of these cells when cocultured with hMSCs, regardless of the presence of SEB. Given that no IL-10 was detected in conditioned media of hMSC cultures (Figure 4C, right panel), these data suggest that coculturing hMSCs with these immune cells induced the secretion of relatively high levels of IL-10 (Figure 4C). To test whether IL-10 contributes to hMSC-derived T-cell inhibition once APCs are added to the system, we determined if addition of neutralizing anti-IL-10 receptor Abs abrogate inhibition and restore T-cell responses. Although addition of anti–IL-10 receptor Abs increased IFN-γ and TNF-α secretion as well as the proliferative response, these responses were still lower compared with control cultures. Thus, the inhibitory effect of hMSCs was only partially reverted by blocking IL-10 activity (Figure 4D).
The inhibitory effect of hMSCs is partially mediated by IL-10 but not by TGF-β. (A) CD4+ T cells, monocytes, and hMSCs were cultured alone (▪) or in combination (▦), in the presence or absence of SEB, as indicated. The levels of TGF-β in the conditioned media were assayed by ELISA after 72 hours. The data represent the mean of triplicate samples and standard deviations. (B) PBMCs were stimulated with SEB in the absence or presence of hMSCs (5 × 104 cells of each cell type). Neutralizing anti–TGF-β1-2 Abs (1 μg/mL) were added and IFN-γ secretion was measured in the conditioned media after 72 hours. The data are presented as percentage of inhibition of IFN-γ secretion in the presence of hMSCs compared with cells stimulated in the absence of hMSCs. The data in panels A and B are from 1 experiment; similar results were obtained in 2 other experiments. (C) Coculturing hMSCs with monocytes induces IL-10 secretion. PBMCs were either left untreated or were stimulated with SEB (1 ng/mL) in the absence (▪) or presence (▦) of hMSCs (left panel). Either monocytes or monocytes and CD4+ T cells that were either untreated or stimulated with SEB (1 ng/mL) were cultured alone (▪) or in the presence (▦) of hMSCs (5 × 104 cells per well; right panel). After 72 hours, the level of IL-10 in the various conditioned media was determined by ELISA. No IL-10 was detected in conditioned media of hMSCs cultured alone (right panel). The data represent the mean values of triplicate samples and standard deviations. (D) Purified CD4+ T cells were stimulated with SEB in the presence of monocytes and in the absence or presence of hMSCs (5 × 104 cells of each cell type were plated). Neutralizing anti–IL-10 receptor Abs (1 μg/mL) were added and either proliferation (right panel) or IFN-γ (left panel) and TNF-α (middle panel) secretion was measured in the conditioned media after 72 hours. Similar results were obtained when whole PBMCs were used (data not shown). The data are presented as percentage of inhibition of proliferation and cytokine secretion in the presence of hMSCs compared with cells stimulated in the absence of hMSCs. The results shown in panels C and D are representative of 3 separate experiments.
The inhibitory effect of hMSCs is partially mediated by IL-10 but not by TGF-β. (A) CD4+ T cells, monocytes, and hMSCs were cultured alone (▪) or in combination (▦), in the presence or absence of SEB, as indicated. The levels of TGF-β in the conditioned media were assayed by ELISA after 72 hours. The data represent the mean of triplicate samples and standard deviations. (B) PBMCs were stimulated with SEB in the absence or presence of hMSCs (5 × 104 cells of each cell type). Neutralizing anti–TGF-β1-2 Abs (1 μg/mL) were added and IFN-γ secretion was measured in the conditioned media after 72 hours. The data are presented as percentage of inhibition of IFN-γ secretion in the presence of hMSCs compared with cells stimulated in the absence of hMSCs. The data in panels A and B are from 1 experiment; similar results were obtained in 2 other experiments. (C) Coculturing hMSCs with monocytes induces IL-10 secretion. PBMCs were either left untreated or were stimulated with SEB (1 ng/mL) in the absence (▪) or presence (▦) of hMSCs (left panel). Either monocytes or monocytes and CD4+ T cells that were either untreated or stimulated with SEB (1 ng/mL) were cultured alone (▪) or in the presence (▦) of hMSCs (5 × 104 cells per well; right panel). After 72 hours, the level of IL-10 in the various conditioned media was determined by ELISA. No IL-10 was detected in conditioned media of hMSCs cultured alone (right panel). The data represent the mean values of triplicate samples and standard deviations. (D) Purified CD4+ T cells were stimulated with SEB in the presence of monocytes and in the absence or presence of hMSCs (5 × 104 cells of each cell type were plated). Neutralizing anti–IL-10 receptor Abs (1 μg/mL) were added and either proliferation (right panel) or IFN-γ (left panel) and TNF-α (middle panel) secretion was measured in the conditioned media after 72 hours. Similar results were obtained when whole PBMCs were used (data not shown). The data are presented as percentage of inhibition of proliferation and cytokine secretion in the presence of hMSCs compared with cells stimulated in the absence of hMSCs. The results shown in panels C and D are representative of 3 separate experiments.
Notwithstanding that this hMSC-induced IL-10 is not inhibitory on its own (given that hMSCs on their own activate T cells, despite the presence of IL-10 in the conditioned media of this coculture) (data not shown) and the moderate effect of the neutralizing anti–IL-10 receptor Ab, there remains the possibility that IL-10 works in concert with other immunoinhibitory factor(s) derived from hMSC-conditioned APCs.
Discussion
Previous studies have suggested that MSCs inhibit T-cell activation and can induce immune tolerance. However, the precise mechanism underlying this phenomenon is still unclear. While one study suggested that hMSCs interfere with T cell–APC contact,10 a second study suggested that the hMSCs themselves act as veto cells.17 The present data offer a different view, namely, an indirect inhibitory effect of hMSCs mediated by the APCs. Accordingly, the primary effect of hMSCs is to convert APCs into what is effectively a “deletional APC” with active immunoregulatory properties, as opposed to simply being activation incompetent. The critical observation here is that hMSCs can efficiently activate highly purified T cells (possibly due to expression of HLA-DR17 and CD8010 by the hMSCs) and surprisingly, addition of APCs attenuates this activation event. Obviously, our findings differ from those described by Krampera et al.10 Using mouse MSCs, Krampera et al10 have demonstrated contact-dependent inhibition of T-cell activation. However, the authors of that study have concluded that MSCs hinder T cells from the contact with APCs in a noncognate fashion, and that the presence of APCs is not required for MSCs to inhibit. At this stage, it is not clear why our data depart from the previous report (perhaps a species human versus mouse difference), but in any case, our results, using human cells, support the hypothesis proposed here, namely that in the presence of hMSCs, APCs are transformed into regulatory APCs.
There is a large and growing literature bearing upon the regulatory APC concept (reviewed in Steinman et al14 and Shortman and Liu18 ). In the past several years, the limited conception of APCs, and DCs in particular, as positive regulators (ie, as natural “adjuvants” that promote immune responses to foreign antigens) has been expanded to encompass the notion of APCs as negative regulators, with the recognition that APCs involved in immune induction are likely to also be involved in the induction of tolerance to self-antigens.19 Two general mechanisms have been proposed by which DCs might maintain peripheral tolerance. The first is that a subtype of specialized regulatory DCs maintains peripheral tolerance. The second is that all DCs have the ability to induce tolerance, with the capacity to induce tolerance or immunity dependent on the maturation or activation state of the DCs.14,15 According to this mechanism, the functional activities of DCs are dependent mainly on their state of activation and maturation; that is, terminally differentiated, mature DCs can efficiently induce the development of T-effector cells, whereas “immature” or “semimature” DCs maintain peripheral tolerance. Full APC maturation can be triggered by Toll-like receptors (TLRs; eg, with LPS, CpG oligodexynucleotides) or by ligation of CD40 by CD40L. Given our observation that LPS or anti-CD40 mAb can partially override hMSC-driven T-cell inhibition (Figure 1D), we have tested the possibility that hMSCs somehow disrupt APC maturation. The data presented establish that via cell-to-cell contact, hMSCs convert APCs into T-cell inhibitors (as opposed to APCs that are lacking T-cell–activating capacity) essentially by locking them in a semimature or in an aberrant maturation phenotype. This unique developmental stage includes not only low levels of expression of several proinflammatory molecules (such as IL-12, TNF-α, and major histocompatibility complex [MHC] class II expression), despite high expression of IL-1β and the costimulatory molecule, CD86, but also the increased expression of the anti-inflammatory cytokine IL-10. Further work will be required to clarify how these phenotypes are associated with each other, and how these intriguing developmental alternations in APC maturation contribute to their capacity to regulate T-cell responses. Interestingly, while the regulatory APC literature deals almost exclusively with DCs, in this study we demonstrate that like DCs, under specific conditions, monocytes can also develop into functionally regulatory APCs that actively inhibit T-cell activation.
As an aside, the model suggested here whereby hMSCs' immunoregulatory activity is mediated by the induction of regulatory APCs links 2 previous seemingly unrelated findings. On the one hand, regulatory DCs have been shown to protect mice from acute GVHD and leukemia relapse,20 and on the other hand, hMSCs have been successfully used in leukemic patients as a treatment of acute GVHD resulting from bone marrow transplantation.12 Thus, this model provides a possible mechanism through which hMSCs can alleviate GVHD.
This description of the outcome of stem cell interface with immune cells resembles the effect of apoptotic cells on APC activation.14,21 Whereas necrotic cells induce an immune response by activating APCs, exposure of APCs to apoptotic cells results in an APC maturation blockade. In this way, apoptotic material may play a role in the induction of peripheral tolerance to self-antigens derived from apoptotic cells. Specifically, uptake of apoptotic material by APCs (macrophages, monocytes, and DC) decreases the secretion of pro-inflammatory cytokine (TNF-α, IL-1β, and IL-12) while increasing the secretion of the anti-inflammatory cytokine, IL-10. Similar to our findings, antigen presentation and consequently the secretion of IFN-γ, which is induced by IL-12 and inhibited by IL-10, was also reduced in the presence of apoptotic cells, and could be partially restored by the addition of neutralizing anti–IL-10 antibodies.22-24
What locks APCs in a distinct differentiation and functional stage in situ has not yet been determined. The data presented in this study suggest that in at least a certain cellular microenvironment, cell-to-cell interactions may uncouple APC activation events and drive the differentiation of tolerogenic APCs with an intermediate phenotype. Thus, the plasticity of the APC maturation program allows them to modulate their function according to the nature and condition of the tissue, which in turn shapes T-cell response, giving rise to distinct functional outputs such as effector T-cells, memory T-cell generation, or T-cell tolerance.25-28 As suggested, we favor the hypothesis that APCs induce and maintain tolerance in the periphery by mechanisms that uncouple APC activation events and involve cell-to-cell interaction within the tissue microenvironment. This may provide an explanation for other examples whereby in certain in vivo milieus, APC maturation is altered acquiring a tolerogenic function. For example, Suter and colleagues29 have demonstrated that DCs isolated from mice with experimental autoimmune encephalomyeolitis (EAE) exhibit a maturational phenotype similar to that of immature bone DCs. In particular, these central nervous system (CNS)–DCs are unable to prime naive T cells and inhibit T-cell priming by bone marrow–derived DCs. TGF-β, IL-10, and TNF-related apoptosis-inducing ligand (TRAIL) were found to significantly contribute to the CNS-DC–mediated inhibition of T-cell proliferation. Thus, under the conditions of the privileged site of the brain, a special DC subset exists with an immature phenotype that secretes IL-10 and TGF-β.29 Interestingly, reminiscent of our LPS data, infectious diseases of the CNS overrule the standard immunosuppressive program of CNS-DCs, and under this condition CNS-DCs function as potent APCs.30 Other examples include tumors that have been shown to promote altered maturation of DCs,31,32 possibly representing a mechanism for tumors to evade immune detection, and pathogens shown to evade the immune responses by inducing IL-10 production by APCs (DCs and macrophages).33-35 Our experimental system may offer a unique setting to study how the development of a distinct APC population is regulated and to look for hMSC surface ligand(s) and their corresponding receptor(s) on the APCs that are linked to induction of the regulatory APC phenotype.
Prepublished online as Blood First Edition Paper, October 28, 2004; DOI 10.1182/blood-2004-07-2921.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Characterization of hMSC-induced inhibition of IFN-γ secretion. (A) hMSCs inhibit the response of SEB-stimulated T cells in a dose-dependent fashion. PBMCs were stimulated with SEB (1 ng/mL) in the absence or presence of increasing numbers of hMSCs. The levels of IFN-γ in the culture media were determined by ELISA (left panel). Proliferation was determined by [3H]thymidine incorporation (right panel). The data represent the mean values of triplicate samples and standard deviations. (B) The inhibitory activity of hMSCs do not correlate with SEB concentration. PBMCs were stimulated with various concentrations of SEB in the absence or presence of hMSCs (5 × 104 cells). IFN-γ secretion was determined in the conditioned medium. (C) Inhibition of IFN-γ secretion by hMSCs requires cell contact. hMSCs and PBMCs were cocultured together or on opposite sides of a transwell in the presence of SEB (1 ng/mL) for 72 hours. IFN-γ secretion was measured in the conditioned media. (D) Proliferation and IFN-γ inhibition by hMSCs are partially restored by adding LPS or anti-CD40 monoclonal antibodies. PBMCs were cocultured with hMSCs (5 × 104 cells) and SEB (1 ng/mL). Either LPS (1μg/mL; left and right panels) or anti-CD40 mAb (1 μg/mL; middle panel) were added and either IFN-γ secretion or incorporation of [3H]thymidine was determined after 72 hours. The data in panels B-D were calculated as the percent inhibition of IFN-γ secretion in the presence of hMSCs compared with PBMCs stimulated in the absence of hMSCs. The results shown are representative of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-07-2921/6/m_zh80050574820001.jpeg?Expires=1765032478&Signature=x~ek42q-g-q88JlHCSzR2HZO1YbZWgk3rL5kGIRBX58gZcWm9rrWbRplQX7r2tz5eun9yrZA1tuBwCvjpzPjiIFB9n7890GVzSB71nabLc2YJfw5kfB4L66i1-y0-SrgfAe6RbmMRFUeIdfP91oaKgYHHLLdSE2qt55uQA4CvkOpMLkocxHbGR5NFMd3GmNuq1nxVnX5JF5UuRwzbodLfs3KX15wr0~PYbGUPCxU0Ju91lz7Q2NZFHORT6g~-9Y9nZdhPi9QoDWFxgb7atUvkiuZR9iBZyz8shbjSJDwbm5EB1HAsyUFEqLDLHgOwwes1USAdb~dw7Cp-gGNzdtoyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. hMSCs convert activating APCs into T-cell inhibitors. Either CD4+ T cells (A,C) or CD8+ T cells (B) were stimulated with SEB (1 ng/mL) in the absence (□) or presence (▪) of hMSCs (5 × 104 cells) and increasing numbers of either monocytes (A,B) or DCs (C). T-cell activation was followed by either IFN-γ secretion, incorporation of [3H]thymidine, or IL-5 secretion to the media. Each panel shows 1 representative experiment of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-07-2921/6/m_zh80050574820002.jpeg?Expires=1765032478&Signature=TptM2eSo1u7LhzQhb8fIFnoTfpXSYikTAWQn-nEWzZfETYj0pUdCA2ksJleKx-D6zssqYznW6czBupER6SfOruG54A-cfvNS6GGOpTWSqKWRD-WQxu4MqxGsi3RouZ6SWb73jiP0mIuofxo642ieVLvGB405SerUIkYtigGaRd31OWDRItMCFXzzmSRDfigXTygjS~3VlfhvZ9KUQpU6Djz6DMgEbhl1Hi2oAcQQoHb0~aKsyfpRIy7siVKRPQAMhH3fb1qBGYn273dUuDGgiOM4sJcahYLodU4y3NUeSqCFBzWRJP9DuNec38QyEwirdT0wwEhHACb57-JmMAxlzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)